Abstract
Prolactin stimulates dopamine release from neuroendocrine dopaminergic (NEDA) neurons in the hypothalamic arcuate nucleus (ARC) to maintain low levels of serum prolactin. Elevated prolactin levels during pregnancy and lactation may mediate actions in other hypothalamic regions such as the paraventricular nucleus (PVN) and rostral preoptic area (rPOA). We predicted that NEDA neurons would be more sensitive prolactin targets than neurons in other regions because they are required to regulate basal prolactin secretion. Moreover, differences in the accessibility of the ARC to prolactin in blood may influence the responsiveness of this population. Therefore, we compared prolactin-induced signaling in different hypothalamic neuronal populations following either systemic or intracerebroventricular (icv) prolactin administration. Phosphorylation of the signal transduction factor, STAT5 (pSTAT5), was used to identify prolactin-responsive neurons. In response to systemic prolactin, pSTAT5-labeled cells were widely observed in the ARC but absent from the rPOA and PVN. Many of these responsive cells in the ARC were identified as NEDA neurons. The lowest icv prolactin dose (10 ng) induced pSTAT5 in the ARC, but with higher doses (>500 ng) pSTAT5 was detected in numerous regions, including the rPOA and PVN. NEDA neurons were maximally labeled with nuclear pSTAT5 in response to 500 ng prolactin and appeared to be more sensitive than dopaminergic neurons in the rPOA. Subpopulations of oxytocin neurons in the hypothalamus were also found to be differentially sensitive to prolactin. These data suggest that differences in the accessibility of the arcuate nucleus to prolactin, together with intrinsic differences in the NEDA neurons, may facilitate homeostatic feedback regulation of prolactin release.
INDEXING TERMS: prolactin, NEDA neurons, pSTAT5, hypothalamus, oxytocin
Prolactin is an anterior pituitary hormone that is classically recognized for its crucial role in the production of milk at the mammary gland. It is now well established that this hormone also exerts a range of actions in the central nervous system (Bole-Feysot et al., 1998). The best-characterized action in the brain is the control of its own secretion, with prolactin levels in the blood tightly regulated by short-loop negative feedback (Freeman et al., 2000). Prolactin stimulates dopamine release from discrete populations of neurons in the mediobasal hypothalamus; the tuberoinfundibular, the tuberohypophyseal, and the periventricular hypothalamic dopaminergic neurons (DeMaria et al., 2000), collectively called neuroendocrine dopaminergic (NEDA) neurons. The released dopamine travels in the portal blood to bind to dopamine receptors on the pituitary lactotropes, thereby inhibiting prolactin secretion (Gudelsky and Porter, 1980; Foord et al., 1983). In the rat, prolactin levels are low for the majority of the reproductive cycle except for acute surges that occur during the afternoons of proestrus (Smith et al., 1975; Arbogast and Voogt, 1994) and possibly estrus (Leite et al., 2010). These prolactin surges occur in response to decreased NEDA neuronal activity resulting from alterations to estrogen and progesterone levels at these times, and are terminated several hours later due to increased dopamine release from the NEDA neurons caused by prolactin feedback stimulation (DeMaria et al., 2000). Therefore, the NEDA neurons must be highly sensitive to the low levels of prolactin experienced during this time in order to maintain prolactin secretion at low basal levels, and to limit the duration of prolactin surges during the estrous cycle.
In mammals, prolactin levels are markedly increased during pregnancy and lactation to mediate adaptations that are crucial for successful reproduction (Grattan et al., 2008). Mating in the rat induces twice-daily surges of prolactin from the pituitary that are necessary for maintaining pregnancy by stimulating progesterone secretion from the newly formed corpus luteum (Smith et al., 1976; Albarracin et al., 1994). The placenta also produces large amounts of prolactin-like peptides, known as placental lactogens, which can also activate prolactin-sensitive targets in the brain from day 10 of pregnancy until term (Arbogast et al., 1992; Bridges et al., 1997; Lee and Voogt, 1999). Like prolactin, placental lactogens are capable of stimulating dopamine release from the NEDA neurons. The resulting increase of dopamine in the portal blood inhibits pituitary prolactin secretion for most of the remainder of pregnancy. Interestingly, pituitary prolactin secretion is elevated for the last 24 hours of pregnancy in the rat (Grattan and Averill, 1990, 1991). This prepartum prolactin surge results from the failure of the NEDA neurons to release dopamine in response to stimulation by placental lactogens and prolactin (Grattan and Averill, 1995; Fliestra and Voogt, 1997). The inhibition of prolactin secretion from the anterior pituitary is therefore reduced and prolactin secretion rises (Andrews et al., 2001; Grattan et al., 2008). The loss of feedback inhibition over pituitary prolactin release at the end of pregnancy also extends into lactation, when high levels of prolactin are secreted in response to suckling (Terkel et al., 1972; Demarest et al., 1983; Arbogast and Voogt, 1996). These mechanisms allow the sequential elevation of lactogenic hormones throughout most of pregnancy and lactation, whether they are produced by the placenta or pituitary. It is likely that the high levels of prolactin at these times coordinate changes simultaneously in the body and brain that are critical for successful reproduction (Grattan and Kokay, 2008). For example, prolactin actions in the preoptic area are important for the development of the appropriate maternal behavior required for the survival of offspring, postpartum (Bridges et al., 1990, 2001). Prolactin has also been shown to stimulate neurogenesis in the rostral forebrain during pregnancy (Shingo et al., 2003), which is likely to be important in the development of appropriate mother-infant bonding. The paraventricular nucleus may mediate many important maternal adaptations in response to prolactin during pregnancy and lactation, including suppressing physiological responses to stress (Torner et al., 2001) and increasing appetite in the female rat (Naef and Woodside, 2007; Augustine and Grattan, 2008; Ladyman et al., 2010). Thus, in contrast to the NEDA neurons, it is possible that these neuronal populations may only respond to high levels of prolactin to ensure that these important maternal adaptations manifest during pregnancy and lactation when prolactin levels are elevated, and yet prevent physiologically inappropriate changes from occurring in the nonpregnant rat when prolactin levels are low.
We hypothesized that the NEDA neurons would be highly sensitive to prolactin levels circulating in the bloodstream to maintain tight regulation over pituitary prolactin secretion, while neurons in other nuclei known to mediate prolactin-induced adaptations in the brain during pregnancy and lactation would only respond to prolactin at higher concentrations. This could be achieved by the arcuate nucleus being more accessible to hormones present in the bloodstream than other brain areas due to the fact that blood vessels and neural tissue in the adjacent median eminence are not separated by a complete blood–brain barrier (Peruzzo et al., 2000). Alternatively, it is possible that the NEDA neurons are inherently more sensitive than other populations due to increased levels of prolactin receptors expressed by these cells, or by intracellular changes that facilitate prolactin-induced signaling. To investigate these possibilities we used nuclear translocation of the transcription factor, phosphorylated signal transducer and activator of transcription 5 (pSTAT5), as an index to quantify prolactin responsiveness between four major prolactin-sensitive regions in the hypothalamus: the arcuate nucleus, the rostral preoptic area, the paraventricular nucleus, and the supraoptic nucleus. pSTAT5 is an accepted marker of prolactin-induced signaling in the rodent forebrain (Brown et al., 2010) and results from activation of the long-form of the prolactin receptor, the predominant receptor isoform found in the rodent hypothalamus (Nagano and Kelly, 1994; DaSilva et al., 1996). STAT5, specifically the STAT5b isoform, is critical for normal prolactin negative feedback (Grattan et al., 2001; Ma et al., 2005). First, we injected rats with prolactin systemically to investigate differences in the ability of prolactin from the bloodstream to access the arcuate nucleus compared with other hypothalamic areas. Second, to directly examine whether the NEDA neurons are intrinsically more sensitive to prolactin than other populations, we injected a range of prolactin doses directly into the cerebrospinal fluid (CSF). To identify prolactin-responsive NEDA populations in the hypothalamus, we used dual-label immunohistochemistry to visualize neurons colocalizing pSTAT5 and tyrosine hydroxylase (TH). Oxytocin was used as a marker to identify whether changes existed in the responsiveness of neurons to prolactin in the paraventricular and supraoptic nuclei.
MATERIALS AND METHODS
Animals
Ten-week-old female Sprague-Dawley rats (weighing 200–250 g) were obtained from the Taieri Resource Unit, University of Otago. Rats were group housed (n = 6) under controlled temperature (22 ± 1°C) and lighting conditions (14:10-hour light:dark cycle, with lights on at 0600 and off at 2000) except following intracerebroventricular (icv) cannula implantation, when they were individually housed. Rats received pelleted food and tap water ad libitum. All experimental procedures were approved by the University of Otago Animal Ethics Committee (AEC 96/05-003).
Estrous cycle monitoring and administration of prolactin
Each day at ≈0900 a vaginal smear was obtained from each rat and the stage of the estrous cycle was determined by light microscopy. In order to deliver prolactin specifically on the day of diestrus (to minimize the confounding affects of endogenous hormones) smears were collected from all rats for two consecutive cycles.
In the systemic prolactin-treated groups, rats received 500 μg of bromocriptine (500 μg/250 μl saline containing 10% ethanol) at 0900 and 1700 on metestrus, and again at 0900 on the morning of diestrus to block the release of endogenous prolactin secretion. At 1300 on diestrus, groups of rats were injected with 1 mg/kg b.w. ovine prolactin (Sigma, St. Louis, MO; 30 IU/mg) into the peritoneal cavity (in saline; n = 5) or vehicle (saline; n = 4). Rats were anesthetized 55 minutes later with an intraperitoneal injection of pentobarbitone (300 mg/kg b.w. containing 1,000 IU heparin) and transcardially perfused with ≈50 ml of saline followed by 200 ml of 4% paraformaldehyde in 0.1 M phosphate buffer (PB) pH 7.3. Two further groups of rats were anesthetized 115 (n = 5) and 175 (n = 4) minutes after prolactin injection and perfused as above. A blood sample was drawn from the left ventricle immediately prior to perfusion and plasma was aspirated and stored at −20°C until radioimmunoassay for ovine prolactin.
For icv prolactin treatment, rats were anesthetized under 2% halothane and placed in a stereotaxic apparatus on the morning of proestrus. A 22G guide cannula (PlasticsOne, Roanoke, VA) was implanted into the left ventricle, 1.3 mm lateral to the midline at bregma and 3 mm below the level of the skull. The cannula was fixed to the skull with three stainless steel screws and dental cement. The cannula was sealed with a plastic cap until the time of injection. Three or four days later, to verify the correct placement of the cannula, water intake was recorded following injection of 10 ng angiotensin II (10 ng diluted in 2 μl artificial CSF). A positive response was indicated by the rat consuming more than 5 ml of water in the 30 minutes subsequent to the delivery of angiotensin II. Six or 7 days following surgery (after one full estrous cycle) on the day of metestrus, rats received 500 μg of bromocriptine at 0900 and 1700, and again at 0900 on the morning of diestrus, to block the release of endogenous prolactin. At 1300 on diestrus, groups of rats received either one of three doses of prolactin: 10 ng (n = 7), 500 ng (n = 7) or 5 μg (n = 6) diluted in 2 μl of vehicle (aCSF) into the lateral ventricle. A fourth group received vehicle only (n = 6). Injections were performed using a 2-μl Hamilton syringe connected to a 28G stainless steel injection cannula, which was designed to protrude 2 mm beyond the tip of the guide cannula. Injections were performed over a 30-second period and the injection cannula remained in the guide cannula for a further 30 seconds before the sealing cap was replaced. Twenty-five minutes later, rats were anesthetized with an intraperitoneal injection of pentobarbitone and killed by transcardial perfusion.
Brains from both the systemic and icv-injected rats were postfixed at room temperature in the same fixative overnight, then infiltrated with 30% sucrose until they sank. Brains were then quickly frozen on powdered dry ice and stored at −80°C until required for immunohistochemistry.
Radioimmunoassay
Ovine prolactin (NIDDK-oPrl-1-3, batch number AFP-10789B) was iodinated by the chloramine-T method and diluted to a concentration of 20,000 cpm/50 μL assay buffer. Primary antibody (NIDDK rabbit anti-oPRL-2, batch number AFP-C358106) was added to give a final dilution of 1:600,000. This radioimmunoassay for ovine prolactin does not crossreact with rat prolactin (Gregory et al., 2004). Data are expressed in terms of an ovine prolactin standard curve generated using the same prolactin preparation as the one used for the iodination. Prolactin levels are reported as the mean ± SEM (standard error of the mean).
Immunohistochemistry
Coronal sections (40-μm thick) were cut on a sliding microtome with a freezing stage, from the septal-preoptic area through to the emergence of the pituitary stalk. Consecutive sections were collected at 120-μm intervals through the rostral preoptic area, paraventricular nucleus, and the supraoptic nucleus, and at 200-μm intervals in the arcuate nucleus to provide three and five sets of sections for each area, respectively. The rostral preoptic area included sections beginning at the level of the anteroventral periventricular nucleus and extending caudally through the periventricular nucleus adjacent to the third ventricle (Herbison, 2008). Sections were then stored in cryoprotectant at −20°C (Watson et al., 1986).
Single-label immunohistochemistry for pSTAT5
Serial sets of sections, containing either the arcuate nucleus or the paraventricular nucleus, or the rostral preoptic area, from both systemic and icv-treated rats, were single-labeled for pSTAT5. Sections were washed six times in 0.05 M TBS (50 mM Tris, 150 mM NaCl, pH 7.6) to remove cryoprotectant, then heated for 10 minutes at 90°C in 0.01 M Tris (pH 10) to retrieve pSTAT5 antigenic sites. Endogenous peroxidases were quenched in 40% methanol containing 0.9% hydrogen peroxide and sections were then incubated in rabbit anti-pSTAT5 (Tyr694, Cell Signaling Technology, Beverly, MA) diluted 1:1,000 in TBS with 0.3% Triton-X and 2% normal goat serum for 36 hours at 4°C. Sections were washed in TBS then incubated with biotinylated secondary antibody (BA-1000, Vector Laboratories, Burlingame, CA) diluted 1:300 for 90 minutes at room temperature. After a further wash, sections were incubated with avidin-biotin complex (ABC Elite kit, Vector) for 1 hour. Finally, sections were reacted with nickel-enhanced 3,3′-diaminobenzidine (Vector) to visualize pSTAT5 staining as a dark blue/black nuclear precipitate. Sections were mounted onto gelatin-coated slides and air-dried overnight. Slides were then passed through graded alcohols, cleared in xylene, and coverslipped with DPX-mounting medium.
Dual-label immunohistochemistry for pSTAT5 and TH or oxytocin
Another set of sections for the arcuate and paraventricular nucleus, along with sections containing periventricular nucleus at the level of the medial preoptic area, were washed three times in TBS following the development of pSTAT5 staining. Endogenous peroxidase activity was requenched by incubating sections in TBS containing 40% methanol and 0.9% hydrogen peroxide. Sections were then incubated in blocking solution (0.05 M TBS containing 2% normal goat serum, 0.3% Triton × 100, and 0.25% bovine serum albumin [BSA]) containing either mouse monoclonal anti-TH (1:40,000, Chemicon, Temecula, CA) or mouse monoclonal anti-oxytocin (1:25,000, Millipore, Bedford, MA) for 36 hours at 4°C. After a TBS wash, sections were incubated with horseradish peroxidase-conjugated secondary antibody diluted at 1:200 for 4 hours at room temperature. Sections were then reacted with non-nickel-enhanced 3,3′-diaminobenzidine to produce a brown cytoplasmic precipitate. The slides were air-dried overnight, dehydrated in alcohols, cleared in xylene, and coverslipped.
Antibodies
Details regarding all primary antibodies used are shown in Table 1. The rabbit polyclonal antiserum for pSTAT5 (anti phospho-STAT5, Tyr 694, Cell Signaling) was raised against a synthetic phosphopeptide. This antiserum recognizes a single band of 90 kD molecular weight on western blots from extracts from TF-1 cells (manufacturer’s technical information) and this result was repeatable on mouse hypothalamic tissue in our laboratory. We have previously reported that prolactin-induced pSTAT5 is absent in mediobasal forebrain neurons of mice with a conditional deletion of the STAT5 locus (Brown et al., 2010).
TABLE 1.
Primary Antibodies
| Antibody | Host | Antigen | Supplier | Dilution |
|---|---|---|---|---|
| pSTAT5 | Rabbit polyclonal | Synthetic phosphopeptide corresponding to the residues surrounding tyrosine 694 of mouse STAT5 (AVDGyVKPQC; where y is phosphorylated tyrosine) | Cell Signaling, #9351 | 1:1,000 |
| Tyrosine hydroxylase | Mouse monoclonal | Tyrosine hydroxylase from PC-12 cells | Chemicon, MAB318 | 1:40,000 |
| Oxytocin | Mouse monoclonal | Synthetic, full-length oxytocin | Millipore, MAB5296 | 1:25,000 |
Mouse monoclonal anti-TH (clone LNC1, MAB318, Chemicon) was raised against TH that was purified from PC12 cells. The antiserum recognizes an epitope on the outside of the regulatory N-terminus of this enzyme. This antiserum recognizes an intensive band of 60 kDa band by western blotting corresponding to the TH monomer in human brain (Wolf et al., 1991). This antibody has been frequently used to label dopaminergic neurons in the rat brain. The distribution of labeling observed in the hypothalamus in the current study was consistent with the well-known pattern of TH cell body and fiber labeling reported previously (Chan-Palay et al., 1984; Okamura et al., 1988).
Mouse monoclonal anti-oxytocin (clone 4G11, MAB-5296, Chemicon) was raised against full-length synthetic oxytocin coupled to a carrier-protein, thyroglobulin. This antibody was characterized by competitive enzyme-linked immunosorbent assay (ELISA) and does not crossreact with the oxytocin-related peptides arginine vasopressin, vasotocin, or [Asu1,6,Arg8]-vasopressin (Liu et al., 2002). The immunolabeling observed in the hypothalamus with this oxytocin antisera is consistent with the distribution of oxytocin protein reported by other studies (Xiong and Hatton, 1996; Simmons and Swanson, 2009) and is identical to the distribution of oxytocin mRNA reported by our laboratory (Kokay et al., 2006) and by other groups (Hallbeck et al., 2001).
Statistics and analysis
For the single-label immunohistochemistry analysis, three sections per region from each animal were photographed under 200× magnification using a Spot RT digital camera (Diagnostic Instruments, Sterling Heights, MI). Sections were matched between animals using anatomical landmarks to ensure consistency in the analysis procedure. Each region was analyzed by counting the total number of pSTAT5-positive nuclei within its boundaries on both sides of the ventricle using the particle counting function of ImageJ software (NIH, Bethesda, MD). The numbers of pSTAT5 labeled nuclei in each area were corrected for double-counts (Abercrombie, 1946). The data for each region are presented as the mean number of pSTAT5-positive nuclei per section. For analysis of the dual-label immunohistochemistry, the percentage of oxytocin- and TH-positive immunoreactive neurons that colocalized with pSTAT5 were counted under 40× objective from at least two, but in the majority of cases from three sections per animal on both sides of the third ventricle. Colocalization of pSTAT5 with TH was performed on sections through the arcuate nucleus and the periventricular nucleus of the rostral preoptic area. TH-positive neurons in the arcuate nucleus were subdivided into those present in the dorsomedial or the ventrolateral division by tracing a 35° line relative to the intersection of the midline of the brain and the horizontal base of the hypothalamus (see Fig. 4A). TH-positive neurons in the dorsomedial arcuate nucleus are believed to be involved in the regulation of prolactin secretion, but those in the ventrolateral region may not synthesize dopamine and their function is largely unknown (Cheung et al., 1997). Colocalization for pSTAT5 and oxytocin was performed on sections from the supraoptic nucleus and the paraventricular nucleus. In the paraventricular nucleus, oxytocin-immunoreactive neurons, some of which have been shown to express prolactin receptors (Kokay et al., 2006), were subdivided into those found in the lateral magnocellular division or those found in the parvocellular region that is relatively medial to the third ventricle (Swanson et al., 1980; Hallbeck et al., 2001). Both oxytocin- and TH-positive neurons were counted pSTAT5-positive if they displayed a distinct dark blue/black nucleus surrounded by brown cytoplasm. Data for each region are presented as the mean percentage of dual-labeled neurons per section.
All data were analyzed by one-way analysis of variance followed by Bonferroni’s post-hoc analysis to identify significant differences between groups. All differences were considered significant if P < 0.05. Data are displayed as the mean ±SEM.
Photomicrograph production
Representative images were taken using an Olympus BX51 microscope and a Spot RT digital camera. The size, brightness, and contrast of these images was adjusted using Adobe Photoshop CS4 (San Jose, CA).
RESULTS
Single-label pSTAT5 immunohistochemistry comparing icv and systemic prolactin administration in prolactin-sensitive hypothalamic nuclei
Following both icv or systemic administration of prolactin, immunoreactivity for pSTAT5 was discretely localized, being present at high levels in specific hypothalamic nuclei known to express long-form prolactin receptor mRNA and protein, or present in very low levels only in other regions. Staining was confined to the cell nucleus in each cell examined. Activation of STAT5 was apparent following intraperitoneal injection of prolactin at all three timepoints examined, but as maximal levels were observed after 120 minutes, with no further significant increase in numbers of activated cells seen after 180 minutes (data not shown), data presented are from the 120-minute group. The systemic dose of ovine prolactin used significantly increased the levels of this hormone in plasma 60 and 120 minutes after injection (P < 0.05) versus vehicle, reaching peak levels of ≈110 ng/ml plasma after 120 minutes and by 180 minutes following injection, levels had fallen to less than 10 ng/ml. Virtually no pSTAT5 staining was observed throughout the mediobasal hypothalamus in vehicle-treated rats, confirming the efficacy of bromocriptine in suppressing endogenous prolactin secretion (data not shown). Figure 1 compares the distribution of nuclear pSTAT5 at the level of the organum vasculosum of the lamina terminalis (Fig. 1A,A′), rostral periventricular nucleus of the third ventricle (1B,B′), medial amygdala (1C,C′), paraventricular nucleus (1D,D′), arcuate nucleus (1E,E′), and supraoptic nucleus (1F,F′) 120 minutes following systemic (left panels) prolactin (1 mg/kg b.w.) delivery or 30 minutes following icv (right panels) prolactin (5 μg) delivery. Nuclear pSTAT5 was observed in all regions in response to icv prolactin treatment, while pSTAT5 labeling was only consistently seen at the level of the organum vasculosum of the lamina terminalis and throughout the arcuate nucleus in response to systemic prolactin delivery. As illustrated in Figure 2, our treatment regime consistently induced nuclear pSTAT5 in the NEDA neurons.
Figure 1.
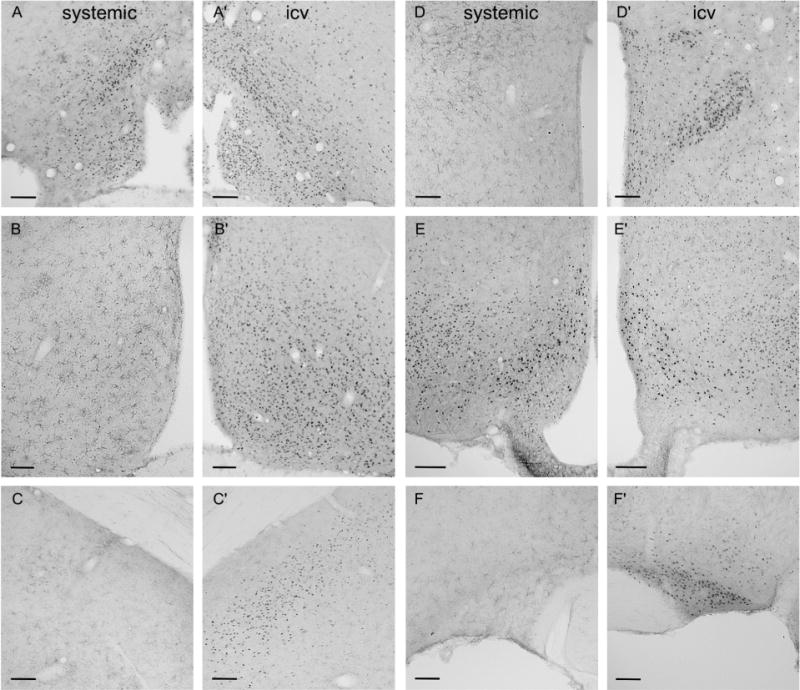
Comparison of prolactin-induced pSTAT5 in the female rat forebrain in response to systemic and icv prolactin delivery. Representative images show pSTAT5 immunohistochemistry at the level of the organum vasculosum of the lamina terminalis (A,A′), rostral periventricular nucleus of the third ventricle (B,B′), medial amygdala (C,C′), paraventricular nucleus (D,D′), arcuate nucleus (E,E′), and supraoptic nucleus (F,F′) 120 minutes following systemic (A–F) prolactin delivery (1 mg/kg b.w.) and 30 minutes following icv (A′–F′) prolactin delivery (5 μg). Scale bars = 100 μm.
Figure 2.
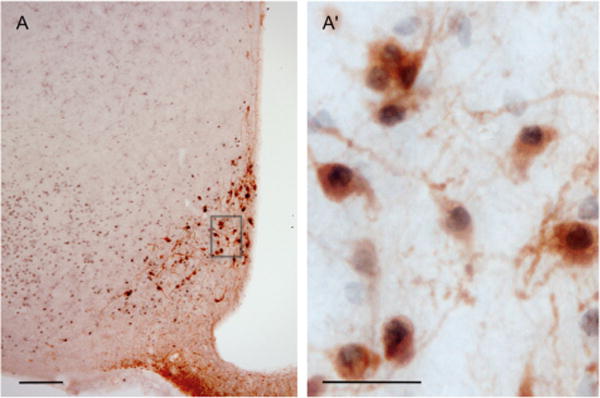
A: Low-power image of double-label immunohistochemistry for pSTAT5 and tyrosine hydroxylase in the arcuate nucleus 120 minutes following systemic injection of prolactin. A′: Highpower image of box in (A). Scale bars = 25 μm.
Single-label pSTAT5 following a range of icv prolactin doses
To investigate whether there are intrinsic differences in the sensitivity of cells in different hypothalamic nuclei to prolactin, irrespective of possible differences in access from systemic circulation, groups of diestrus rats were injected with a range of prolactin doses directly into the lateral ventricle. pSTAT5 immunohistochemical labeling in response to these prolactin doses at the level of the anteroventral periventricular nucleus, paraventricular nucleus, and arcuate nucleus is shown in Figure 3, and the total number of pSTAT5-positive nuclei labeled in response to each dose is summarized in Figure 3M–O. In the anteroventral periventricular nucleus of the rostral preoptic area (Fig. 3A–D), few pSTAT5 immunoreactive cells were present in sections in both the vehicle- and 10 ng prolactin-treated groups. The 500 ng prolactin dose, however, produced a significant induction of pSTAT5 staining, which was further increased after the 5 μg dose of prolactin (P < 0.05 vs. 500 ng dose) where strong staining was evident throughout the anteroventral periventricular nucleus and in the adjacent preoptic area.
Figure 3.
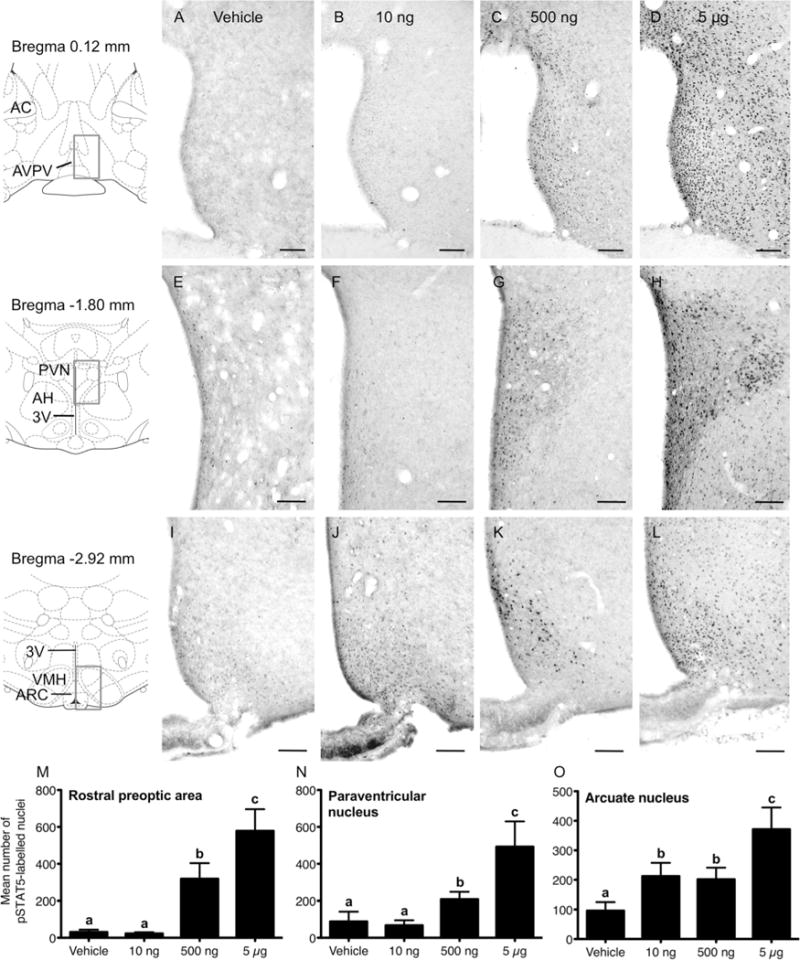
Prolactin-induced pSTAT5 in the female rat forebrain. A–L: Representative images showing pSTAT5 immunohistochemistry in the rostral preoptic area (A–D), paraventricular nucleus (E–H), and arcuate nucleus (I–L) in response to vehicle (A,E,I), 10 ng (B,F,J), 500 ng (C,G,K), or 5 μg icv prolactin doses (D,H,L). M–O: Graphs summarizing the mean number of pSTAT5-labeled nuclei per brain section in response to 10 ng (n = 7), 500 ng (n = 7), or 5 μg (n = 6) prolactin icv, or vehicle (n = 6) in each area. Bars with different letters are significantly different compared with vehicle (P < 0.05). Scale bars = 100 μm in A–L.
In the paraventricular nucleus (Fig. 3,E–H,N), pSTAT5-immunoreactive nuclei were detected in all treatment groups. The number of labeled nuclei significantly increased in response to the 500 ng dose of prolactin (P < 0.05 vs. 10 ng dose), particularly in cells located throughout the medial parvocellular region of this nucleus. Increased levels of pSTAT5-labeled cells were observed in response to the 5 μg prolactin dose (P < 0.05 vs. 500 ng dose), where labeled nuclei were distributed widely throughout the more lateral magnocellular region of this nucleus.
In the arcuate nucleus (Fig. 3I–L,O), administration of the 10 ng dose of prolactin significantly increased the number of pSTAT5-positive nuclei (P < 0.05 vs. vehicle), particularly in cells located in the ventrolateral region. The total number of pSTAT5-positive nuclei was unchanged between the low and medium dose-treated groups. While we have not attempted to quantify any difference, it did appear that nuclear staining was much stronger in response to the medium prolactin dose, especially in cells located in the dorsomedial division of the arcuate nucleus. The number of pSTAT-positive nuclei in the arcuate nucleus was further increased in response to the 5 μg prolactin dose (P < 0.05 vs. 500 ng dose).
Dual-label immunohistochemistry
To begin to identify the specific neuronal populations that are responsive to prolactin, sections that were anatomically matched between prolactin treatment groups were subjected to dual-label immunohistochemistry for pSTAT5 and either TH or oxytocin.
Colocalization of pSTAT5 with TH
Representative images illustrating the distribution of TH-labeled cells (brown cytoplasmic staining) and nuclear pSTAT5 (blue/black staining) in the arcuate nucleus and rostral preoptic area are shown in Figures 4 and 5, respectively. In the dorsomedial and ventrolateral regions of the arcuate nucleus, very few TH-positive neurons contained nuclear pSTAT5 in response to the lowest dose (10 ng) of prolactin (Fig. 4). In the dorsomedial arcuate, after administration of a 500 ng dose of prolactin, over 84% of neurons were dual-labeled for TH and pSTAT5. Following the 5 μg dose of prolactin, no further increase in the number of colocalized neurons was observed (Fig. 4E). In the ventrolateral arcuate nucleus, a different pattern of activation was observed. In response to 500 ng of prolactin, only 56% percent of TH-positive cells contained nuclear pSTAT5 and this increased to 87% after delivery of the highest dose (Fig. 4F).
Figure 4.
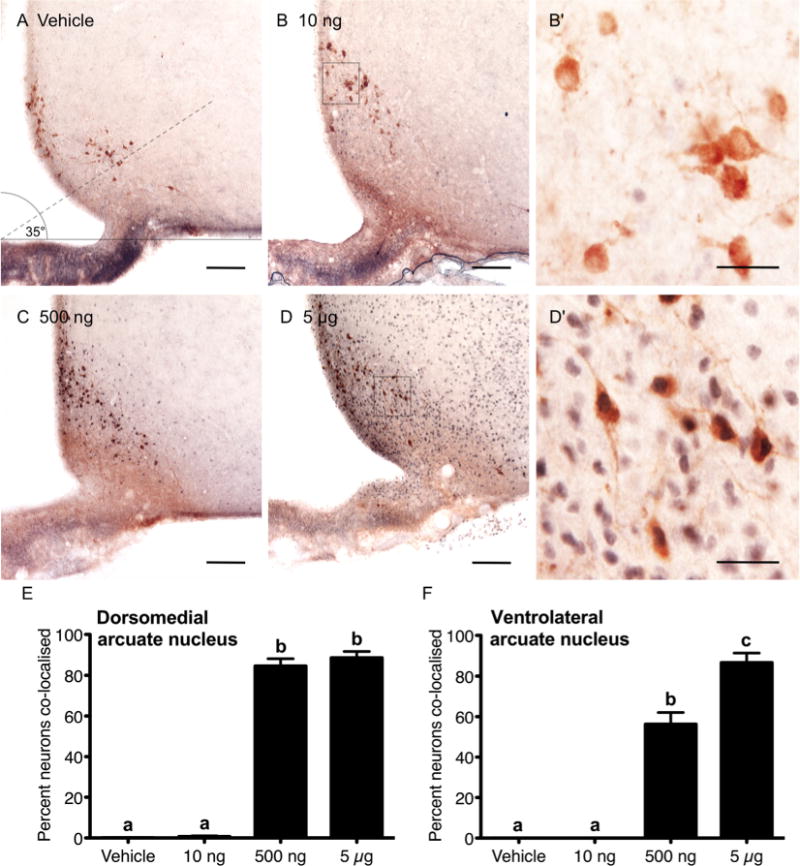
Prolactin induction of nuclear pSTAT5 in neuroendocrine dopaminergic neurons of the arcuate nucleus. Representative images of vehicle (A), 10 ng (B,B′), 500 ng (C), and 5 μg icv prolactin dose-treated brains (D,D′), showing the distribution of nuclear pSTAT5 (blue/black) and cytoplasmic tyrosine hydroxylase (brown) staining in arcuate nucleus sections. The percentage of TH-positive neurons displaying nuclear pSTAT5 in response to 10 ng (n = 7), 500 ng (n = 7), or 5 μg (n = 6) prolactin icv, or vehicle (n = 6) in the dorsomedial and ventrolateral divisions of the arcuate nucleus is summarized in E,F, respectively. Bars with different letters are significantly different compared with vehicle (P < 0.05). Scale bars = 100 μm in A–D; 25 μm in B′,D′.
Figure 5.
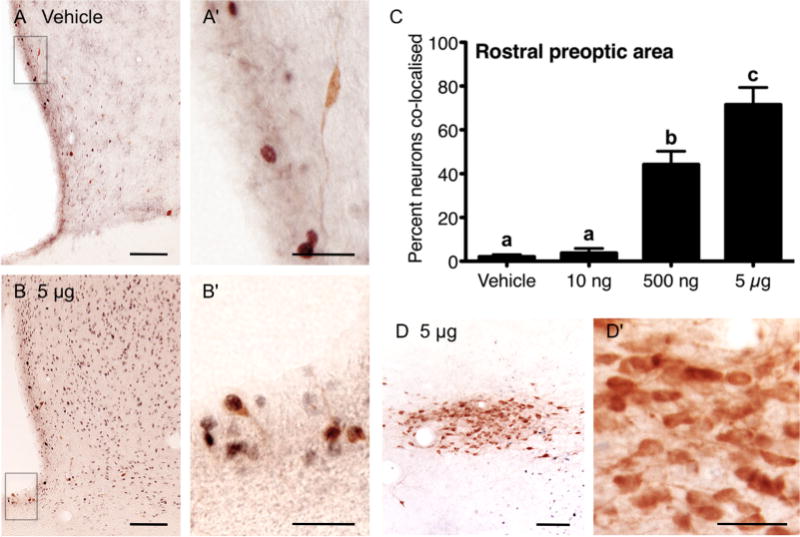
Prolactin induction of nuclear pSTAT5 in TH-positive neurons in the rostral preoptic area. Representative images of vehicle (A,A′) and 5 μg (B,B′) icv prolactin-treated animals, showing the distribution of nuclear pSTAT5 (blue/black) and cytoplasmic TH (brown) staining in rostral preoptic area sections. The percentage of TH-positive neurons displaying nuclear pSTAT5 in response to 10 ng (n = 7), 500 ng (n = 7), or 5 μg (n = 6) icv prolactin, or vehicle (n = 6) in the rostral preoptic area is summarized in C. Bars with different letters are significantly different compared with vehicle (P < 0.05). The absence of pSTAT5 and TH colocalization in the zona incerta (D,D′) is included as a negative control. Scale bars = 100 μm in A,B,D; 30 μm in A′,B′,D′.
The pattern of colocalization of TH with pSTAT5 observed in the rostral preoptic area following acute prolactin administration (Fig. 5A–C), was similar to that seen in the ventrolateral arcuate nucleus. Dual-labeled neurons were rarely recorded in the vehicle (Fig. 5A′) and lowest dose prolactin-treated groups. The 500 ng dose of prolactin caused a significant increase in the percentage of dual-labeled cells in the periventricular region of the rostral preoptic area (P < 0.05 vs. low dose) which were further increased after the 5 μg dose of prolactin (P < 0.05 vs. 500 ng dose) (5B,B′). In populations of TH-positive neurons that are known to be not prolactin-responsive, such as the zona incerta, no nuclear pSTAT5 was observed (Fig. 5D,D′).
Colocalization of nuclear pSTAT5 with oxytocin
Neurons immunoreactive for oxytocin were abundant in both the paraventricular nucleus and the supraoptic nucleus. Representative images for sections dual-labeled with oxytocin and pSTAT5 in the paraventricular nucleus in response to each treatment group are presented in Figure 6. These data are summarized in Figure 6E,F and high-power images of representative sections are shown of parvocellular oxytocin (6G,H,K,L) and magnocellular oxytocin neurons (6I,J,M,N). In the parvocellular region, very few oxytocin neurons showed phosphorylation of nuclear STAT5 in response to vehicle or the lowest dose of prolactin. 65% of oxytocin neurons were dual-labeled following the 500 ng prolactin dose (P < 0.05 vs. vehicle) and this percentage increased further after the highest prolactin dose (P < 0.05 vs. medium dose). In the magnocellular division of the paraventricular nucleus, while the percentage of dual-labeled cells appeared to increase in response to the 500 ng prolactin dose, this was not significant. However, the percentage of dual-labeled magnocellular neurons significantly increased after the highest dose of prolactin (P < 0.05 vs. 500 ng dose).
Figure 6.
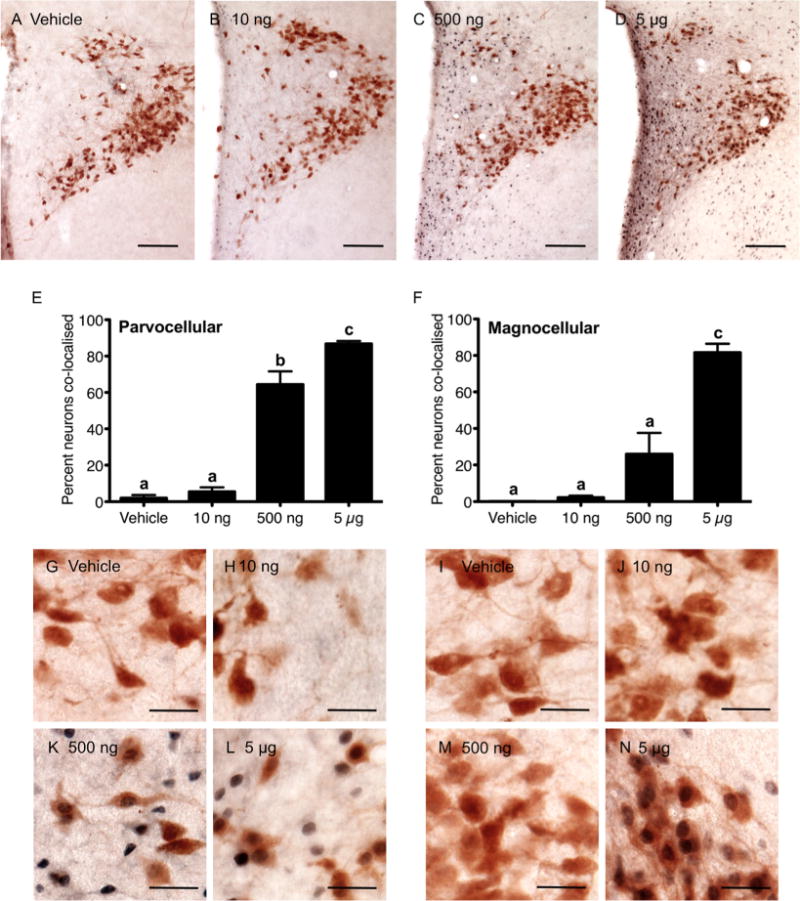
Prolactin induction of nuclear pSTAT5 in oxytocin-positive neurons in the paraventricular nucleus. A–D: Representative images of prolactin-treated animals, showing the distribution of nuclear pSTAT5 (blue/black) and cytoplasmic oxytocin (brown) staining in paraventricular nucleus sections. The percentage of oxytocin-positive neurons displaying nuclear pSTAT5 in response to 10 ng (n = 7), 500 ng (n = 7), and 5 μg (n = 6) icv prolactin, or vehicle (n = 6) in the parvocellular and magnocellular divisions of the paraventricular nucleus is summarized in E,F, respectively. Bars with different letters are significantly different compared with vehicle (P < 0.05). G–N: Representative images of pSTAT5 and oxytocin staining in the parvocellular division (G,H,K,L) and magnocellular division (I,J,M,N) of the paraventricular nucleus in response to vehicle (G,I), 10 ng (H,J), 500 ng (K,M), or 5 μg doses (L,N) of prolactin. Scale bars = 100 μm in A–D; 25 μm in G–N.
Oxytocin immunoreactivity was predominantly located in the dorsal region of the supraoptic nucleus. Neurons dual-labeled for oxytocin and pSTAT5 were rarely observed in response to the 500 ng prolactin dose (Fig. 7A,A′). However, after 5 μg of prolactin icv, 85% of these oxytocin neurons were dual-labeled (Fig. 7B,B′). The data for each treatment group are summarized in Figure 7C.
Figure 7.
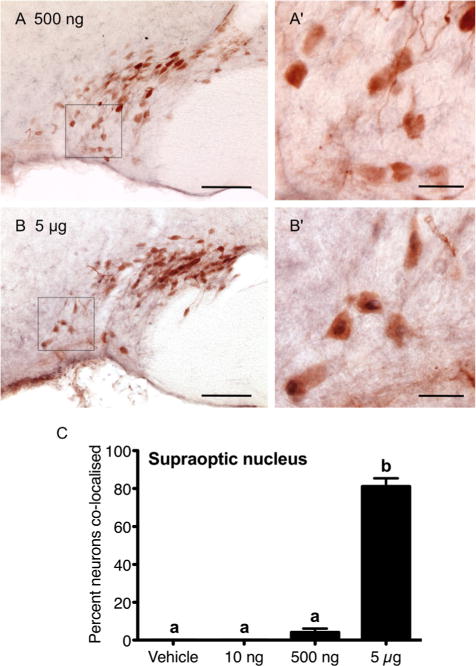
Prolactin induction of nuclear pSTAT5 in oxytocin neurons in the supraoptic nucleus. Representative images of 500 ng (A,A′) and 5 μg (B,B′) icv prolactin-treated animals, showing the distribution of nuclear pSTAT5 (blue/black) and cytoplasmic oxytocin (brown) staining in supraoptic nucleus sections. The percentage of oxytocin-positive neurons displaying nuclear pSTAT5 in response to 10 ng (n = 7), 500 ng (n = 7), and 5 μg (n = 6) icv prolactin, or vehicle (n = 6) in the supraoptic nucleus is summarized in C. Bars with different letters are significantly different compared with vehicle (P < 0.05). Scale bars = 100 μm in A,B; 25 μm in A′,B′.
DISCUSSION
Under most conditions, prolactin secretion is tightly regulated by a homeostatic feedback mechanism. Prolactin stimulates dopamine release from NEDA neurons located in the arcuate nucleus of the hypothalamus, and this dopamine travels to the anterior pituitary to strongly inhibit prolactin secretion. We hypothesized that the NEDA neurons would therefore exhibit a high degree of sensitivity to prolactin due to their role in mediating negative feedback over basal prolactin release. We further hypothesized that neurons in regions such as the rostral preoptic area and paraventricular nucleus, which have also been shown to express prolactin receptors (Bakowska and Morrell, 1997, 2003; Pi and Grattan, 1998b, 1999; Brown et al., 2010), would require higher levels of prolactin to induce a response because these areas typically mediate crucial functions in the maternal brain during late pregnancy and lactation when prolactin levels are elevated (Grattan, 2002). Our results showed that the systemic dose of prolactin increased the numbers of pSTAT5-labeled nuclei in the arcuate nucleus, but not in the paraventricular nucleus or rostral preoptic area. Consistent with our hypothesis, these pSTAT5-labeled cells in the arcuate nucleus included the NEDA neurons. These data indicate that the NEDA neurons of the arcuate nucleus are highly sensitive to prolactin feedback. This could be because the arcuate nucleus is more accessible to prolactin present in the bloodstream than the other hypothalamic regions examined. Alternatively, it is possible that the NEDA neurons are intrinsically more sensitive to prolactin than other hypothalamic neurons. To test the latter hypothesis, we examined responses to icv prolactin, where prolactin should gain access to different hypothalamic nuclei equally because it is delivered directly into the ventricular system. These results also showed that cells in the arcuate nucleus were more sensitive to prolactin stimulation delivered by an icv route than cells found in the paraventricular nucleus or rostral preoptic area. Moreover, dual-label immunohistochemistry confirmed that the NEDA neurons were significantly more responsive to central prolactin than dopamine neurons of the rostral preoptic area, or oxytocin neurons located in the paraventricular and supraoptic nuclei. These data suggest that differences in the intrinsic responsiveness of different hypothalamic neuronal populations to prolactin may exist.
Prolactin transport into the CSF of the central nervous system from the systemic bloodstream is believed to require a carrier-mediated transport mechanism, possibly involving prolactin receptors in the choroid plexus (Walsh et al., 1987). The results of the current study, however, demonstrate that cells in the arcuate nucleus were more sensitive to peripheral prolactin than cells in the paraventricular nucleus and rostral preoptic area. One interpretation of these data is that the arcuate nucleus is more accessible to large proteins such as prolactin present in blood than other hypothalamic regions. The increased sensitivity of the arcuate nucleus to hormones in the blood could arise due to the fact that the arcuate nucleus is adjacent to the median eminence; the latter is known to lack a complete blood–brain barrier (Peruzzo et al., 2000). This means that the arcuate nucleus could be relatively accessible to prolactin either diffusing or actively transported from blood vessels present in this area, in addition to prolactin traveling to the arcuate nucleus indirectly, following transport into the CSF at the choroid plexus. The concept that prolactin may preferentially access brain areas lacking a blood–brain barrier was supported by the fact that, after systemic administration, the only other region exhibiting significant levels of nuclear pSTAT5 was the rostral preoptic area immediately surrounding the organum vasculosum of the lamina terminalis (OVLT), another region lacking a complete blood–brain barrier (Kaplan et al., 1981). However, despite the proximity to these circumventricular organs, both the arcuate nucleus and preoptic area are considered to be within the blood–brain barrier (Rethelyi, 1984; Katsuura et al., 1990). An alternative mechanism might be preferential transport from the third ventricle. The ependyma cell layer lining the third ventricle at the level of the arcuate nucleus contains more specialized cells of glial origin, called tanycytes, than it does in other areas of the hypothalamus (Mullier et al., 2010; Rodriguez et al., 2010). Adjacent tanycytes at the level of the arcuate nucleus are joined by far fewer tight junctions than regular ependymal cells, meaning that the arcuate nucleus is in open communication with the CSF due to the lack of a functional cellular barrier separating the third ventricle and the adjacent neural tissue. In theory, this would enable very low levels of prolactin in the CSF to enter the intercellular space of the arcuate nucleus freely, while the regular ependymal cells at the level of the paraventricular nucleus and rostral preoptic area may limit the access of prolactin more. Despite possible differences in access of prolactin into the different nuclei, the absence of any response in the paraventricular nucleus and rostral preoptic area following systemic administration of prolactin was surprising, as prolactin would be expected to reach these nuclei after transport into the CSF. It is possible that the dose delivered was not large enough to increase the concentration of prolactin in the CSF to a level sufficient to affect cell signaling in these regions.
The original intention of the icv prolactin administration was to test the second possibility that the NEDA neurons are intrinsically more sensitive to prolactin than the other neuronal populations in the hypothalamus. All of the prolactin-responsive NEDA neurons showed activation of pSTAT5 in response to the 500 ng dose of prolactin, whereas 5 μg of prolactin was required to dual-label all of the prolactin-responsive dopamine neurons found in the periventricular area of the rostral preoptic area, and to induce nuclear pSTAT5 in all prolactin-responsive oxytocin neurons located in both the paraventricular and supraoptic nuclei. The mechanism that enables the NEDA neurons, and other cells in the arcuate nucleus, to be more responsive to prolactin stimulation than cells in the other regions examined is intriguing. It is possible that a differential transport of prolactin into the arcuate nucleus, as discussed above, is the major factor involved. Alternatively, there may be intrinsic differences in the cellular responsiveness, such as increased expression of prolactin receptors compared to cells in other nuclei. There is limited immunohistochemical evidence that prolactin-receptor-expressing cells are more readily detectable in the arcuate nucleus, particularly in the dorsomedial division of this nucleus occupied by the tuberoinfundibular dopaminergic (TIDA) neurons than in the rostral preoptic area or paraventricular nucleus (Pi and Grattan, 1998b). It is also possible that differences in the number of nuclear pSTAT5-labeled cells between different hypothalamic regions involves changes downstream of prolactin receptor activation. For example, there may be differences in the expression of proteins that inhibit JAK2/STAT5 signaling, such as suppressors of cytokine signaling proteins (Anderson et al., 2006), or factors that enhance JAK2/STAT5 activation, such as Src kinases (Garcia-Martinez et al., 2010) between cells in different hypothalamic nuclei. The balance of the expression of such factors could favor the activation of cells in the arcuate nucleus to prolactin stimulation while dampening the responsiveness of cells in other regions. These changes at the cellular level, and those that affect the access of prolactin from the CSF into the underlying neuronal tissue, may preferentially enhance prolactin actions on cells in the arcuate nucleus, particularly when prolactin levels are low.
In the arcuate nucleus there were some unexpected results following icv prolactin treatment. First, the lowest icv dose of prolactin increased the number of pSTAT5-labeled cells, suggesting a population of highly sensitive prolactin-responsive cells. Surprisingly, these cells did not colocalize with TH, indicating that they were not the NEDA neurons involved in the regulation of prolactin secretion. It must be emphasized that the relative amount of pSTAT5 in these cells was low compared with the strongly labeled NEDA neurons seen with the higher dose. The identity of these cells is unclear, and it is possible that they are not even neurons. Second, in the single label pSTAT5 study there were no detectable differences in the total number of cells responding to the 10 ng and 500 ng doses, despite our dual-label data showing that all of the prolactin-responsive NEDA neurons were double-labeled at the latter dose. This suggests that there was in fact a decrease in the number of non-TH prolactin-responsive cells in response to the 500 ng dose. However, it is important to note that the pattern of staining between these doses was different (Fig. 3) and it is possible that in response to the 500 ng prolactin dose the intensely stained NEDA neurons in the dorsomedial region of this nucleus precluded the counting of the other less-stained neurons scattered throughout this region. Although this result is interesting, we have focused on the dual-label immunohistochemistry data in response to icv prolactin treatment to infer potential differences in the sensitivity of different hypothalamic neuronal populations to prolactin.
Our results demonstrate that prolactin induces the expression of nuclear pSTAT5 in oxytocin neurons located in both the paraventricular nucleus and the supraoptic nucleus of the rodent brain. These data are in agreement with a previous report from our laboratory indicating that oxytocin neurons in both these nuclei express long-form prolactin receptor mRNA (Kokay et al., 2006), and indicates that prolactin is capable of directly affecting intracellular signal transduction in oxytocin neurons. In the supraoptic nucleus, few non-oxytocin immunoreactive cells expressed nuclear pSTAT5, suggesting that this is an exclusive effect on oxytocin neurons found in this nucleus. A recent report has shown that central prolactin delivery activates the mitogen-activated protein kinase (MAPK) pathway members ERK1/2 in oxytocin and vasopressin neurons in the rat, predominantly those located in the supraoptic nucleus (Blume et al., 2009). This is intriguing, especially in the case of the vasopressin cells, because very few of these neurons in either the paraventricular nucleus or the supraoptic nucleus express long-form prolactin receptor mRNA, nor do those neurons in the supraoptic nucleus change their electrophysiological response after central prolactin treatment (Kokay et al., 2006). These data suggest the involvement of the alternative isoform of the prolactin receptor, the short-form prolactin receptor, that has been identified in the paraventricular and supraoptic nuclei (Pi and Grattan, 1998a; Bakowska and Morrell, 2003) and has also been shown to be coexpressed with vasopressin (Mejia et al., 1997; Bakowska and Morrell, 2003). The short-form prolactin receptor has a truncated intracellular domain and lacks the ability to induce nuclear localization of pSTAT5, but is capable of mediating signaling through the MAPK pathway (Lebrun et al., 1995; Goupille et al., 1997). Further work is required to characterize the expression of the short-form prolactin receptor in the paraventricular nucleus, as well as the consequences of the activation of each pathway on the function of the oxytocin neurons in this region.
The results show that the anatomically distinct oxytocin neuronal populations in the paraventricular nucleus and supraoptic nucleus respond differently to prolactin, and this differential sensitivity may have implications on the function of each population. The oxytocin neurons located medially to the third ventricle in the paraventricular nucleus, the parvocellular oxytocin neurons, were more sensitive than the magnocellular neurons located more laterally in the paraventricular nucleus and in the supraoptic nucleus. Parvocellular oxytocin neurons project to the median eminence as well as to other brain regions such as the spinal cord and medulla (Swanson et al., 1980; Sofroniew and Schrell, 1981; Sofroniew, 1983), while the magnocellular oxytocin neurons project to the posterior pituitary to release oxytocin into the bloodstream (Swanson and Sawchenko, 1983). Prolactin could possibly influence a variety of changes in the maternal brain though actions on parvocellular oxytocin neurons (Brunton and Russell, 2008), while at higher concentrations prolactin may influence the release of oxytocin into the bloodstream (Sarkar, 1989; Popeski et al., 2003; Shahrokh et al., 2010). In the parvocellular region of the paraventricular nucleus, the number of pSTAT5-labeled cells in response to prolactin treatment indicates that neuronal phenotypes in addition to oxytocin are prolactin-sensitive in this region. Given the lack of long-form prolactin receptor in vasopressin neurons, the most likely candidates found here include corticotrophin-releasing and thyrotropin-releasing hormone neurons (Blume et al., 2009; Simmons and Swanson, 2009).
The data show that there are many prolactin-responsive cells in the rostral preoptic area; however, a high icv dose of prolactin was required to induce pSTAT5-labeled nuclei throughout this brain region. As yet, we have not identified the phenotype of the majority of these prolactin-responsive neurons, but we have recently shown by in situ hybridization that kisspeptin and GABA neurons in the rostral preoptic area express long-form prolactin receptor mRNA (Kokay et al., 2011). The requirement for very high levels of prolactin to influence cells in the rostral preoptic area may serve to limit the metabolically demanding cost of the onset of maternal behavior in the virgin female, but enables this crucial behavior to develop during pregnancy when prolactin levels are chronically elevated in the maternal bloodstream (Loundes and Bridges, 1986; Bridges et al., 1990).
In conclusion, the current study identified that cells in the arcuate nucleus are more sensitive to prolactin stimulation than cells in other regions of the hypothalamus. This was apparent whether prolactin was delivered by either an icv or a systemic route. The data support the hypothesis that the NEDA neurons situated in the arcuate nucleus, which are involved in the regulation of prolactin secretion, are more sensitive than a range of other prolactin-responsive neurons. Future work will investigate the consequences that prolactin actions in the paraventricular nucleus and preoptic area have on the physiology of the maternal female, particularly during pregnancy and lactation when prolactin levels are chronically elevated.
Acknowledgments
Grant sponsor: National Institutes of Health; Grant number: HD039895 (to R.S.B.); Grant sponsor: Health Research Council; Grant number: HRC 08/076D (to D.R.G., I.C.K.); Grant sponsor: University of Otago Postgraduate Scholarship (to T.J.S.).
LITERATURE CITED
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. doi: 10.1002/ar.1090940210. [DOI] [PubMed] [Google Scholar]
- Albarracin CT, Parmer TG, Duan WR, Nelson SE, Gibori G. Identification of a major prolactin-regulated protein as 20 alpha-hydroxysteroid dehydrogenase: coordinate regulation of its activity, protein content, and messenger ribonucleic acid expression. Endocrinology. 1994;134:2453–2460. doi: 10.1210/endo.134.6.8194472. [DOI] [PubMed] [Google Scholar]
- Anderson GM, Beijer P, Bang AS, Fenwick MA, Bunn SJ, Grattan DR. Suppression of prolactin-induced signal transducer and activator of transcription 5b signaling and induction of suppressors of cytokine signaling messenger ribonucleic acid in the hypothalamic arcuate nucleus of the rat during late pregnancy and lactation. Endocrinology. 2006;147:4996–5005. doi: 10.1210/en.2005-0755. [DOI] [PubMed] [Google Scholar]
- Andrews ZB, Kokay IC, Grattan DR. Dissociation of prolactin secretion from tuberoinfundibular dopamine activity in late pregnant rats. Endocrinology. 2001;142:2719–2724. doi: 10.1210/endo.142.6.8196. [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL. Progesterone suppresses tyrosine hydroxylase messenger ribonucleic acid levels in the arcuate nucleus on proestrus. Endocrinology. 1994;135:343–350. doi: 10.1210/endo.135.1.7912184. [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Voogt JL. The responsiveness of tuberoinfundibular dopaminergic neurons to prolactin feedback is diminished between early lactation and midlactation in the rat. Endocrinology. 1996;137:47–54. doi: 10.1210/endo.137.1.8536641. [DOI] [PubMed] [Google Scholar]
- Arbogast LA, Soares MJ, Tomogane H, Voogt JL. A trophoblast-specific factor(s) suppresses circulating prolactin levels and increases tyrosine hydroxylase activity in tuberoinfundibular dopaminergic neurons. Endocrinology. 1992;131:105–113. doi: 10.1210/endo.131.1.1351837. [DOI] [PubMed] [Google Scholar]
- Augustine RA, Grattan DR. Induction of central leptin resistance in hyperphagic pseudopregnant rats by chronic prolactin infusion. Endocrinology. 2008;149:1049–1055. doi: 10.1210/en.2007-1018. [DOI] [PubMed] [Google Scholar]
- Bakowska JC, Morrell JI. Atlas of the neurons that express mRNA for the long form of the prolactin receptor in the forebrain of the female rat. J Comp Neurol. 1997;386:161–177. doi: 10.1002/(sici)1096-9861(19970922)386:2<161::aid-cne1>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Bakowska JC, Morrell JI. The distribution of mRNA for the short form of the prolactin receptor in the forebrain of the female rat. Brain Res Mol Brain Res. 2003;116:50–58. doi: 10.1016/s0169-328x(03)00213-4. [DOI] [PubMed] [Google Scholar]
- Blume A, Torner L, Liu Y, Subburaju S, Aguilera G, Neumann ID. Prolactin activates mitogen-activated protein kinase signaling and corticotropin releasing hormone transcription in rat hypothalamic neurons. Endocrinology. 2009;150:1841–1849. doi: 10.1210/en.2008-1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bole-Feysot C, Goffin V, Edery M, Binart N, Kelly PA. Prolactin (PRL) and its receptor: actions, signal transduction pathways and phenotypes observed in PRL receptor knockout mice. Endocr Rev. 1998;19:225–268. doi: 10.1210/edrv.19.3.0334. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Numan M, Ronsheim PM, Mann PE, Lupini CE. Central prolactin infusions stimulate maternal behavior in steroid-treated, nulliparous female rats. Proc Natl Acad Sci U S A. 1990;87:8003–8007. doi: 10.1073/pnas.87.20.8003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges RS, Robertson MC, Shiu RP, Sturgis JD, Henriquez BM, Mann PE. Central lactogenic regulation of maternal behavior in rats: steroid dependence, hormone specificity, and behavioral potencies of rat prolactin and rat placental lactogen I. Endocrinology. 1997;138:756–763. doi: 10.1210/endo.138.2.4921. [DOI] [PubMed] [Google Scholar]
- Bridges RS, Rigero BA, Byrnes EM, Yang LL, Walker AM. Central infusions of the recombinant human prolactin receptor antagonist, S179D-PRL, delay the onset of maternal behavior in steroid-primed, nulliparous female rats. Endocrinology. 2001;142:730–739. doi: 10.1210/endo.142.2.7931. [DOI] [PubMed] [Google Scholar]
- Brown RS, Kokay IC, Herbison AE, Grattan DR. Distribution of prolactin-responsive neurons in the mouse forebrain. J Comp Neurol. 2010;518:92–102. doi: 10.1002/cne.22208. [DOI] [PubMed] [Google Scholar]
- Brunton PJ, Russell JA. The expectant brain: adapting for motherhood. Nat Rev Neurosci. 2008;9:11–25. doi: 10.1038/nrn2280. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V, Zaborszky L, Kohler C, Goldstein M, Palay SL. Distribution of tyrosine-hydroxylase-immunoreactive neurons in the hypothalamus of rats. J Comp Neurol. 1984;227:467–496. doi: 10.1002/cne.902270403. [DOI] [PubMed] [Google Scholar]
- Cheung S, Will YM, Hentschel K, Moore KE, Lookingland KJ. Role of gonadal steroids in determining sexual differences in expression of Fos-related antigens in tyrosine hydroxylase-immunoreactive neurons in subdivisions of the hypothalamic arcuate nucleus. Endocrinology. 1997;138:3804–3810. doi: 10.1210/endo.138.9.5411. [DOI] [PubMed] [Google Scholar]
- DaSilva L, Rui H, Erwin RA, Howard OM, Kirken RA, Malabarba MG, Hackett RH, Larner AC, Farrar WL. Prolactin recruits STAT1, STAT3 and STAT5 independent of conserved receptor tyrosines TYR402, TYR479, TYR515 and TYR580. Mol Cell Endocrinol. 1996;117:131–140. doi: 10.1016/0303-7207(95)03738-1. [DOI] [PubMed] [Google Scholar]
- Demarest KT, McKay DW, Riegle GD, Moore KE. Biochemical indices of tuberoinfundibular dopaminergic neuronal activity during lactation: a lack of response to prolactin. Neuroendocrinology. 1983;36:130–137. doi: 10.1159/000123449. [DOI] [PubMed] [Google Scholar]
- DeMaria JE, Livingstone JD, Freeman ME. Ovarian steroids influence the activity of neuroendocrine dopaminergic neurons. Brain Res. 2000;879:139–147. doi: 10.1016/s0006-8993(00)02763-3. [DOI] [PubMed] [Google Scholar]
- Fliestra RJ, Voogt JL. Lactogenic hormones of the placenta and pituitary inhibit suckling-induced prolactin (PRL) release but not the ante-partum PRL surge. Proc Soc Exp Biol Med. 1997;214:258–264. doi: 10.3181/00379727-214-44094. [DOI] [PubMed] [Google Scholar]
- Foord SM, Peters JR, Dieguez C, Scanlon MF, Hall R. Dopamine receptors on intact anterior pituitary cells in culture: functional association with the inhibition of prolactin and thyrotropin. Endocrinology. 1983;112:1567–1577. doi: 10.1210/endo-112-5-1567. [DOI] [PubMed] [Google Scholar]
- Freeman ME, Kanyicska B, Lerant A, Nagy G. Prolactin: structure, function, and regulation of secretion. Physiol Rev. 2000;80:1523–1631. doi: 10.1152/physrev.2000.80.4.1523. [DOI] [PubMed] [Google Scholar]
- Garcia-Martinez JM, Calcabrini A, Gonzalez L, Martin-Forero E, Agullo-Ortuno MT, Simon V, Watkin H, Anderson SM, Roche S, Martin-Perez J. A non-catalytic function of the Src family tyrosine kinases controls prolactin-induced Jak2 signaling. Cell Signal. 2010;22:415–426. doi: 10.1016/j.cellsig.2009.10.013. [DOI] [PubMed] [Google Scholar]
- Goupille O, Daniel N, Bignon C, Jolivet G, Djiane J. Prolactin signal transduction to milk protein genes: carboxy-terminal part of the prolactin receptor and its tyrosine phosphorylation are not obligatory for JAK2 and STAT5 activation. Mol Cell Endocrinol. 1997;127:155–169. doi: 10.1016/s0303-7207(97)04005-7. [DOI] [PubMed] [Google Scholar]
- Grattan DR. Behavioural significance of prolactin signalling in the central nervous system during pregnancy and lactation. Reproduction. 2002;123:497–506. doi: 10.1530/rep.0.1230497. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Averill RL. Effect of ovarian steroids on a nocturnal surge of prolactin secretion that precedes parturition in the rat. Endocrinology. 1990;126:1199–1205. doi: 10.1210/endo-126-2-1199. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Averill RL. Role of the placenta in the control of the ante-partum surge of prolactin in the rat. J Endocrinol. 1991;130:401–407. doi: 10.1677/joe.0.1300401. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Averill RL. Absence of short-loop autoregulation of prolactin during late pregnancy in the rat. Brain Res Bull. 1995;36:413–416. doi: 10.1016/0361-9230(94)00216-n. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Kokay IC. Prolactin: a pleiotropic neuroendocrine hormone. J Neuroendocrinol. 2008;20:752–763. doi: 10.1111/j.1365-2826.2008.01736.x. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Xu JJ, McLachlan MJ, Kokay IC, Bunn SJ, Hovey RC, Davey HW. Feedback regulation of PRL secretion is mediated by the transcription factor, signal transducer, and activator of transcription 5b. Endocrinology. 2001;142:3935–3940. doi: 10.1210/endo.142.9.8385. [DOI] [PubMed] [Google Scholar]
- Grattan DR, Steyn FJ, Kokay IC, Anderson GM, Bunn SJ. Pregnancy-induced adaptation in the neuroendocrine control of prolactin secretion. J Neuroendocrinol. 2008;20:497–507. doi: 10.1111/j.1365-2826.2008.01661.x. [DOI] [PubMed] [Google Scholar]
- Gregory SJ, Townsend J, McNeilly AS, Tortonese DJ. Effects of prolactin on the luteinizing hormone response to gonadotropin-releasing hormone in primary pituitary cell cultures during the ovine annual reproductive cycle. Biol Reprod. 2004;70:1299–1305. doi: 10.1095/biolreprod.103.022806. [DOI] [PubMed] [Google Scholar]
- Gudelsky GA, Porter JC. Release of dopamine from tuberoinfundibular neurons into pituitary stalk blood after prolactin or haloperidol administration. Endocrinology. 1980;106:526–529. doi: 10.1210/endo-106-2-526. [DOI] [PubMed] [Google Scholar]
- Hallbeck M, Larhammar D, Blomqvist A. Neuropeptide expression in rat paraventricular hypothalamic neurons that project to the spinal cord. J Comp Neurol. 2001;433:222–238. doi: 10.1002/cne.1137. [DOI] [PubMed] [Google Scholar]
- Herbison AE. Estrogen positive feedback to gonadotropin-releasing hormone (GnRH) neurons in the rodent: the case for the rostral periventricular area of the third ventricle (RP3V) Brain Res Rev. 2008;57:277–287. doi: 10.1016/j.brainresrev.2007.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan GP, Hartman BK, Creveling CR. Immunohistochemical localization of catechol-O-methyltransferase in circumventricular organs of the rat: potential variations in the blood-brain barrier to native catechols. Brain Res. 1981;229:323–335. doi: 10.1016/0006-8993(81)90997-5. [DOI] [PubMed] [Google Scholar]
- Katsuura G, Arimura A, Koves K, Gottschall PE. Involvement of organum vasculosum of lamina terminalis and preoptic area in interleukin 1 beta-induced ACTH release. Am J Physiol. 1990;258(1 Pt 1):E163–171. doi: 10.1152/ajpendo.1990.258.1.E163. [DOI] [PubMed] [Google Scholar]
- Kokay IC, Bull PM, Davis RL, Ludwig M, Grattan DR. Expression of the long form of the prolactin receptor in magnocellular oxytocin neurons is associated with specific prolactin regulation of oxytocin neurons. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1216–1225. doi: 10.1152/ajpregu.00730.2005. [DOI] [PubMed] [Google Scholar]
- Kokay IC, Petersen SL, Grattan DR. Identification of prolactin-sensitive GABA and kisspeptin neurons in regions of the rat hypothalamus involved in the control of fertility. Endocrinology. 2011;152:526–535. doi: 10.1210/en.2010-0668. [DOI] [PubMed] [Google Scholar]
- Ladyman SR, Augustine RA, Grattan DR. Hormone interactions regulating energy balance during pregnancy. J Neuroendocrinol. 2010;22:805–817. doi: 10.1111/j.1365-2826.2010.02017.x. [DOI] [PubMed] [Google Scholar]
- Lebrun JJ, Ali S, Ullrich A, Kelly PA. Proline-rich sequence-mediated Jak2 association to the prolactin receptor is required but not sufficient for signal transduction. J Biol Chem. 1995;270:10664–10670. doi: 10.1074/jbc.270.18.10664. [DOI] [PubMed] [Google Scholar]
- Lee Y, Voogt J. Feedback effects of placental lactogens on prolactin levels and fos-related antigen immunoreactivity of tuberoinfundibular dopaminergic neurons in the arcuate nucleus during pregnancy in the rat. Endocrinology. 1999;140:2159–2166. doi: 10.1210/endo.140.5.6730. [DOI] [PubMed] [Google Scholar]
- Leite CM, Ribeiro AB, Szawka RE, Anselmo-Franci JA. Activity of hypothalamic dopaminergic neurones during the day of oestrus: involvement in prolactin secretion. J Neuroendocrinol. 2010;22:1052–1060. doi: 10.1111/j.1365-2826.2010.02057.x. [DOI] [PubMed] [Google Scholar]
- Liu HL, Cao R, Jin L, Chen LW. Immunocytochemical localization of substance P receptor in hypothalamic oxytocin-containing neurons of C57 mice. Brain Res. 2002;948:175–179. doi: 10.1016/s0006-8993(02)03146-3. [DOI] [PubMed] [Google Scholar]
- Loundes DD, Bridges RS. Length of prolactin priming differentially affects maternal behavior in female rats. Biol Reprod. 1986;34:495–501. doi: 10.1095/biolreprod34.3.495. [DOI] [PubMed] [Google Scholar]
- Ma FY, Anderson GM, Gunn TD, Goffin V, Grattan DR, Bunn SJ. Prolactin specifically activates signal transducer and activator of transcription 5b in neuroendocrine dopaminergic neurons. Endocrinology. 2005;146:5112–5119. doi: 10.1210/en.2005-0770. [DOI] [PubMed] [Google Scholar]
- Mejia S, Morales MA, Zetina ME, Martinez de la Escalera G, Clapp C. Immunoreactive prolactin forms colocalize with vasopressin in neurons of the hypothalamic paraventricular and supraoptic nuclei. Neuroendocrinology. 1997;66:151–159. doi: 10.1159/000127233. [DOI] [PubMed] [Google Scholar]
- Mullier A, Bouret SG, Prevot V, Dehouck B. Differential distribution of tight junction proteins suggests a role for tanycytes in blood-hypothalamus barrier regulation in the adult mouse brain. J Comp Neurol. 2010;518:943–962. doi: 10.1002/cne.22273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naef L, Woodside B. Prolactin/Leptin interactions in the control of food intake in rats. Endocrinology. 2007;148:5977–5983. doi: 10.1210/en.2007-0442. [DOI] [PubMed] [Google Scholar]
- Nagano M, Kelly PA. Tissue distribution and regulation of rat prolactin receptor gene expression. Quantitative analysis by polymerase chain reaction. J Biol Chem. 1994;269:13337–13345. [PubMed] [Google Scholar]
- Okamura H, Kitahama K, Nagatsu I, Geffard M. Comparative topography of dopamine- and tyrosine hydroxylase-immunoreactive neurons in the rat arcuate nucleus. Neurosci Lett. 1988;95:347–353. doi: 10.1016/0304-3940(88)90683-0. [DOI] [PubMed] [Google Scholar]
- Peruzzo B, Pastor FE, Blazquez JL, Schobitz K, Pelaez B, Amat P, Rodriguez EM. A second look at the barriers of the medial basal hypothalamus. Exp Brain Res. 2000;132:10–26. doi: 10.1007/s002219900289. [DOI] [PubMed] [Google Scholar]
- Pi XJ, Grattan DR. Differential expression of the two forms of prolactin receptor mRNA within microdissected hypothalamic nuclei of the rat. Brain Res Mol Brain Res. 1998a;59:1–12. doi: 10.1016/s0169-328x(98)00109-0. [DOI] [PubMed] [Google Scholar]
- Pi XJ, Grattan DR. Distribution of prolactin receptor immunoreactivity in the brain of estrogen-treated, ovariectomized rats. J Comp Neurol. 1998b;394:462–474. doi: 10.1002/(sici)1096-9861(19980518)394:4<462::aid-cne5>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Pi XJ, Grattan DR. Increased expression of both short and long forms of prolactin receptor mRNA in hypothalamic nuclei of lactating rats. J Mol Endocrinol. 1999;23:13–22. doi: 10.1677/jme.0.0230013. [DOI] [PubMed] [Google Scholar]
- Popeski N, Amir S, Woodside B. Prolactin and oxytocin in the paraventricular and supraoptic nuclei: effects on oxytocin mRNA and nitric oxide synthase. J Neuroendocrinol. 2003;15:687–696. doi: 10.1046/j.1365-2826.2003.01048.x. [DOI] [PubMed] [Google Scholar]
- Rethelyi M. Diffusional barrier around the hypothalamic arcuate nucleus in the rat. Brain Res. 1984;307:355–358. doi: 10.1016/0006-8993(84)90494-3. [DOI] [PubMed] [Google Scholar]
- Rodriguez EM, Blazquez JL, Guerra M. The design of barriers in the hypothalamus allows the median eminence and the arcuate nucleus to enjoy private milieus: the former opens to the portal blood and the latter to the cerebrospinal fluid. Peptides. 2010;31:757–776. doi: 10.1016/j.peptides.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Sarkar DK. Evidence for prolactin feedback actions on hypothalamic oxytocin, vasoactive intestinal peptide and dopamine secretion. Neuroendocrinology. 1989;49:520–524. doi: 10.1159/000125161. [DOI] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ. Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology. 2010;151:2276–2286. doi: 10.1210/en.2009-1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Gregg C, Enwere E, Fujikawa H, Hassam R, Geary C, Cross JC, Weiss S. Pregnancy-stimulated neurogenesis in the adult female forebrain mediated by prolactin. Science. 2003;299:117–120. doi: 10.1126/science.1076647. [DOI] [PubMed] [Google Scholar]
- Simmons DM, Swanson LW. Comparison of the spatial distribution of seven types of neuroendocrine neurons in the rat paraventricular nucleus: toward a global 3D model. J Comp Neurol. 2009;516:423–441. doi: 10.1002/cne.22126. [DOI] [PubMed] [Google Scholar]
- Smith MS, Freeman ME, Neill JD. The control of progesterone secretion during the estrous cycle and early pseudopregnancy in the rat: prolactin, gonadotropin and steroid levels associated with rescue of the corpus luteum of pseudopregnancy. Endocrinology. 1975;96:219–226. doi: 10.1210/endo-96-1-219. [DOI] [PubMed] [Google Scholar]
- Smith MS, McLean BK, Neill JD. Prolactin: the initial luteotropic stimulus of pseudopregnancy in the rat. Endocrinology. 1976;98:1370–1377. doi: 10.1210/endo-98-6-1370. [DOI] [PubMed] [Google Scholar]
- Sofroniew Vasopressin and oxytocin in the mammalian brain and spinal cord. Trends Neurosci. 1983;6:467–472. [Google Scholar]
- Sofroniew MV, Schrell U. Evidence for a direct projection from oxytocin and vasopressin neurons in the hypothalamic paraventricular nucleus to the medulla oblongata: immunohistochemical visualisation of both the horse radish peroxidase transported and the peptide produced by the same neurons. Neurosci Lett. 1981;22:211–217. [Google Scholar]
- Swanson LW, Sawchenko PE. Hypothalamic integration: organization of the paraventricular and supraoptic nuclei. Annu Rev Neurosci. 1983;6:269–324. doi: 10.1146/annurev.ne.06.030183.001413. [DOI] [PubMed] [Google Scholar]
- Swanson LW, Sawchenko PE, Wiegand SJ, Price JL. Separate neurons in the paraventricular nucleus project to the median eminence and to the medulla or spinal cord. Brain Res. 1980;198:190–195. doi: 10.1016/0006-8993(80)90354-6. [DOI] [PubMed] [Google Scholar]
- Terkel J, Blake CA, Sawyer CH. Serum prolactin levels in lactating rats after suckling or exposure to ether. Endocrinology. 1972;91:49–53. doi: 10.1210/endo-91-1-49. [DOI] [PubMed] [Google Scholar]
- Torner L, Toschi N, Pohlinger A, Landgraf R, Neumann ID. Anxiolytic and anti-stress effects of brain prolactin: Improved efficacy of antisense targeting of the prolactin receptor by molecular modeling. J Neurosci. 2001;21:3207–3214. doi: 10.1523/JNEUROSCI.21-09-03207.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RJ, Slaby FJ, Posner BI. A receptor-mediated mechanism for the transport of prolactin from blood to cerebrospinal fluid. Endocrinology. 1987;120:1846–1850. doi: 10.1210/endo-120-5-1846. [DOI] [PubMed] [Google Scholar]
- Watson RE, Jr, Wiegand SJ, Clough RW, Hoffman GE. Use of cryoprotectant to maintain long-term peptide immunoreactivity and tissue morphology. Peptides. 1986;7:155–159. doi: 10.1016/0196-9781(86)90076-8. [erratum, Peptides 1986 May–Jun;7:545] [DOI] [PubMed] [Google Scholar]
- Wolf ME, LeWitt PA, Bannon MJ, Dragovic LJ, Kapatos G. Effect of aging on tyrosine hydroxylase protein content and the relative number of dopamine nerve terminals in human caudate. J Neurochem. 1991;56:1191–1200. doi: 10.1111/j.1471-4159.1991.tb11410.x. [DOI] [PubMed] [Google Scholar]
- Xiong JJ, Hatton GI. Differential responses of oxytocin and vasopressin neurons to the osmotic and stressful components of hypertonic saline injections: a Fos protein double labeling study. Brain Res. 1996;719:143–153. doi: 10.1016/0006-8993(95)01466-7. [DOI] [PubMed] [Google Scholar]


