Abstract
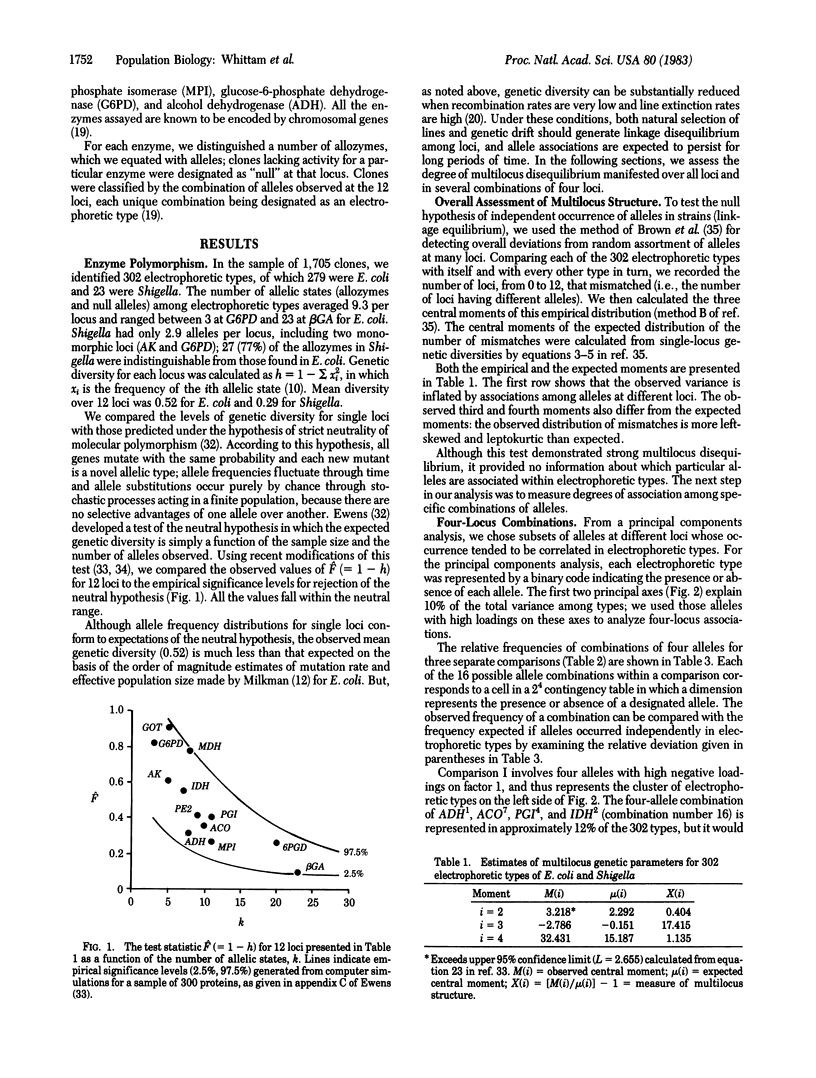
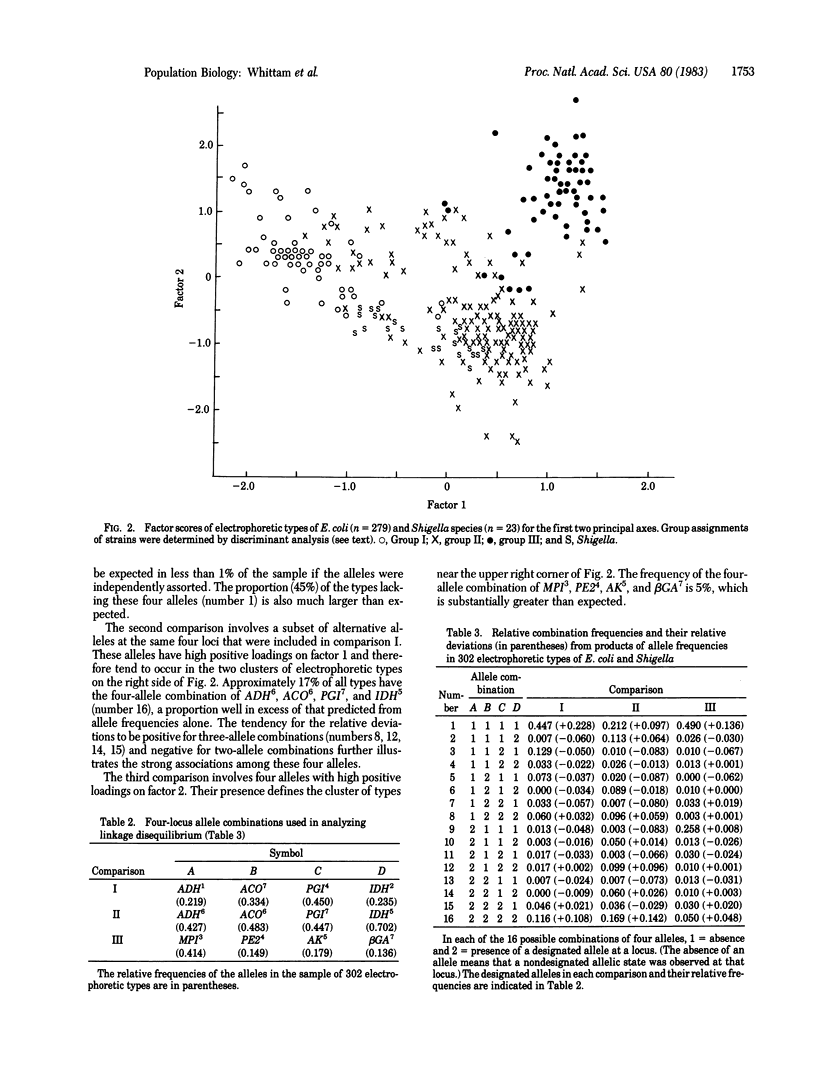
A survey of allozyme variation at 12 enzyme loci in 1,705 clones of the genetic species Escherichia coli (including four species of Shigella) from natural populations revealed 302 unique allele combinations (electrophoretic types). Single-locus diversity estimates fall within the range predicted by the neutral allele theory of molecular evolution, but the combination of alleles in electrophoretic types are highly nonrandom, as indicated by a test of association over all loci and by evidence of complex linkage disequilibria in several four-locus combinations. These linkage disequilibria reflect genetic differentiation of E. coli into three groups of strains. Because of restricted recombination, both the stochastic extinction of lines and selective differences between particular genetic combinations may have contributed to the evolution of subspecific structure in E. coli.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bachmann B. J., Low K. B. Linkage map of Escherichia coli K-12, edition 6. Microbiol Rev. 1980 Mar;44(1):1–56. doi: 10.1128/mr.44.1.1-56.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J. Characterization and clinical identification of Enterobacteriaceae by DNA hybridization. Prog Clin Pathol. 1978;7:71–117. [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Johnson K. E., Citarella R. V., Falkow S. Polynucleotide sequence relationships among members of Enterobacteriaceae. J Bacteriol. 1969 May;98(2):637–650. doi: 10.1128/jb.98.2.637-650.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Skerman F. J., Falkow S. Polynucleotide sequence divergence among strains of Escherichia coli and closely related organisms. J Bacteriol. 1972 Mar;109(3):953–965. doi: 10.1128/jb.109.3.953-965.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner D. J., Fanning G. R., Steigerwalt A. G., Orskov I., Orskov F. Polynucleotide sequence relatedness among three groups of pathogenic Escherichia coli strains. Infect Immun. 1972 Sep;6(3):308–315. doi: 10.1128/iai.6.3.308-315.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown A. H., Feldman M. W., Nevo E. Multilocus Structure of Natural Populations of HORDEUM SPONTANEUM. Genetics. 1980 Oct;96(2):523–536. doi: 10.1093/genetics/96.2.523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Lidin-Janson G., Whittam T. S., Svanborg Edén C., Selander R. K. Genetic diversity and relationships among strains of Escherichia coli in the intestine and those causing urinary tract infections. Prog Allergy. 1983;33:203–227. doi: 10.1159/000318331. [DOI] [PubMed] [Google Scholar]
- Caugant D. A., Levin B. R., Selander R. K. Genetic diversity and temporal variation in the E. coli population of a human host. Genetics. 1981 Jul;98(3):467–490. doi: 10.1093/genetics/98.3.467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dykhuizen D., Hartl D. L. Selective neutrality of 6PGD allozymes in E. coli and the effects of genetic background. Genetics. 1980 Dec;96(4):801–817. doi: 10.1093/genetics/96.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ewens W. J. The sampling theory of selectively neutral alleles. Theor Popul Biol. 1972 Mar;3(1):87–112. doi: 10.1016/0040-5809(72)90035-4. [DOI] [PubMed] [Google Scholar]
- Hartl D. L., Dykhuizen D. E. Potential for selection among nearly neutral allozymes of 6-phosphogluconate dehydrogenase in Escherichia coli. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6344–6348. doi: 10.1073/pnas.78.10.6344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin B. R., Stewart F. M., Rice V. A. The kinetics of conjugative plasmid transmission: fit of a simple mass action model. Plasmid. 1979 Apr;2(2):247–260. doi: 10.1016/0147-619x(79)90043-x. [DOI] [PubMed] [Google Scholar]
- Maruyama T., Kimura M. Genetic variability and effective population size when local extinction and recolonization of subpopulations are frequent. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6710–6714. doi: 10.1073/pnas.77.11.6710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkman R. Electrophoretic variation in Escherichia coli from natural sources. Science. 1973 Dec 7;182(4116):1024–1026. doi: 10.1126/science.182.4116.1024. [DOI] [PubMed] [Google Scholar]
- Nei M. F-statistics and analysis of gene diversity in subdivided populations. Ann Hum Genet. 1977 Oct;41(2):225–233. doi: 10.1111/j.1469-1809.1977.tb01918.x. [DOI] [PubMed] [Google Scholar]
- Nei M., Li W. H. Linkage disequilibrium in subdivided populations. Genetics. 1973 Sep;75(1):213–219. doi: 10.1093/genetics/75.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nevo E. Genetic variation in natural populations: patterns and theory. Theor Popul Biol. 1978 Feb;13(1):121–177. doi: 10.1016/0040-5809(78)90039-4. [DOI] [PubMed] [Google Scholar]
- Ohta T. Linkage disequilibrium due to random genetic drift in finite subdivided populations. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1940–1944. doi: 10.1073/pnas.79.6.1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohta T. Linkage disequilibrium with the island model. Genetics. 1982 May;101(1):139–155. doi: 10.1093/genetics/101.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orskov I., Orskov F., Jann B., Jann K. Serology, chemistry, and genetics of O and K antigens of Escherichia coli. Bacteriol Rev. 1977 Sep;41(3):667–710. doi: 10.1128/br.41.3.667-710.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selander R. K., Levin B. R. Genetic diversity and structure in Escherichia coli populations. Science. 1980 Oct 31;210(4469):545–547. doi: 10.1126/science.6999623. [DOI] [PubMed] [Google Scholar]
- Smith J. M., Haigh J. The hitch-hiking effect of a favourable gene. Genet Res. 1974 Feb;23(1):23–35. [PubMed] [Google Scholar]
- Smouse P. E. Likelihood analysis of recombinational disequilibrium in multiple-locus gametic frequencies. Genetics. 1974 Mar;76(3):557–565. doi: 10.1093/genetics/76.3.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomson G. The effect of a selected locus on linked neutral loci. Genetics. 1977 Apr;85(4):753–788. doi: 10.1093/genetics/85.4.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watterson G. A. The homozygosity test of neutrality. Genetics. 1978 Feb;88(2):405–417. doi: 10.1093/genetics/88.2.405. [DOI] [PMC free article] [PubMed] [Google Scholar]



