Abstract
Background
Bleeding is a common, noncardiac, preventable complication of PCI. We compared the relative safety of of radial access and bivalirudin in PCI.
Methods and Results
From CathPCI Registry®, we determined the association between the site of arterial access, bivalirudin and periprocedural bleeding rates in 501,017 patients. Radial access patients receiving heparin (RA) were compared with those receiving bivalirudin (RAB). Femoral access patients who had bivalirudin and a vascular closure device (VCD) served as a reference group (FA). An inverse probability weighting analysis incorporating propensity scores was used to compare groups. The overall rate of bleeding was 2.59 %. It was 2.71 % in the FA group, 2.5 % in the RA and 1.82 % in the RAB groups (P < 0.001). When compared with FA, the adjusted odds ratio (OR) for bleeding was significantly lower for patients with RAB (OR = 0.79, 95% CI = 0.72–0.86), but not for RA (OR = 0.96, 95% CI = 0.88–1.05), unless patients treated with IIb/IIIa were excluded (RA-IIb/IIIa OR=0.84, 95% CI=0.75–0.94). The number needed to treat (NNT) to prevent one bleeding event with RAB was 138, whereas NNT to prevent one bleeding event in high bleeding risk patients was 68.
Conclusions
In this observational analysis, the combination of bivalirudin and radial access was associated with reduced bleeding event rate. This benefit was present across the entire spectrum of preprocedural risk of bleeding, with or without exposure to IIb/IIIa inhibitors. These data support an adequately powered randomized trial comparing bleeding avoidance strategies.
Keywords: radial access, bivalirudin, bleeding, PCI
Periprocedural bleeding is a common, yet largely preventable noncardiac complication of PCI that is associated with early mortality (1) and higher costs of care (2). Use of bleeding avoidance strategies (BAS), such as bivalirudin and femoral vascular closure devices, has been associated with lower bleeding rates (3). Radial access is another effective BAS that significantly reduces access site bleeding even in the most challenging clinical scenarios (4). Nevertheless, radial access is used infrequently during PCI in the United States (5). For the femoral operator, bivalirudin along with optimized femoral access represents an attractive bleeding avoidance strategy, as it does not have a significant learning curve that is associated with the radial approach. Moreover, the use of bivalirudin reduces both access-site and non-access site bleeding (6).
Given the significant association between radial access and reduction in vascular complications, and the significant association between bivalirudin and reduction in overall major bleeding, combining radial access with bivalirudin may provide an opportunity to achieve the safest PCI outcomes. Prior trials of bivalirudin had limited radial access, and an analysis of the interaction between pharmacological strategies and radial access did not show an advantage of transradial PCI when bivalirudin was used as the procedural anticoagulant (7). The CathPCI Registry is a large, nationally representative contemporary database of PCI procedures that provides an ideal opportunity to explore the relationship between access site, anticoagulation and patient outcomes. Accordingly, we examined the association between radial access and bivalirudin with periprocedural bleeding rates across a spectrum of patients with varied preprocedural bleeding risk estimates. We hypothesized that the combination of radial access and bivalirudin would be associated with significantly lower bleeding risk compared with the combination of femoral access with bivalirudin and VCD.
Methods
Data Source and Study sample
CathPCI Registry® is an initiative of the American College of Cardiology Foundation and The Society for Cardiovascular Angiography and Interventions.
Descriptions of the NCDR have been published (8–9). Demographic, clinical, procedural, and institutional data elements from diagnostic catheterization and PCI procedures are collected at more than 1340 participating centers. Data are entered into a centralized database via either a secure Web-based platform or software provided by ACC-certified vendors. Data quality assurance includes automatic system validation and reporting of data completeness, random on-site auditing of participating centers, and education and training for site data managers (10). All data elements and definitions were prospectively defined by a committee of the ACC. A comprehensive description of NCDR data elements and definitions is available at https://www.ncdr.com/webncdr/cathpci/home/datacollection.
In order to describe the bleeding avoidance strategies, the most contemporary data from CathPCI Registry version 4 (July 2009 – March 2012) was analyzed. Only patients who underwent PCI via the radial and femoral artery approach were included in this analysis. For the purposes of the present study, we included only patients who underwent transfemoral PCI with bivalirudin anticoagulation and VCD, since a previously published study from the CathPCI registry showed this strategy to be safer than other anticoagulants and manual compression (3). Exclusion criteria consisted of patients with more than 1 PCI procedure during a hospitalization (since bleeding events could not reliably be attributed to a specific procedure), incomplete data for calculation of expected bleeding rates, PCI through access of a nonfemoral and nonradial artery (ie, ulnar, brachial), missing VCD data, death in the catheterization laboratory, or unknown data on bleeding events.
Endpoints and Definitions
The primary endpoint for this analysis was periprocedural bleeding, defined using the CathPCI registry definition as the presence of one or more of the following within 72 hours of PCI: external bleeding at the access site, hematoma, retroperitoneal hemorrhage, gastrointestinal or genitourinary bleeding, cardiac tamponade (to qualify all of the above bleeding events had to be associated with ≥ 3 g/dl hemoglobin drop or blood transfusion or intervention/surgery to stop the bleeding); intracranial hemorrhage, non-bypass surgery related blood transfusion among patients with a pre-procedure hemoglobin >8 g/dl, or an absolute decrease in hemoglobin ≥ 3 g/dl from pre-PCI to post-PCI in patients with a pre-procedure hemoglobin value ≤ 16 g/dl. Access site bleeding event was counted if at least one of the following was present: external bleeding at the access site, hematoma or retroperitoneal hemorrhage. All other periprocedural bleeding events were considered nonaccess site bleeds.
Ethical Considerations
This study was approved by the Duke University Medical Center institutional review board and was determined to meet the definition of research not requiring informed consent.
Statistical Analysis
Patients were grouped according to access site used for PCI (radial or femoral) and procedural anticoagulation (bivalirudin or other). Three bleeding avoidance strategies were considered: radial access(−)bivalirudin – “radial group” (RA), radial access(+) bivalirudin – “radial combination group” (RAB), femoral access(+)bivalirudin(+)vascular closure device – “femoral group”(FA). The femoral group (FA) served as a reference group. Demographic data by group are expressed as median (interquartile range), and compared using Kruskal-Wallis tests for continuous variables; and as a number (%) compared using Pearson χ2 tests for categorical variables. Given our large sample size, some p-values comparing demographic data between groups were highly significant despite a small magnitude of absolute differences. Therefore, we have discussed only differences that are both clinically and statistically significant. Based on individual bleeding risk calculated using the NCDR bleeding risk model (11), patients were categorized into 3 groups based on their risk for periprocedural bleeding events occurring during hospitalization: low (< 1.78 %), intermediate (1.78 % – 5.08 %), and high (> 5.08 %). Bleeding risk scores were generated for all patients in this study using the following baseline pre-PCI variables (c-index: 0.77): age, sex, body mass index (BMI), cerebrovascular disease (CVD), peripheral vascular disease (PVD), chronic lung disease (CLD), prior PCI, diabetes mellitus, renal function, presentation with ST-segment elevation myocardial infarction (STEMI), ejection fraction (EF), use of lytics prior to PCI, cardiogenic shock, pre-procedure cardiac arrest, PCI status (defined as elective, urgent, emergent, or salvage), subacute stent thrombosis, lesion risk as defined by the Society for Cardiovascular Angiography and Intervention (SCAI), lesion location (proximal LAD or Left Main vs. other), pre-procedure Heart Failure NYHA Class IV, pre-procedure TIMI flow, number of diseased vessels, and preprocedure hemoglobin.
To minimize confounding, an inverse probability weighting (IPW) analysis incorporating propensity scores was implemented. Propensity scores for three study groups representing a distinct BAS were derived using a multinomial regression model. Variables used in the propensity model included demographics (age, female gender, white race); clinical characteristics (imputed body mass index, heart failure NYHA class IV); coronary artery disease risk factors (diabetes, imputed hypertension, imputed dyslipidemia, current/recent smoker, family history of coronary artery disease); coronary artery disease history (prior PCI, prior coronary artery bypass graft surgery, prior MI); other cardiovascular disease history (prior CHF, prior CVD, prior PVD, prior valve surgery, cardiac transplantation); other disease history (prior chronic obstructive pulmonary disease, renal failure); and presenting syndrome (no symptoms, atypical chest pain, stable angina, unstable angina, ST-segment elevation MI, and non-ST-segment elevation MI). The effectiveness of the IPW (i.e. better balance among the patients that received different bleeding avoidance therapies) was evaluated using Cramer’s Phi (V) and R-squared and displayed graphically; values closer to zero demonstrate a more balanced cohort (12) (13). The model for bleeding was weighted using the inverse propensity score for getting each BAS and also included insurance status (none, other, Medicaid, Medicare), PCI type (elective, urgent, emergency), hospital region (West, Northeast, Midwest, South), community type (rural, suburban, urban), profit type (government, private, university), and each site’s average annual PCI volume. The model was fit using generalized estimating equations (GEEs) to account for the correlation between patients at the same center.
Odds ratios from the outcome model were obtained to estimate the number of patients needed to treat (NNT) to prevent 1 bleeding event for RA and RAB compared with FA (14). Statistical significance was defined as P<0.05. All statistical analyses were performed by the Duke Clinical Research Institute using SAS version 9.2 (SAS Institute, Cary, North Carolina).
Results
Study Sample and Efficacy of BAS
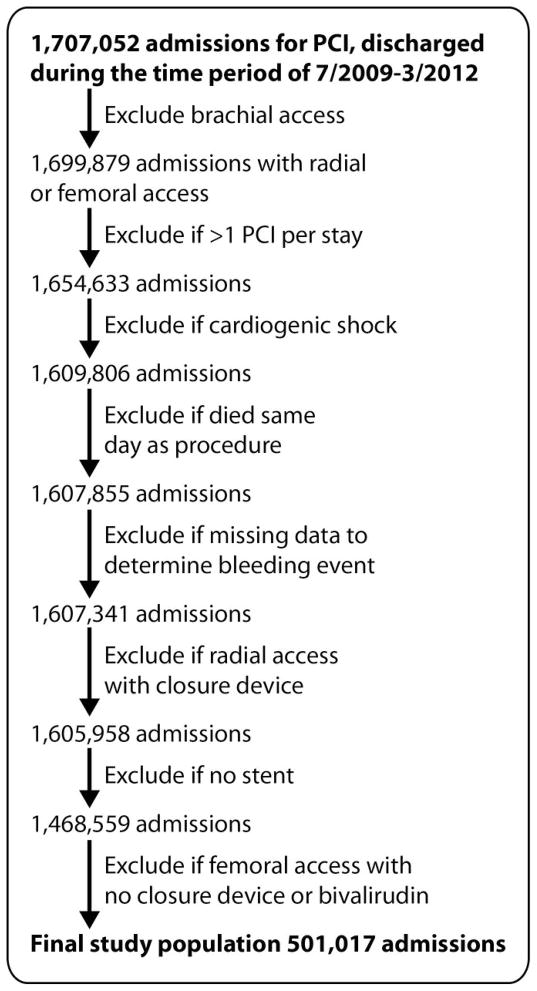
From July 1, 2009 to March 31, 2012, PCI procedures in 1,707,052 patients were reported to Cath PCI Registry. After applying the exclusions, 501,017 patients were available for this analysis (Figure 1). Baseline patient characteristics for the entire study sample and by BAS group are shown in the Table. During the study period, FA (with bivalirudin and VCD) was four times more common in the U.S. than radial access with or without bivalirudin.
Figure 1.
This figure shows the Consort diagram with patient population selection for the study. Abbreviation: PCI – percutaneous coronary intervention.
Table.
Baseline characteristics of the study sample
| Variable | Overall Cohort (N=501,017) 100% |
Femoral access*, bivalirudin (N=382,792) 76% |
Radial access, bivalirudin (N=55,188) 11% |
Radial access, heparin (N=63,037) 13% |
|---|---|---|---|---|
| Age | 65 (56,73) | 65 (56,74) | 64 (55,72) | 63 (55,72) |
| Male gender | 68.87 | 68.44 | 69.81 | 70.70 |
| Race - white | 88.86 | 88.83 | 88.92 | 89.01 |
| BMI (kg/m^2) | 29.3 (25.9,33.6) | 29.2 (25.8,33.4) | 29.9 (26.4,34.8) | 29.7 (26.2,34.4) |
| Previous MI | 29.15 | 29.68 | 28.37 | 26.62 |
| Previous CHF | 10.65 | 10.85 | 10.36 | 9.70 |
| Previous Valve Surgery | 1.35 | 1.42 | 1.12 | 1.12 |
| Diabetes | 35.89 | 33.93 | 36.81 | 34.82 |
| Cerebrovascular Disease | 11.18 | 11.34 | 10.92 | 10.44 |
| Peripheral Vascular Disease | 10.62 | 10.29 | 12.15 | 11.13 |
| Chronic Lung Disease | 13.98 | 13.89 | 14.92 | 13.72 |
| Hypertension | 82.36 | 82.54 | 82.85 | 80.87 |
| Current/Recent Smoker | 25.36 | 24.59 | 27.52 | 28.18 |
| Dyslipidemia | 81.34 | 81.59 | 81.54 | 79.66 |
| Family History of CAD | 25.85 | 25.55 | 26.91 | 26.70 |
| Previous PCI | 40.64 | 41.56 | 39.28 | 36.25 |
| Previous CABG | 16.31 | 18.66 | 8.93 | 8.45 |
| Renal Dialysis | 1.75 | 1.98 | 1.00 | 1.02 |
| Presentation Non-STEMI | 17.08 | 16.04 | 18.77 | 21.90 |
| Presentation STEMI | 9.11 | 9.41 | 6.10 | 9.96 |
| NYHA Class 4 | 18.88 | 18.65 | 19.14 | 20.07 |
| EF | 55 (50,60) | 55 (50,60) | 57 (50,60) | 55 (50,60) |
| GP IIb/IIIa | 12.31 | 8.53 | 7.39 | 39.57 |
| Low bleeding risk | 25.00 | 24.56 | 28.06 | 24.96 |
| Med bleeding risk | 50.00 | 49.69 | 51.26 | 50.98 |
| High bleeding risk | 25.00 | 25.74 | 20.69 | 24.07 |
All femoral access patients had a vascular closure device. Abbreviations: BMI – body mass index. MI – myocardial infarction. CHF – congestive heart failure. CAD – coronary artery disease. PCI – percutaneous coronary intervention. CABG – coronary artery bypass graft surgery. STEMI – ST segment elevation myocardial infarction. EF – ejection fraction. GP IIb/IIIa – glycoprotein IIb/IIIa.
The overall rate of bleeding was 2.59 %. Bleeding was reported in 2.71 % of patients in the FA group, compared with 2.5 % in RA group, and 1.82 % in RAB group (P < .0001). Bleeding rates by bleeding avoidance strategy and proportions of patients with access (vs. nonaccess) site bleeding are presented in Figure 2. Among patients without IIb/IIIa inhibitors, when compared with FA, the adjusted odds ratio (OR) for bleeding was significantly lower for patients with RAB (OR=0.78, 95% CI=0.71–0.86), and for patients with RA (OR=0.84, 95% CI=0.75–0.94). However, when compared with FA without IIb/IIIa inhibitor, the OR for bleeding was significantly higher for patients with RA and IIb/IIIa inhibitor (OR=1.41, 95% CI=1.26–1.58).
Figure 2.
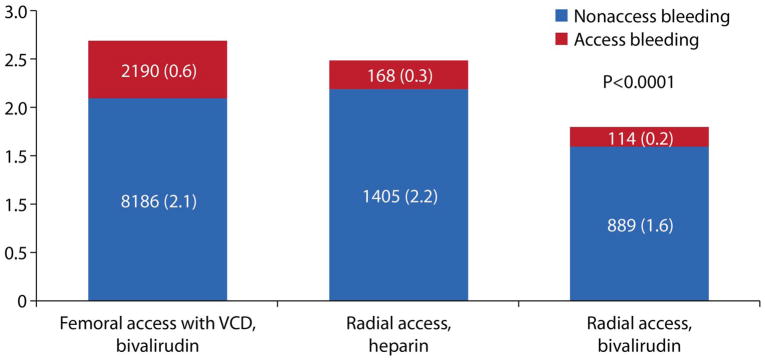
This figure demonstrates the rate of PCI – associated bleeding in 501,017 patients grouped by vascular access and anticoagulation. The numbers in columns are numbers of patients with bleeding and % (in parenthesis). Abbreviation: VCD – vascular closure device.
There was no difference in inhospital death (FA 0.27%, RA 0.26%, RAB 0.22%), stroke (FA 0.14%, RA 0.17%, RAB 0.16%) or periprocedural infarction (FA 1.77%, RA 1.73%, RAB 1.66%), P=NS.
Use of Bleeding Avoidance Strategies by Strata of Bleeding Risk
Among patients with FA, 25.74 % were at high risk for bleeding, whereas among patients with RA the proportion of high risk patients was lower − 24.07 %; among patients with RAB it was the lowest− 20.69 %, P < .0001. The proportion of low risk of bleeding patients was 24.56 % among patients with FA, 28.06 % - among patients with RA, and 24.96 % in patients with RAB, P < .0001.
Inverse Probability Weighting and Center-Adjusted Analysis
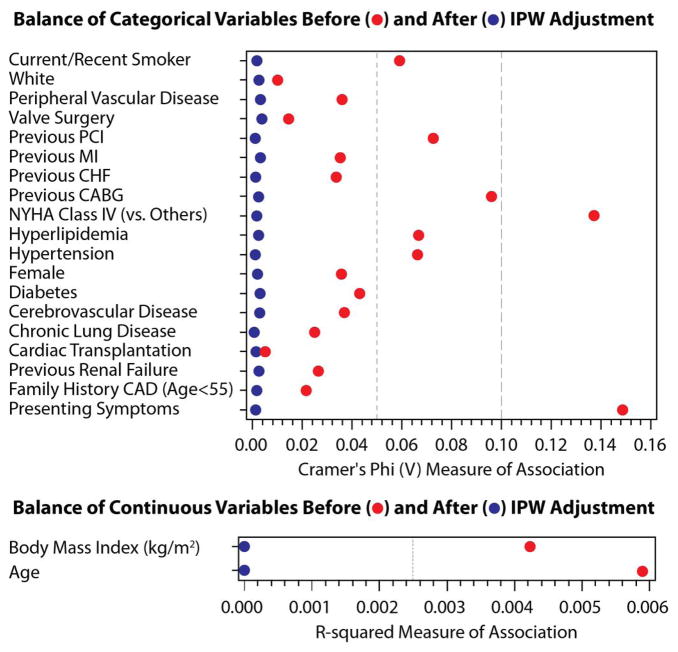
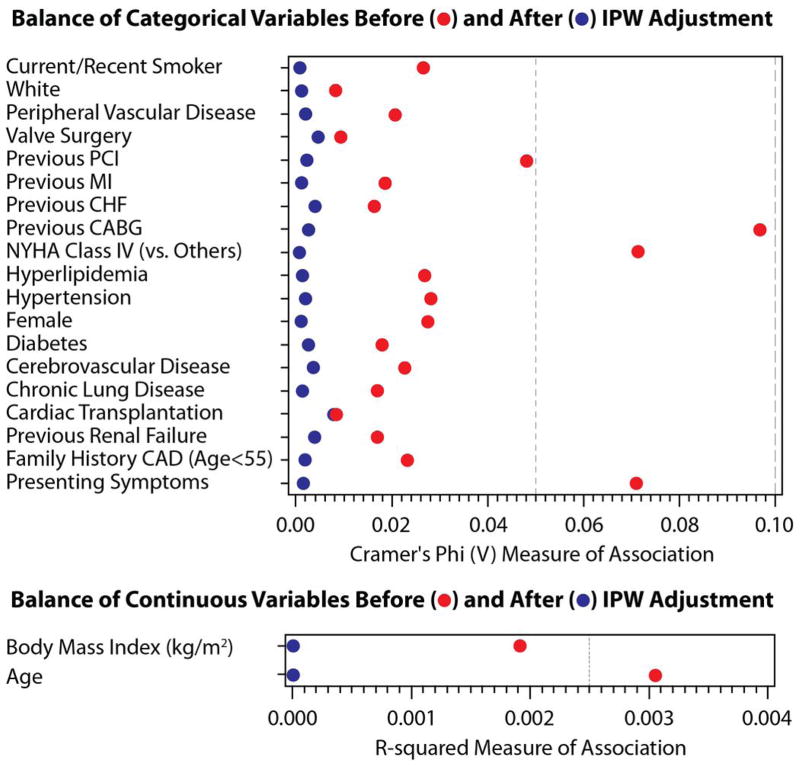
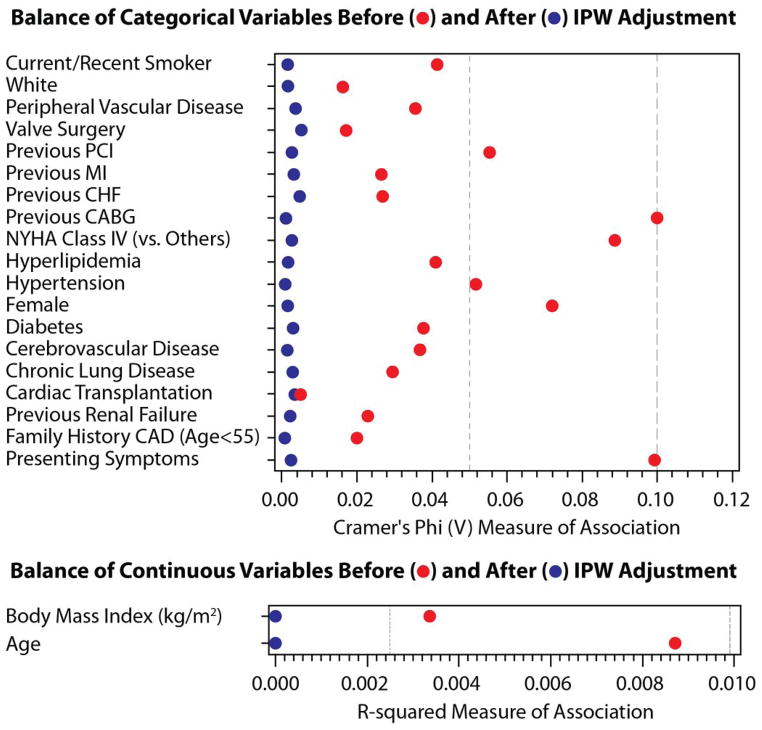
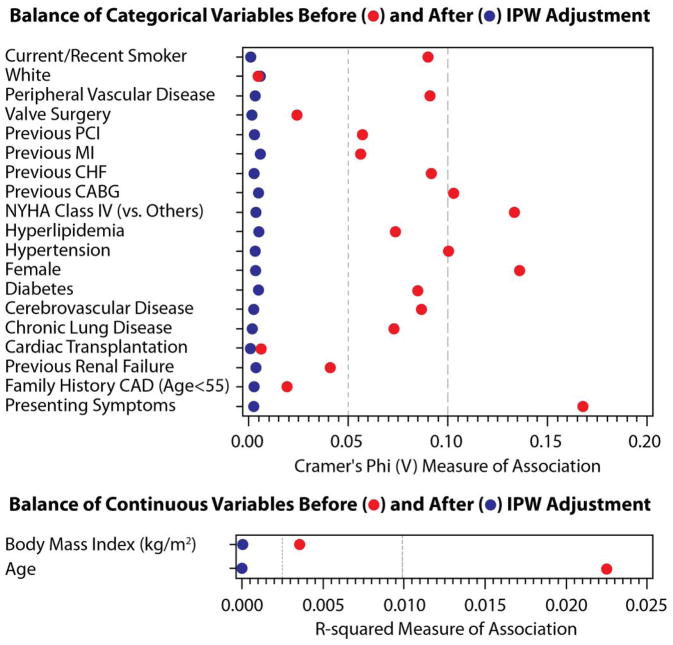
The effectiveness of inverse probability weighting (IPW) was assessed in the propensity model. Figure 3 displays the results of IPW adjustment for categorical variables (Cramer’s Phi V plot) and continuous variables (R-squared plot) for the total cohort and for the low, intermediate and high bleeding risk subsets. After adjustment, each measure was less than 0.01 for Cramer’s Phi, and was less than 0.001 for R-squared.
Figure 3.
This figure demonstrates the effectiveness of Inverse Probability Weighting adjustment. Bleeding risks cohorts: total – Figure 3A, low – Figure 3B, intermediate – Figure 3C, high – Figure 3D. The effectiveness of the IPW was evaluated using Cramer’s Phi (V) and R-squared and displayed graphically; values closer to zero demonstrate a more balanced cohort. Abbreviations: IPW – inverse probability weighting. PCI – percutaneous coronary intervention. MI – myocardial infarction. CHF – congestive heart failure. NYHA – New York Hospital Association. CAD – coronary artery disease.
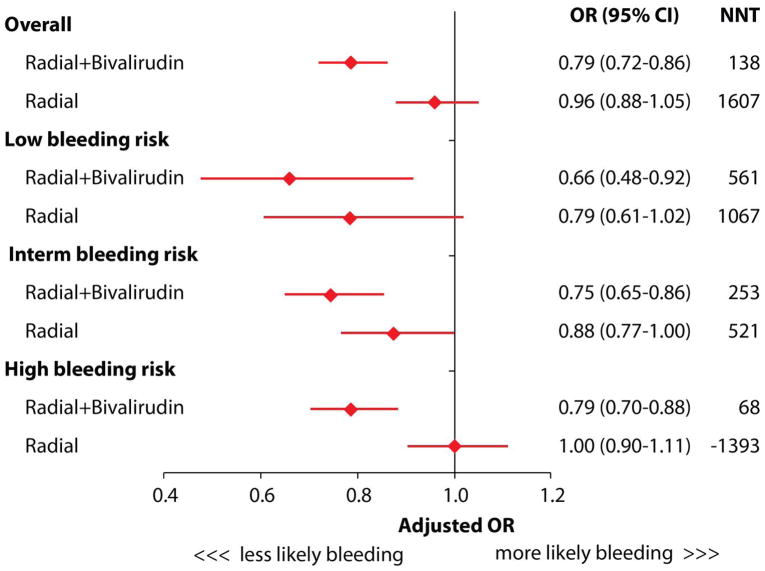
The adjusted odds ratio and corresponding 95% confidence intervals for periprocedural bleeding across the spectrum of bleeding risk, as well as NNT to prevent one bleeding complication, are presented in Figure 4. Overall, compared with patients in the reference FA group, the adjusted odds ratio (OR) for bleeding was significantly lower for patients with RAB (OR = 0.79, 95% CI = 0.72–0.86), but not for patients with RA alone (OR = 0.96, 95% CI = 0.88–1.05). The association between RAB and reduced risk for bleeding was present across the spectrum of bleeding risk, with NNT of 561 in low risk patients, 253 in medium risk, and 68 in high risk patients. The overall NNT to prevent one bleeding event with RAB was 138.
Figure 4.
This figure demonstrates the adjusted odds ratios (OR) of bleeding events and Number Needed to Treat (NNT) to prevent one bleeding in PCI. The interaction p-value between BAS and pre-PCI bleeding risk was less than 0.0001.
Discussion
Using data from a broadly representative contemporary CathPCI Registry, we found that the combination of radial access and bivalirudin anticoagulation is associated with a significant reduction in post-PCI bleeding compared with either radial access alone or the combination of femoral access, bivalirudin, and vascular closure devices. This was true across the entire spectrum of procedural bleeding risk, and our finding that patients at highest bleeding risk were least likely to undergo the radial combination strategy suggest that wider adoption of this approach, particularly in high risk patients, may significantly improve the safety of PCI.
The findings of this study add considerably to the previously published data on the use of radial access alone and bivalirudin alone for reducing PCI-related bleeding. Radial access has been associated with reduced rates of bleeding during PCI for acute coronary syndromes (ACS), but this is primarily at the access site (7, 15–16). A significant proportion of bleeding events in ACS patients also occur remote from the access site, and the radial approach would not be expected to have a significant effect on this type of systemic bleeding. For example, in the largest randomized clinical trial of radial vs. femoral approach in patients with both non-ST-segment and ST-segment elevation ACS, non-access site bleeding accounted for two-thirds of the bleeding events (17). Accordingly, there was no difference in the rate of non-CABG related major bleeding between patients assigned to radial or femoral access. Interestingly, we observed that the use of glycoprotein IIb/IIIa inhibitors and unfractionated heparin was much more common among patients undergoing transradial PCI, which may reflect a sense of false security that the radial approach affords a reduction in total bleeding. Our study showed that when IIb/IIIa inhibitors are used, the risk of bleeding is increased, which is consistent with the prior studies. Radial access has not been shown to reduce non-access site bleeding. However, when a subgroup if patients without IIb/IIIa inhibitors was analyzed, radial access alone, and more so in combination with bivalirudin, was associated with reduced bleeding event rate.
The impact of bivalirudin on the incidence of periprocedural bleeding has been also evaluated in clinical trials (6, 18–23) and registries (3, 24–25), consistently demonstrating lower bleeding risk in all studied clinical scenarios. Adding vascular closure devices to bivalirudin in the setting femoral access is associated with even better safety. For this reason we elected to exclude patients with femoral access without closure device and non-bivalirudin anticoagulation, in order to compare radial access with the default U.S. “state of the art” bleeding avoidance strategy.
A risk-treatment paradox in the use of the vascular closure device – bivarirudin combination has been previously reported (3), and we have found that a similar paradox exists with the radial-bivalirudin combination strategy. Potential reasons why this paradox exists for radial-bivalirudin combination in clinical practice are multiple. There is a significant learning curve for the radial approach and most diagnostic procedures require use of unfractionated heparin to reduce the risk of radial artery occlusion. Therefore, there may be reluctance to switch from heparin to bivalirudin for ad hoc PCI due to concerns over increasing bleeding risk, incurring higher costs, or insufficiently developed clinical protocols. While the present study cannot address these issues directly, it does clearly show an association between radial access and bivalirudin and the lowest rate of bleeding, which is particularly pronounced in high-risk patients.
There are some potential limitations to this study. First, as with any other observational study, associations between treatments and outcomes cannot prove causality. A limitation of the adjustment methodology is that there is always a chance of unmeasured confounding. There might be residual factors that are not known or captured by the registry that may bias the results in favor of the femoral or radial access. However, operators and hospital systems in the United States are much more familiar with femoral access, and any advantage of the radial access shown by providers early in the learning curve would likely become more evident with more experience in radial procedures. Second, while an auditing program is used to verify data accuracy in the registry, outcomes are not adjudicated and thus there may be underreporting or misclassification of complications (26). Third, we could not examine bleeding by other definitions, including the Bleeding Academic Research Consortium (BARC) definition (27), since particular data elements necessary are not present in the CathPCI registry. The definition of bleeding used in version 4 of the CathPCI data collection form (used for the present analysis), approximates BARC Type 2 and 3 bleeding. Fourth, patients without VCDs were excluded from this analysis, but such patients have been shown to have higher rates of bleeding in the CathPCI registry, and the association of the radial access with reduced bleeding would likely have been even higher, if compared against those who did not have VCDs.
Conclusion
In this observational analysis, the combination of bivalirudin and radial access was associated with reduced bleeding, when compared with either radial access alone or the combination of femoral access, vascular closure devices, and bivalirudin. Radial access-bivalirudin combination was used less frequently among patients at highest risk for bleeding. Given the limitations of these observational data, randomized clinical trials are needed to confirm the benefit of radial access combined with bivalirudin. Until these data are available, the wider application of a radial combination strategy, particularly among high risk patients, may further improve the safety of PCI.
Supplementary Material
What is Known
Bleeding is a relatively common, morbid complication of PCI
Use of bivalirudin and femoral closure devices has been associated with reduced bleeding
Radial access lowers the rate of vascular and bleeding complications in PCI
Radial access is used infrequently in the United States (16.1% of all PCI)
What this Article Adds
Observational analysis of bleeding avoidance strategies from a broadly representative contemporary CathPCI Registry
The combination of radial access and bivalirudin was associated with a significant reduction in post-PCI bleeding, as compared with the best practice of femoral access including use of bivalirudin and closure devices
In patients with radial access not exposed to IIb/IIIa inhibitors, the benefit of bivalirudin over heparin was still present, but very small
Patients at highest bleeding risk were least likely to receive radial access and bivalirudin
Acknowledgments
Sources of Funding
This research was supported by the American College of Cardiology Foundation’s National Cardiovascular Data Registry (NCDR). The views expressed in this manuscript represent those of the author(s), and do not necessarily represent the official views of the NCDR or its associated professional societies identified at www.ncdr.com.
ABBREVIATIONS LIST
- NCDR®
National Cardiovascular Data Registry®
- PCI
Percutaneous Coronary Intervention
- BAS
Bleeding Avoidance Strategy
- NNT
Number Needed to Treat
- BMI
Body Mass Index
- CVD
Cerebrovascular Disease
- PVD
Peripheral Vascular Disease
- STEMI
ST segment Elevation Myocardial Infarction
- SCAI
Society for Cardiovascular Angiography or Intervention
- NYHA
New York Heart Association
- BARC
Bleeding Academic Research Consortium
- TIMI
Thrombolysis in Acute Myocardial Infarction
- MI
Myocardial Infarction
- OR
Odds Ratio
- CABG
Coronary Artery Bypass Surgery
- ACS
Acute Coronary Syndrome
Footnotes
Disclosures
Dr. Baklanov reports research funding from Saint-Lukes Hospital Foundation of Kansas City, Blue Cross and Blue Shield of Kansas City; consultancy for TheHeart.org. Dr. Subherwal and Ms. Kim report no disclosures. Dr. Marso reports no personal conflicts of interest during the previous 12 months; all his compensation for his research activities, including research grants and consulting fees from The Medicines Company, Novo Nordisk, Abbott Vascular, Amylin Pharmaceuticals, Boston Scientific, Volcano Corporation, and Terumo Medical, are paid directly to the Saint Luke’s Hospital Foundation of Kansas City. Dr. Rao reports consultancy (modest) for Terumo Medical, The Medicines Company; Research funding from Sanofi-Aventis, St. Jude Medical.
References
- 1.Eikelboom JW, Mehta SR, Anand SS, Xie C, Fox KA, Yusuf S. Adverse impact of bleeding on prognosis in patients with acute coronary syndromes. Circulation. 2006;114:774–82. doi: 10.1161/CIRCULATIONAHA.106.612812. [DOI] [PubMed] [Google Scholar]
- 2.Pinto DS, Stone GW, Shi C, Dunn ES, Reynolds MR, York M, Walczak J, Berezin RH, Mehran R, McLaurin BT, Cox DA, Ohman EM, Lincoff AM, Cohen DJ. Economic evaluation of bivalirudin with or without glycoprotein IIb/IIIa inhibition versus heparin with routine glycoprotein IIb/IIIa inhibition for early invasive management of acute coronary syndromes. J Am Coll Cardiol. 2008;52:1758–68. doi: 10.1016/j.jacc.2008.08.021. [DOI] [PubMed] [Google Scholar]
- 3.Marso SP, Amin AP, House JA, Kennedy KF, Spertus JA, Rao SV, Cohen DJ, Messenger JC, Rumsfeld JS. Association between use of bleeding avoidance strategies and risk of periprocedural bleeding among patients undergoing percutaneous coronary intervention. JAMA. 2010;303:2156–64. doi: 10.1001/jama.2010.708. [DOI] [PubMed] [Google Scholar]
- 4.Pancholy S, Patel T, Sanghvi K, Thomas M. Comparison of door-to-balloon times for primary PCI using transradial versus transfemoral approach. Catheter Cardiovasc Interv. 2010;75:991–5. doi: 10.1002/ccd.22425. [DOI] [PubMed] [Google Scholar]
- 5.Rao SV, Ou FS, Wang TY, Roe MT, Brindis R, Rumsfeld JS, Peterson ED. Trends in the prevalence and outcomes of radial and femoral approaches to percutaneous coronary intervention: a report from the National Cardiovascular Data Registry. JACC Cardiovasc Interv. 2008;1:379–86. doi: 10.1016/j.jcin.2008.05.007. [DOI] [PubMed] [Google Scholar]
- 6.Verheugt FW, Steinhubl SR, Hamon M, Darius H, Steg PG, Valgimigli M, Marso SP, Rao SV, Gershlick AH, Lincoff AM, Mehran R, Stone GW. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4:191–7. doi: 10.1016/j.jcin.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 7.Hamon M, Mehta S, Steg PG, Faxon D, Kerkar P, Rupprecht HJ, Tanguay JF, Afzal R, Yusuf S. Impact of transradial and transfemoral coronary interventions on bleeding and net adverse clinical events in acute coronary syndromes. EuroIntervention: journal of EuroPCR in collaboration with the Working Group on Interventional Cardiology of the European Society of Cardiology. 2011;7:91–7. doi: 10.4244/EIJV7I1A16. [DOI] [PubMed] [Google Scholar]
- 8.Weintraub WS, McKay CR, Riner RN, Ellis SG, Frommer PL, Carmichael DB, Hammermeister KE, Effros MN, Bost JE, Bodycombe DP. The American College of Cardiology National Database: progress and challenges. American College of Cardiology Database Committee. J Am Coll Cardiol. 1997;29:459–65. doi: 10.1016/s0735-1097(96)00545-1. [DOI] [PubMed] [Google Scholar]
- 9.Brindis RG, Fitzgerald S, Anderson HV, Shaw RE, Weintraub WS, Williams JF. The American College of Cardiology-National Cardiovascular Data Registry (ACC-NCDR): building a national clinical data repository. J Am Coll Cardiol. 2001;37:2240–5. doi: 10.1016/s0735-1097(01)01372-9. [DOI] [PubMed] [Google Scholar]
- 10.CathPCI Registry Companion Guide to Your NCDR Data Quality Report. Washington, DC: American College of Cardiology Foundation; 2008. [Google Scholar]
- 11.Rao SV, Kaltenbach LA, Spertus J, Krone RJ, Singh M, Peterson ED. Abstract 13479: Contemporary Predictors of Post-Procedural Bleeding Complications Among Patients Undergoing Percutaneous Coronary Intervention (PCI): Results from the National Cardiovascular Data Registry (NCDR) Circulation. 2011;124:A13479. [Google Scholar]
- 12.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Statistics in medicine. 2009;28:3083–107. doi: 10.1002/sim.3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Austin PC. Using the Standardized Difference to Compare the Prevalence of a Binary Variable Between Two Groups in Observational Research. Communications in Statistics - Simulation and Computation. 2009;38:1228–34. [Google Scholar]
- 14.Austin PC. Absolute risk reductions and numbers needed to treat can be obtained from adjusted survival models for time-to-event outcomes. Journal of clinical epidemiology. 2010;63:46–55. doi: 10.1016/j.jclinepi.2009.03.012. [DOI] [PubMed] [Google Scholar]
- 15.Genereux P, Mehran R, Palmerini T, Caixeta A, Kirtane AJ, Lansky AJ, Brodie BR, Witzenbichler B, Mockel M, Guagliumi G, Peruga JZ, Dudek D, Fahy MP, Dangas G, Stone GW. Radial access in patients with ST-segment elevation myocardial infarction undergoing primary angioplasty in acute myocardial infarction: the HORIZONS-AMI trial. EuroIntervention. 2011;7:905–16. doi: 10.4244/EIJV7I8A144. [DOI] [PubMed] [Google Scholar]
- 16.Barthelemy O, Silvain J, Brieger D, Mercadier A, Lancar R, Bellemain-Appaix A, Beygui F, Collet JP, Costagliola D, Montalescot G. Bleeding complications in primary percutaneous coronary intervention of ST-elevation myocardial infarction in a radial center. Catheter Cardiovasc Interv. 2012;79:104–12. doi: 10.1002/ccd.23164. [DOI] [PubMed] [Google Scholar]
- 17.Jolly SS, Yusuf S, Cairns J, Niemela K, Xavier D, Widimsky P, Budaj A, Niemela M, Valentin V, Lewis BS, Avezum A, Steg PG, Rao SV, Gao P, Afzal R, Joyner CD, Chrolavicius S, Mehta SR. Radial versus femoral access for coronary angiography and intervention in patients with acute coronary syndromes (RIVAL): a randomised, parallel group, multicentre trial. Lancet. 2011;377:1409–20. doi: 10.1016/S0140-6736(11)60404-2. [DOI] [PubMed] [Google Scholar]
- 18.Lincoff AM, Bittl JA, Harrington RA, Feit F, Kleiman NS, Jackman JD, Sarembock IJ, Cohen DJ, Spriggs D, Ebrahimi R, Keren G, Carr J, Cohen EA, Betriu A, Desmet W, Kereiakes DJ, Rutsch W, Wilcox RG, de Feyter PJ, Vahanian A, Topol EJ. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289:853–63. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 19.Stone GW, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Dangas G, Wong SC, Kirtane AJ, Parise H, Mehran R. Bivalirudin during primary PCI in acute myocardial infarction. N Engl J Med. 2008;358:2218–30. doi: 10.1056/NEJMoa0708191. [DOI] [PubMed] [Google Scholar]
- 20.Kastrati A, Neumann FJ, Mehilli J, Byrne RA, Iijima R, Buttner HJ, Khattab AA, Schulz S, Blankenship JC, Pache J, Minners J, Seyfarth M, Graf I, Skelding KA, Dirschinger J, Richardt G, Berger PB, Schomig A. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008;359:688–96. doi: 10.1056/NEJMoa0802944. [DOI] [PubMed] [Google Scholar]
- 21.Mehran R, Lansky AJ, Witzenbichler B, Guagliumi G, Peruga JZ, Brodie BR, Dudek D, Kornowski R, Hartmann F, Gersh BJ, Pocock SJ, Wong SC, Nikolsky E, Gambone L, Vandertie L, Parise H, Dangas GD, Stone GW. Bivalirudin in patients undergoing primary angioplasty for acute myocardial infarction (HORIZONS-AMI): 1-year results of a randomised controlled trial. Lancet. 2009;374:1149–59. doi: 10.1016/S0140-6736(09)61484-7. [DOI] [PubMed] [Google Scholar]
- 22.Schulz S, Mehilli J, Ndrepepa G, Neumann FJ, Birkmeier KA, Kufner S, Richardt G, Berger PB, Schomig A, Kastrati A. Bivalirudin vs. unfractionated heparin during percutaneous coronary interventions in patients with stable and unstable angina pectoris: 1-year results of the ISAR-REACT 3 trial. Eur Heart J. 2010;31:582–7. doi: 10.1093/eurheartj/ehq008. [DOI] [PubMed] [Google Scholar]
- 23.Kumar D, Dangas G, Mehran R, Kirtane A, Bertrand M, Ebrahimi R, Guagliumi G, Brar S, Fahy M, Heller E, Moses J, Stone G. Comparison of Bivalirudin versus Bivalirudin plus glycoprotein IIb/IIIa inhibitor versus heparin plus glycoprotein IIb/IIIa inhibitor in patients with acute coronary syndromes having percutaneous intervention for narrowed saphenous vein aorto-coronary grafts (the ACUITY trial investigators) Am J Cardiol. 2010;106:941–5. doi: 10.1016/j.amjcard.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 24.Bangalore S, Cohen DJ, Kleiman NS, Regev-Beinart T, Rao SV, Pencina MJ, Mauri L. Bleeding risk comparing targeted low-dose heparin with bivalirudin in patients undergoing percutaneous coronary intervention: results from a propensity score-matched analysis of the Evaluation of Drug-Eluting Stents and Ischemic Events (EVENT) registry. Circ Cardiovasc Interv. 2011;4:463–73. doi: 10.1161/CIRCINTERVENTIONS.111.961912. [DOI] [PubMed] [Google Scholar]
- 25.Lindsey JB, Cohen DJ, Stolker JM, Meht SK, Mahoney E, Robertus K, House JA, Kennedy K, Riggs L, Rao SV, Marso SP. The impact of bivalirudin on percutaneous coronary intervention-related bleeding. EuroIntervention. 2010;6:206–13. [PubMed] [Google Scholar]
- 26.Mehta SK, Frutkin AD, Lindsey JB, House JA, Spertus JA, Rao SV, Ou FS, Roe MT, Peterson ED, Marso SP. Bleeding in patients undergoing percutaneous coronary intervention: the development of a clinical risk algorithm from the National Cardiovascular Data Registry. Circ Cardiovasc Interv. 2009;2:222–9. doi: 10.1161/CIRCINTERVENTIONS.108.846741. [DOI] [PubMed] [Google Scholar]
- 27.Ndrepepa G, Schuster T, Hadamitzky M, Byrne RA, Mehilli J, Neumann FJ, Richardt G, Schulz S, Laugwitz KL, Massberg S, Schomig A, Kastrati A. Validation of the Bleeding Academic Research Consortium definition of bleeding in patients with coronary artery disease undergoing percutaneous coronary intervention. Circulation. 2012;125:1424–31. doi: 10.1161/CIRCULATIONAHA.111.060871. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.