Abstract
Soluble adenylyl cyclase (sAC) is a recently recognized source of the signaling molecule cyclic AMP (cAMP) that is genetically and biochemically distinct from the classic G-protein-regulated transmembrane adenylyl cyclases (tmACs). Mammalian sAC is distributed throughout the cytoplasm and it may be present in the nucleus and inside mitochondria. sAC activity is directly stimulated by HCO3−, and sAC has been confirmed to be a HCO3− sensor in a variety of mammalian cell types. In addition, sAC can functionally associate with carbonic anhydrases to act as a de facto sensor of pH and CO2. The two catalytic domains of sAC are related to HCO3−-regulated adenylyl cyclases from cyanobacteria, suggesting the cAMP pathway is an evolutionarily conserved mechanism for sensing CO2 levels and/or acid/base conditions. Reports of sAC in aquatic animals are still limited but are rapidly accumulating. In shark gills, sAC senses blood alkalosis and triggers compensatory H+ absorption. In the intestine of bony fishes, sAC modulates NaCl and water absorption. And in sea urchin sperm, sAC may participate in the initiation of flagellar movement and in the acrosome reaction. Bioinformatics and RT-PCR results reveal that sAC orthologs are present in most animal phyla. This review summarizes the current knowledge on the physiological roles of sAC in aquatic animals and suggests additional functions in which sAC may be involved.
Key words: V-ATPase, Acid/base, cAMP, Carbonic anhydrase, pH sensing, Proton pump
Introduction
Cyclic adenosine monophosphate (cAMP) is the signaling molecule of one of the most versatile and evolutionarily conserved signaling pathways. cAMP is produced by adenylyl cyclase enzymes that use ATP as substrate. There are six different classes of adenylyl cyclases distributed throughout Bacteria, Archaea and Eukarya; these classes are unrelated in sequence and structure but all produce cAMP as a result of convergent evolution (Linder and Schultz, 2008). All known eukaryotic adenylyl cyclases, including soluble adenylyl cyclase (sAC) and transmembrane adenylyl cyclase (tmAC) from animals, belong to Class III.
Discovery of mammalian sAC
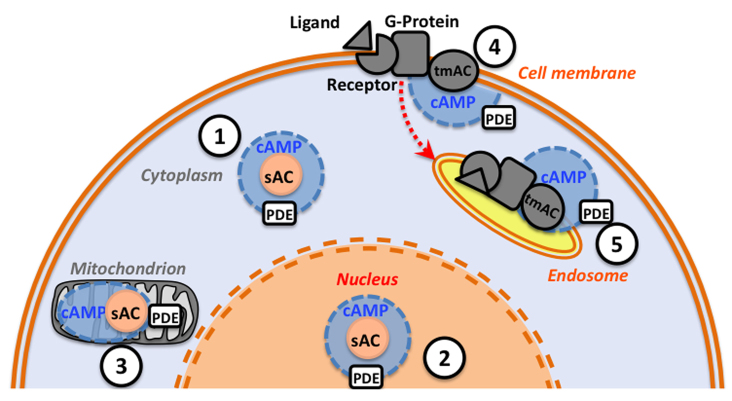
Until recently, vertebrate animals were believed to have only one type of adenylyl cyclase, a family of hormone and G-protein-regulated tmACs. Mammals have nine tmAC genes (ADCY1–9), which differ in their tissue and developmental expression as well as in some of their regulatory properties (Cooper, 2003; Hanoune and Defer, 2001). A genetically unrelated, novel adenylyl cyclase (ADCY10) was recently identified by Levin and Buck (Buck et al., 1999; Chen et al., 2000), following on from earlier reports of Mn2+-stimulated cAMP activity in rat testis homogenates (Braun, 1974; Braun, 1975; Braun, 1990; Braun, 1991; Braun and Dods, 1975). This enzyme was termed ‘soluble adenylyl cyclase’ (sAC) because its activity is preferentially found in the cytosolic fraction, although some activity is also found associated with membranes. Subsequent immunolocalization and biochemical studies found sAC in the cell cytoplasm and in organelles (Zippin et al., 2003; Zippin et al., 2004). The intracellular localization of sAC changed the assumption that cAMP is produced exclusively in the proximity of the cell membrane by tmACs. Production of cAMP in various focal points within cells supports the model of cAMP-signaling microdomains (Fig. 1), which entails tmACs and sAC as sources of cAMP, phosphodiesterases (PDEs) as barriers for cAMP diffusion, and the cAMP-activated targets protein kinase A (PKA), cyclic nucleotide-gated channels and exchange protein activated by cAMP (EPAC) (reviewed in Cooper, 2003; Tresguerres et al., 2011; Zaccolo, 2009; Zaccolo et al., 2006). The most widely studied cAMP effector is PKA, which modulates the activity of multiple downstream proteins by phosphorylation. PKA and PDE are tethered to specific intracellular compartments by A-kinase anchoring proteins (AKAPs), which also coordinate PKA and PDE activities, thus allowing the spatio-temporal dynamics of cAMP signaling (reviewed in Wong and Scott, 2004). The cAMP-signaling microdomain model has changed the cAMP-signaling paradigm and causing researchers to revisit previous studies on signal transduction pathways.
Fig. 1.
Intracellular cAMP-signaling microdomains. cAMP production may occur in discrete intracellular compartments such as (1) focal points throughout the cytoplasm, (2) the nucleus, (3) mitochondria, (4) the cell membrane vicinity and (5) internalized endosomes. Additional regulation might involve the movement of soluble adenylyl cyclase (sAC) between compartments (not shown). Each microdomain contains a source of cAMP [sAC or transmembrane adenylyl cyclase (tmAC)]; phosphodiesterases (PDEs) that degrade cAMP, thus acting as barriers for cAMP diffusion; and cAMP targets such as protein kinase A (PKA) or exchange protein activated by cAMP (EPAC) (not depicted). Production of cAMP by sAC is stimulated by increased [HCO3−] (and in some cases Ca2+, see ‘Discovery of mammalian sAC’ and Fig. 2 for details). Production of cAMP by tmAC occurs in response to various extracellular ligands and it requires modulation by G-protein-coupled receptors and G-protein.
The paramount feature of sAC is direct stimulation by HCO3− to produce cAMP, turning sAC into a putative physiological acid/base (A/B) sensor (Chen et al., 2000). The molecular mechanism of HCO3− stimulation has been elucidated for CyaC, a cyanobacterial adenylyl cyclase related to sAC (Steegborn et al., 2005b); this mechanism is believed to also apply to mammalian sAC (reviewed in Kamenetsky et al., 2006; Tresguerres et al., 2011). Briefly, HCO3− induces an allosteric change that results in an increase in the Vmax of sAC without changing its Km for substrate ATP. The stimulatory effect of HCO3− on sAC and sAC-like enzymes is strictly dependent on a specific amino acid residue that is a threonine in mammals and a serine in bacteria and mollusks. In contrast, the corresponding residue in tmACs, which are not stimulated by HCO3−, is an aspartate (Linder, 2006; Steegborn et al., 2005b).
There are several other biochemical differences between mammalian sAC and tmACs. While tmAC activity is modulated by heterotrimeric G-proteins, sAC is unresponsive. Most tmAC isoforms are stimulated by low micromolar [Ca2+] via calmodulin (Wang and Storm, 2003; Willoughby and Cooper, 2007), but inhibited by millimolar [Ca2+], which displaces the cofactor Mg2+ from the active site (Guillou et al., 1999). In contrast, mammalian sAC (but not necessarily sACs from other organisms, see below) is stimulated by millimolar [Ca2+], which lowers sAC Km for ATP (Jaiswal and Conti, 2003; Litvin et al., 2003); mammalian sAC is additionally insensitive
List of abbreviations
- A/B
acid/base
- AC
adenylyl cyclase
- AKAP
A-kinase anchoring protein
- CA
carbonic anhydrase
- cAMP
cyclic AMP
- CFTR
cystic fibrosis transmembrane conductance regulator
- CyaC
cyanobacterial AC
- EPAC
exchange protein activated by cAMP
- EST
expressed sequence tag
- GPCR
G-protein-coupled receptor
- NBC
sodium/bicarbonate cotransporter
- NHE
Na+/H+ exchanger
- NKCC
Na+/K+/2Cl− cotransporter
- PDE
phosphodiesterase
- PKA
protein kinase A
- sAC
soluble adenylyl cyclase
- sACFL
full-length sAC
- sACT
truncated sAC
- tmAC
transmembrane AC
- TSA
transcriptome shotgun assemblies
- WGS
whole genome shotgun
to calmodulin. Most tmACs are potently activated by forskolin, a plant dipertene that has been classically used to study cAMP function in vivo (Seamon and Daly, 1981; Seamon et al., 1981); however, sAC is insensitive to forskolin (Buck et al., 1999). Mammalian sAC and tmACs also display differential sensitivity to the pharmacological antagonists KH7 and derivatives of catechol estrogen (selective for sAC), and ‘p-site’ inhibitors such as 2′,5′-dideoxyadenosine (selective for tmAC) (Tresguerres et al., 2011).
Mammalian sAC is composed of two catalytic domains in the N-terminus (~50 kDa), and a C-terminus domain with mostly unknown regulatory functions (~140 kDa) (Buck et al., 1999; Chen et al., 2000). Some mammals have multiple sAC genes (e.g. dog, bonobo), while humans have a single sAC gene and one pseudo gene. Human sAC mRNA undergoes extensive alternative splicing (Farrell et al., 2008; Geng et al., 2005; Jaiswal and Conti, 2001; Moore et al., 2008). The better-characterized human sAC splice variant, termed ‘truncated sAC’ (sACT), is essentially composed of just the two catalytic domains and is ~10-fold more active than full-length sAC (sACFL) (reflected as a higher Vmax) (Buck et al., 1999). Most of the difference in specific activity is due to a nine amino acid autoinhibitory domain located C-terminal to the second catalytic domain (Chaloupka et al., 2006). However, the half-maximum stimulation (EC50) for HCO3− and the Km for Mg2+, Mn2+ and Ca2+ of sACT, sACFL and other splice variants are indistinguishable from each other (Chaloupka et al., 2006; Geng et al., 2005), indicating that these parameters are exclusively determined by residues in the two catalytic domains.
Studies on sAC have been challenging because of the intrinsic complexity of sAC and methodological issues. Among the former is that sAC mRNA abundance is typically very low in cells, with the exception of testes bearing maturing sperm (Buck et al., 1999; Farrell et al., 2008; Geng et al., 2005). This trend seems to apply to all vertebrate animals but not necessarily to invertebrates. The low sAC mRNA abundance only implies a low protein turnover rate but it does not preclude robust expression. However, it complicates detection of sAC by RT-PCR and makes it almost impossible to use degenerate PCR to clone sAC from species lacking a sequenced genome (e.g. Tresguerres et al., 2010b). One important methodological issue is that sAC rapidly loses its activity after more than one freeze/thaw cycle (Barott et al., 2013), and that the activity assay conditions that sustain HCO3− stimulation are not universal among species, especially when it comes to the required catalytic metals (Mg2+, Mn2+, Ca2+). Also, the initial studies on sAC were impaired by the lack of pharmacological inhibitors specific for sAC. However, KH7 and derivatives of catechol estrogen are now proving to be effective against sACs from cyanobacteria (Steegborn et al., 2005a), coral (Barott et al., 2013), sea urchin (Beltrán et al., 2007), shark (Tresguerres et al., 2010c) and mammals (Hess et al., 2005; Pastor-Soler et al., 2003).
Mechanisms of sAC activation in vivo
The in vitro activity of sAC is specifically modulated by [HCO3−] and not by pH or CO2 (Chen et al., 2000; Tresguerres et al., 2010c). However, the presence of carbonic anhydrase (CA) and A/B ion-transporting proteins may render sAC a de facto sensor of intra- and extra-cellular CO2, pH and [HCO3−] in vivo (Fig. 2). A straightforward mechanism of sAC stimulation involves extracellular HCO3− entering the cell via ion transporter proteins, for example membrane potential-driven electrogenic Na+/HCO3− cotransporters (NBCs) in astrocytes (Choi et al., 2012) and probably mammalian epididymis and kidney, or CFTR channels in airway cells (Schmid et al., 2010). Another mechanism is the entry of CO2 into the cell (by diffusion or possibly across aquaporins), where it is hydrated into HCO3− and H+ by intracellular CA, as observed in shark gills (Tresguerres et al., 2010c; Tresguerres et al., 2007), and possibly mammalian epididymis (Pastor-Soler et al., 2003) and kidney (Gong et al., 2010; Paunescu et al., 2008). A third source of HCO3− is CO2 from mitochondrial aerobic respiration, which, after CA-catalyzed hydration into HCO3−, may stimulate sAC inside mitochondria (Acin-Perez et al., 2009) or in the cytoplasm (Tresguerres et al., 2010b). Additional mechanisms for sAC stimulation may be more complex. For example, sAC in mammalian epididymis responds to physiologically relevant variations in extracellular pH from 6.6 to 7.8 in HCO3−-free medium (Pastor-Soler et al., 2003) via an unknown mechanism. And although sAC is not directly modulated by hormones, the mechanisms that load HCO3− into a cell may be. For example, in mouse ovarian granulosa cells, the follicle-stimulating hormone activates CFTR channels [via G-protein-coupled receptor (GPCR)/G-protein/tmAC] leading to HCO3− entry and subsequent sAC stimulation (Chen et al., 2012a). This illustrates a potential, more intricate, mechanism of sAC action that depends on the interplay between cAMP from different sources. Finally, sAC in mammalian INS-1 cells is stimulated by Ca2+ that enters the cell across voltage-dependent Ca2+ channels, possibly in conjunction with changes in [HCO3−] and ATP (Ramos et al., 2008).
Fig. 2.
Mechanisms of sAC activation in vivo. (1) sAC in the cytoplasm can be stimulated by HCO3− from various sources. (a) Carbonic anhydrase (CA)-dependent hydration of external CO2. (b) CA-dependent hydration of metabolic CO2. (c) H+-extruding transporters (HE) such as V-type H+-ATPase or Na+/H+ exchangers from the cell may prevent slowing down of the CO2 hydration reaction. (d,e) HCO3− that enters through membrane-transporting proteins such as electrogenic Na+/HCO3−-cotransporters (NBCs), anion exchangers or cystic fibrosis transmembrane conductance regulator (CFTR) channels. (f) The entry of HCO3− across transporters, exchangers and channels can potentially be modulated by hormones. (2) sAC in the nucleus may be stimulated by HCO3− derived from all the sources listed above. (3) sAC in the cytoplasm may be stimulated by catalytic metals (e.g. Ca2+ in mammals), which enter the cell through voltage-dependent Ca2+ channels (VDCC) or potentially by Ca2+ released from the endoplasmic reticulum or mitochondria (not depicted). (4) sAC inside mitochondria may be stimulated by metabolically generated CO2 through CA.
In vitro versus in vivo EC50 for HCO3−
The in vitro EC50 for HCO3− for purified mammalian and shark sAC protein is close to the normal [HCO3−] in the extracellular fluid of each organism (~20 and ~5 mmol l−1, respectively; Table 1). This implies that small changes in [HCO3−] will result in large changes in sAC activity, as the EC50 is the mean point in the steepest part of the dose–response curve. However, sAC is located inside cells, where the average [HCO3−] is somewhat lower than in extracellular fluids. For example, normal intracellular [HCO3−] has been reported to be 10–15 mmol l−1 in mammals (Bettice et al., 1984; Lodish, 1999) and 1–4 mmol l−1 in fish (Hōbe et al., 1984; Strobel et al., 2012; Wood et al., 1990), depending on the species and cell type. Nonetheless, variations in plasma [HCO3−] result in proportional changes in intracellular [HCO3−] during stress conditions (Bettice et al., 1984; Hōbe et al., 1984; Strobel et al., 2012; Wood et al., 1990).
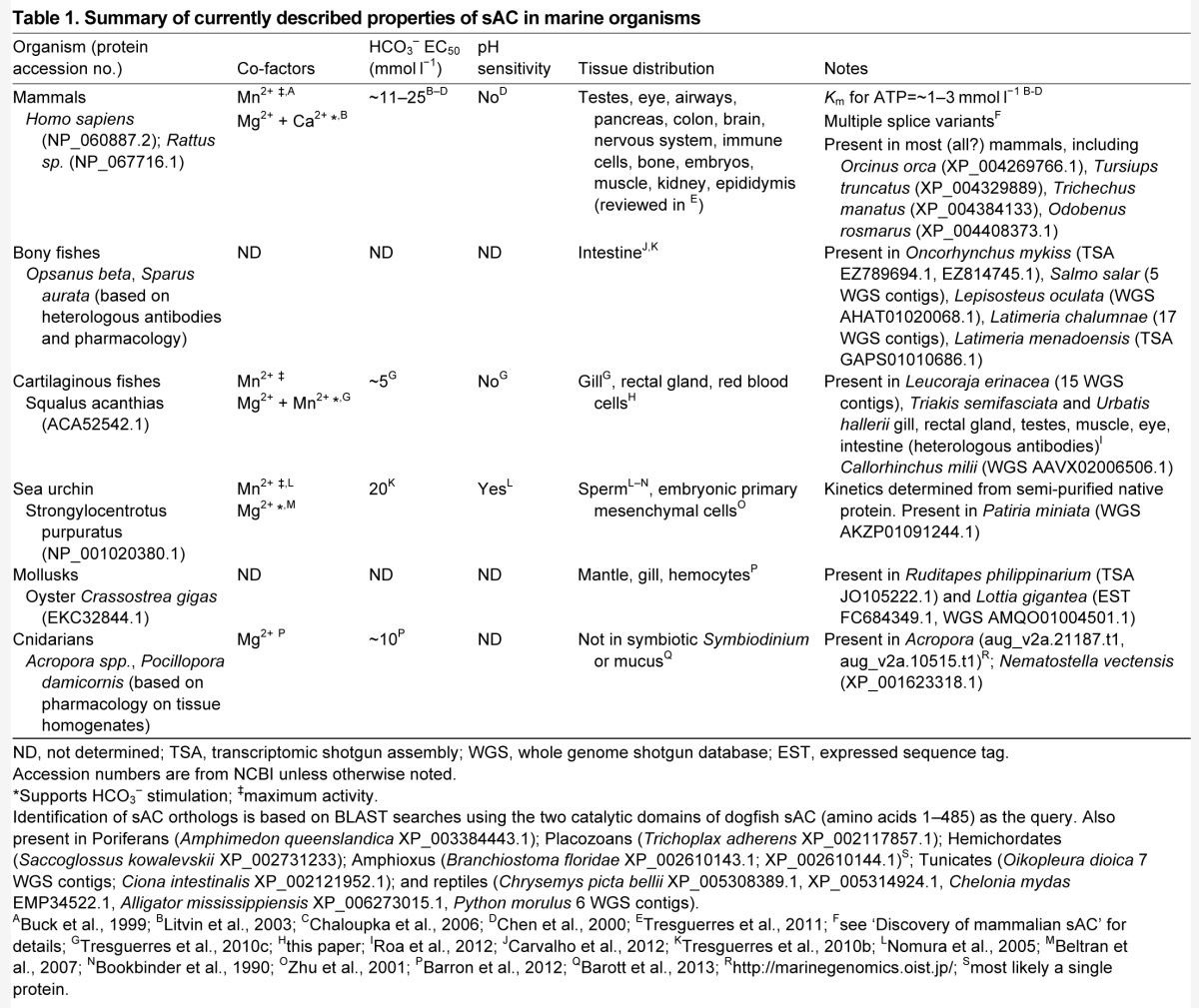
Table 1.
Summary of currently described properties of sAC in marine organisms
There are at least two possible reasons for the small discrepancy between sAC EC50 for HCO3− and reported intracellular [HCO3−]: (1) EC50 is estimated using purified sAC protein in vitro, which does not necessarily match the conditions inside cells (for example, co-factors); (2) the reported intracellular [HCO3−] values represent an average within whole cells. It is thus possible that focal points inside cells have higher local [HCO3−] due to the proximity to mitochondria or to the action of CAs (e.g. Tresguerres et al., 2006a). In support of this possibility, sAC is more often present in cells that also express abundant intracellular CA (reviewed in Tresguerres et al., 2010a; Tresguerres et al., 2011).
Physiological roles of mammalian sAC
In the clear cells of the epididymis, sAC is activated by lumen alkalinization and triggers compensatory H+ secretion via apical V-type H+-ATPases (Pastor-Soler et al., 2003; Pastor-Soler et al., 2008). Similar roles in A/B sensing and homeostasis have been proposed for sAC in kidney intercalated cells (Gong et al., 2010; Paunescu et al., 2008). sAC also regulates other physiological functions in response to A/B status in diverse mammalian systems such as sperm, eye, airways, pancreas, colon, brain, nervous system, immune cells, bone, embryos and several cell lines. These functions include epithelial ion and fluid transport, sperm flagellar movement, gene expression, development and cell differentiation, ciliary beating in airways and oxidative phosphorylation in mitochondria (reviewed in Tresguerres et al., 2010a; Tresguerres et al., 2011). At the time of writing, sAC has additionally been shown to regulate the metabolic coupling between neurons and astrocytes (Choi et al., 2012), intraocular pressure (Lee et al., 2011), survival of retinal ganglion cells and axon growth (Corredor et al., 2012), aromatase gene expression and estradiol production (Chen et al., 2012a), pathologic activation of proteases in pancreatic acinar cells (Kolodecik et al., 2012) and endocytosis of Na+/K+-ATPase in cultured cells exposed to hypercapnia (Lecuona et al., 2013). Moreover, nuclear sAC has been suggested to determine the aggressiveness of certain skin and prostate cancers (Flacke et al., 2013; Magro et al., 2012a; Magro et al., 2012b; Zippin et al., 2010). In the heart, sAC controls mitochondria-dependent apoptosis in both coronary endothelial cells (Kumar et al., 2009) and cardiomyocytes (Appukuttan et al., 2012) (reviewed in Chen et al., 2012b). Lastly, certain sAC splice variants present in mammalian testis and skeletal muscle have been identified to contain a heme-binding domain (Middelhaufe et al., 2012) of still undefined function.
While many of these functions likely also apply to aquatic organisms (especially vertebrates, e.g. boney and cartilaginous fishes), studies on sAC in aquatic organisms have started only recently, so information is still scarce. The concentration of HCO3− in the internal fluids of aquatic organisms is generally much more variable compared with that of mammals, in terms of both stability and the magnitude of the changes. This has two important implications: (1) the physiological roles of sAC may be more critical in aquatic organisms than in mammals, and (2) studying the physiological roles of sAC may be easier in aquatic organisms than in mammals because it is possible to experimentally induce more pronounced A/B stress, which may result in responses that are easier to measure.
sAC in non-mammalian organisms
Mammalian sAC was discovered in the late 1990s (Buck et al., 1999), a few years before the first drafts of the mouse and rat genomes became available. Bioinformatics analyses in the early 2000s revealed sAC orthologs in the slime mold Dictyostelium and in cyanobacteria, but not in the fully sequenced genomes of Drosophila melanogaster, Caenorhabditis elegans, Arabidopsis thaliana or Saccharomyces cerevisiae. This led to the hypothesis that the sAC gene had been lost in multiple lineages (Roelofs and Van Haastert, 2002). The lack of sAC-related genes in drafts of the zebrafish genome that came out several years later further substantiated the belief that sAC was a mammal-specific A/B sensor, and many even believed it to be a mammalian sperm-specific sensor (see Tresguerres et al., 2011).
This view, however, was upended with the discovery of sAC in sperm from the purple sea urchin Strongylocentrotus purpuratus (Nomura et al., 2005), work that relied on traditional protein purification techniques instead of bioinformatics. A few years later, a sAC ortholog was identified from an EST library from the dogfish shark Squalus acanthias, which led to the first characterization of a sAC enzyme from a non-mammalian vertebrate (Tresguerres et al., 2010c). Furthermore, sAC was found to be present and functional in shark gills, demonstrating that sAC is not unique to mammals among animals, nor it is exclusive to sperm.
With the explosion in genomic and transcriptomic information, sAC-related genes can now be found in virtually every animal phylum (Table 1), as well as in other organisms such as diatoms (Matsuda et al., 2011). Although some evidence supports the presence of sAC in plants (Lomovatskaya et al., 2008; Romanenko et al., 2008), even the mere presence of cAMP in plants is controversial and so this topic is not discussed further in this review. BLAST searches of genomes from fruit fly and zebrafish still do not reveal any sAC orthologs; however, sAC genes are found in other insects (e.g. moth, bee, wasp, mosquito) and other fishes (e.g. trout, salmon, gar, coelacanth, chimera, shark, skate). This phylogenetic distribution suggests that either fly and zebrafish sense HCO3− using fundamentally distinct mechanisms, or that the absence of sAC in these species is due to a glitch of sequencing and annotation techniques.
To date, the only published biochemical reports on non-mammalian sACs are those from the dogfish shark (purified recombinant protein) (Tresguerres et al., 2010c) and from purple sea urchin (semi-purified native protein) (Beltrán et al., 2007; Nomura et al., 2005; Nomura and Vacquier, 2006). Like mammalian sAC, dogfish and sea urchin sAC are stimulated by HCO3−, potently stimulated by millimolar concentrations of Mn2+, and inhibited by micromolar concentrations of KH7 and derivatives of catechol estrogens. However, unlike mammalian sAC, dogfish and sea urchin sAC are inhibited by millimolar Ca2+ concentrations. More details about dogfish and sea urchin sAC are described below, as well as reports from marine fish and some information about sAC from other fish, mollusks and coral.
sAC in sharks and boney fishes
The first two reports of sAC in fish described it as an essential sensor for blood A/B homeostasis in dogfish shark (Squalus acanthias) gills (Tresguerres et al., 2010c), and as regulator of NaCl and water absorption in the intestinal epithelium of toadfish (Opsanus beta) (Tresguerres et al., 2010b). This latter role was recently confirmed by another research group working with sea bream (Sparus aurata) (Carvalho et al., 2012).
Dogfish sAC is ~110 kDa (Tresguerres et al., 2010c), but it is still unclear whether sAC splice variants or isoforms exist in dogfish. Studies using purified recombinant protein comprising the two catalytic domains established that dogfish sAC is stimulated by HCO3− to produce cAMP with an EC50 of ~5 mmol l−1. Like purified mammalian sAC, dogfish sAC is insensitive to physiologically relevant changes in pH (Tresguerres et al., 2010c). Purified dogfish sAC is maximally stimulated by millimolar concentrations of Mn2+, while HCO3− stimulation is supported by a combination of Mg2+ and Mn2+, but neither by Mg2+ alone nor a combination of Mg2+ and Ca2+ (unlike mammalian sAC). The identity of the actual catalytic metals and their concentrations in vivo remain to be determined.
The steepest part of the sAC dose–response curve for HCO3− is between 2 and 15 mmol l−1 (Tresguerres et al., 2010c), which matches the normal [HCO3−] in dogfish plasma as well as changes in shark plasma [HCO3−] induced by feeding (metabolic alkalosis) (Wood et al., 2005), by exhaustive exercise (metabolic acidosis) (Richards et al., 2003), in response to hypercapnia (compensatory metabolic alkalosis) (Heisler, 1988), and by temperature changes (increased [HCO3−] at higher temperature, and vice versa) (Heisler, 1988). Therefore, sAC is poised to sense the A/B status and trigger physiological responses during these physiologically relevant situations.
The role of sAC as a sensor of A/B status in shark gills has been confirmed through a series of experiments that induced metabolic alkalosis via continuous intravenous infusion of NaHCO3− (Tresguerres et al., 2005; Tresguerres et al., 2006b; Tresguerres et al., 2010c; Tresguerres et al., 2007). The sAC-dependent mechanism for sensing and counteracting blood alkalosis is summarized in Fig. 3, and is as follows: excess HCO3− in plasma is dehydrated into CO2 by extracellular CA IV located in gill pillar cells (Gilmour et al., 2007). CO2 then enters the gill epithelial cells, including the subpopulation that expresses V-type H+-ATPase, intracellular CA and sAC (Tresguerres et al., 2005; Tresguerres et al., 2010c; Tresguerres et al., 2007). CO2 is hydrated back into H+ and HCO3−, which stimulates sAC, which in turns triggers the microtubule-dependent translocation of V-type H+-ATPases from cytoplasmic vesicles to the basolateral membrane (Tresguerres et al., 2005; Tresguerres et al., 2006b; Tresguerres et al., 2010c; Tresguerres et al., 2007). This mechanism most likely occurs via PKA-dependent phosphorylation of microtubule motor proteins; however, this has not been experimentally confirmed. The now basolateral V-type H+-ATPases pump intracellular H+ into the blood, thus counteracting the alkalosis. At the same time, the excess HCO3− is secreted across the apical membrane into seawater, most likely via Pendrin-like anion transporters (Piermarini et al., 2002). This process seems to work independently of any hormonal cues, and in theory is self-regulatory: once normal blood [HCO3−] has been restored, sAC is no longer stimulated, permitting the retrieval of V-type H+-ATPases away from the basolateral membrane.
Fig. 3.
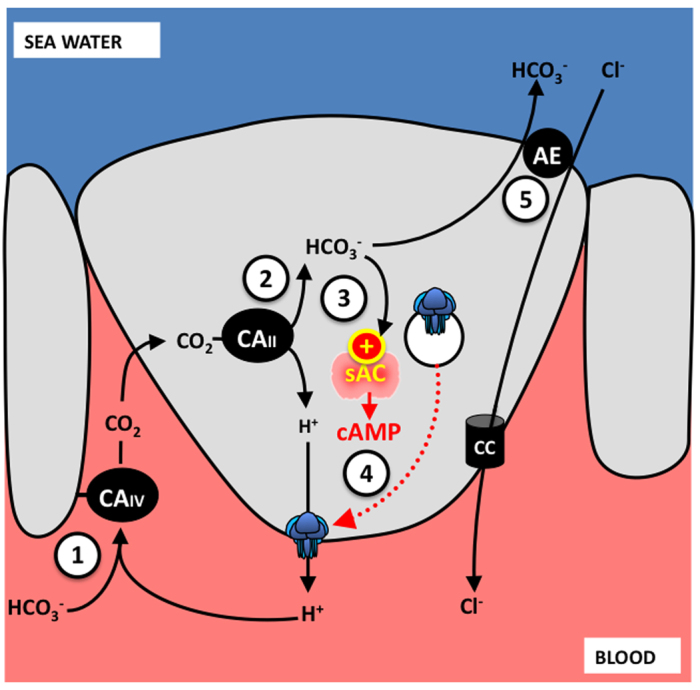
sAC-dependent sensing and compensation of blood alkalosis. During increased blood HCO3− and pH, (1) HCO3− in blood is dehydrated into CO2 by extracellular carbonic anhydrase (CAIV). (2) CO2 enters the V-type H+-ATPase-rich cells, where it is hydrated back into H+ and HCO3− by intracellular CA II (CAII). (3) The elevated intracellular HCO3− stimulates sAC to generate cAMP, which triggers the microtubule-dependent translocation of V-type H+-ATPase (blue icon) containing cytoplasmic vesicles to the basolateral membrane. Basolateral V-type H+-ATPase reabsorbs H+ into the blood to counteract the original alkalosis. (4) The excess HCO3− is secreted to seawater in exchange for chloride via a Pendrin-like anion exchanger (AE). Chloride is probably absorbed into the blood across a basolateral channel (CC).
In the post-feeding period, dogfish gastric cells secrete H+ into the stomach (to aid with food digestion), which is matched by equimolar HCO3− absorption into the blood, resulting in pronounced alkalosis (Wood et al., 2005; Wood et al., 2009). The blood alkalosis is compensated for by an upregulation of branchial HCO3− secretion and H+ absorption across the gills (Wood et al., 2007a), which correlates with significant V-type H+-ATPase translocation in the gill cells (Tresguerres et al., 2007). Altogether, the evidence points to sAC being responsible for sensing post-feeding alkalosis and triggering the A/B compensatory mechanisms. Moreover, sAC is present in most gill epithelial cells and not just in V-type H+-ATPase-rich cells (Tresguerres et al., 2010c), suggesting additional regulatory roles in acid-secreting Na+/K+-ATPase-rich cells, pavement cells and pillar cells. Furthermore, as sAC is also present in multiple other shark tissues (Fig. 4) (see also Roa et al., 2012), it is a good candidate to mediate some of the profound metabolic changes that take place throughout the shark's body during the alkalotic post-feeding period (Walsh et al., 2006; Wood et al., 2008), including the stimulation of NaCl secretion across the rectal gland (Shuttleworth et al., 2006; Wood et al., 2007b).
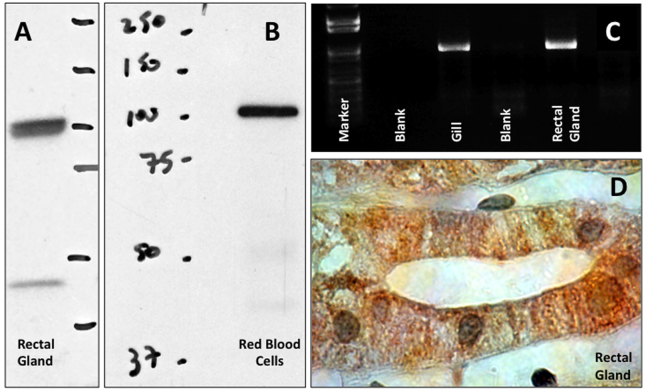
Fig. 4.
Expression of sAC in dogfish shark tissues. (A,B) sAC protein detected by western immunoblot in rectal gland and red blood cells; the band matches the predicted ~110 kDa sAC protein. (C) sAC mRNA detected by RT-PCR in gill (positive control) and rectal gland. (D) Immunolocalization of sAC (brown) in rectal gland cells, showing cytoplasmic and potentially nuclear localization (nuclei stained in green). Methods followed those described previously (Tresguerres et al., 2010c).
The published evidence for sAC presence and activity in teleost fishes is so far limited to immunological detection in toadfish intestine using heterologous antibodies (Tresguerres et al., 2010b) and inhibition of NaCl and water absorption in toadfish and sea bream intestine using sAC antagonists (Carvalho et al., 2012; Tresguerres et al., 2010b). The proposed physiological role of sAC in the teleost intestine is to coordinate intestinal carbonate precipitation with NaCl and water absorption by modulating the activities of Na+/K+-ATPase, Na+/K+/Cl− cotransporter (NKCC), V-type H+-ATPase and anion exchangers. These processes are essential for osmoregulation of marine teleost fishes (Grosell, 2011; Walsh et al., 1991; Wilson et al., 2009; Wilson et al., 2002).
sAC genes are present in the sequenced genomes or whole genome shotgun (WGS) assemblies of salmon (Salmo salar), chimera (Callorhinchus milii), little skate (Leucoraja erinacea), coelacanth (Latimeria chalumnae) and spotted gar (Lepiosteus oculatus), and in transcriptome shotgun assemblies (TSA) of rainbow trout (Oncorhynchus mykiss) (Table 1). Further evidence for the ubiquity of sAC in elasmobranchs includes the detection of sAC protein in gill, rectal gland, white muscle, intestine and eye of leopard shark (Triakis semifasciata) and round ray (Urobatis hallerii) by western blotting using antibodies against dogfish sAC (Roa et al., 2012). We have also detected sAC by RT-PCR in the testis of dogfish shark and rainbow trout (M.T. and J.N.R., unpublished observations), suggesting sAC is important for sperm biology in fishes as it is in mammals. Intriguingly, the initiation of sperm motility depends on cAMP in several fish species (Morisawa and Ishida, 1987; Zilli et al., 2008), and at least in salmonids it is stimulated by HCO3− (Morisawa and Morisawa, 1988).
sAC in sea urchin
Among marine invertebrates, sAC has so far only been characterized in the purple sea urchin, S. purpuratus. High levels of AC activity in urchin spermatozoa (Garbers and Kopf, 1980) led to the identification and purification of an AC enzyme by immunoaffinity chromatography (Bookbinder et al., 1990). The 190 kDa protein, which was the first AC to be identified from animal spermatozoa, was not stimulated by forskolin or by G-proteins, and showed high activity in response to Mn2+. However, as mammalian sAC would not be discovered until almost a decade later, the sea urchin AC was not identified as a sea urchin sAC and its sensitivity to HCO3− was not tested at the time. Eventually, analysis of the sea urchin AC sequence indicated it was a homolog of mammalian sAC (Nomura et al., 2005). Compared with mammalian sAC, sea urchin sAC contains multiple amino acid insertions (16–74 amino acids in length) with several potential phosphorylation sites (Nomura et al., 2005).
In the presence of Mg2+, partially purified sea urchin sAC is stimulated by HCO3− with an EC50 of ~20 mmol l−1, and it reaches its maximum at 50 mmol l−1 (Beltrán et al., 2007). However, these values seem high compared with [HCO3−] in aquatic invertebrates, raising doubts about the physiological significance of sea urchin sAC. Unlike the pH-insensitive mammalian (Chen et al., 2000) and dogfish sAC (Tresguerres et al., 2010c), sea urchin sAC displays a steep sensitivity to pH between 7.0 and 7.5 (Beltrán et al., 2007). However, the high EC50 value and pH sensitivity may reflect artifacts of working with semi-purified sea urchin sAC preparations or of not utilizing physiologically relevant assay conditions (e.g. catalytic metals, salts, etc.).
Initial experiments found sea urchin sAC to be concentrated in the proximal half of the sperm flagellum near the mitochondrial midpiece (Bookbinder et al., 1990); this suggested sea urchin sAC triggers the initiation of sperm motility, which depends on cAMP-dependent phosphorylation of flagella-associated proteins (Bracho et al., 1998). Sea urchin sAC is tightly complexed with several proteins of the plasma membrane and axoneme, including dynein heavy chains 7 and 9, sperm-specific Na+/H+ exchanger (NHE), cyclic nucleotide-gated ion channel, sperm-specific creatine kinase, membrane-bound guanylyl cyclase, cGMP-specific phosphodiesterase 5A, the receptor for the egg peptide speract, and α- and β-tubulins (Nomura and Vacquier, 2006). The authors proposed that this complex modulates sperm motility in response to speract and pH changes. Further research using confocal microscopy revealed sea urchin sAC to also be present in the head and acrosomal area (Beltrán et al., 2007). Indeed, sea urchin sAC is important, but not essential, for the sperm acrosome reaction (Beltrán et al., 2007). Searches of EST and TSA databases reveal sea urchin sAC mRNA is also present in embryonic primary mesenchyme cells (Zhu et al., 2001), suggesting various physiological roles in addition to sperm motility and acrosome reaction.
sAC in mollusks
sAC hits are present in the genome, TSAs and ESTs of the Pacific oyster (Crassostrea gigas). Moreover, we have detected sAC mRNA by RT-PCR in mantle, gill and hemocytes, and have observed KH7-sensitive cAMP production in mantle tissue homogenates (Barron et al., 2012). However, the physiological roles of oyster sAC remain unknown. In addition to oysters, other mollusks with readily identifiable sAC orthologs include the Venus clam (Ruditapes philippinarium) and the owl limpet (Lottia gigantea) (Table 1). The sAC amino acid sequences from these three mollusk species have a serine in the position of the residue suggested to be involved in HCO3− stimulation, which is different from the threonine in sACs from all other animals examined so far, but matches the motifs of HCO3−-sensing bacterial sAC-like enzymes (Linder, 2006; Steegborn et al., 2005b). The implications of these differences are unknown.
In bivalves, pathways involving cAMP have been found or implied to play a role in regulating glycogen breakdown, cilia beating and activation, spawning induction, cardiac contraction, reproduction, mantle and siphon movement, adductor muscle relaxation after the ‘catch response’, and stress response (reviewed in Fabbri and Capuzzo, 2010). Research on cAMP in bivalves has relied heavily on the use of the biogenic amines 5-HT, dopamine, noradrenaline and adrenaline, which are extracellular ligands for GPCRs (Fabbri and Capuzzo, 2010). As a result, cAMP production in mollusks has been exclusively attributed to the activation of tmACs. However, the literature on cAMP and its physiological responses predates the discovery of sAC in mammals. For example, cAMP production in some bivalve species such as Mytilus galloprovincialis and Tapes philippinarum, as well as the sea hare, Aplysia californica, are unresponsive to, or only slightly stimulated by, forskolin (Mancebo et al., 1991; Valbonesi et al., 2004; Weiss and Drummond, 1985). Although the results of these studies have been explained by attributing activity to orthologs related to the forskolin-insensitive tmAC IX isoform, orthologs of sAC could supply an alternative, equally plausible, explanation.
Mollusks typically experience many situations associated with increases in the [HCO3−] in their internal fluids, which could lead to stimulation of sAC. For example, [HCO3−] in the hemolymph of the freshwater clam Anodonta cygnea rises from 5 to 12 mmol l−1 on a seasonal basis (Lopes-Lima et al., 2009), and doubles from 3 to 6.5 mmol l−1 in zebra mussel (Dreissena polymorpha) upon transfer from freshwater to brackish water (Byrne and Dietz, 2006). The cuttlefish (Sepia officinalis) can accumulate HCO3− in plasma from ~2 mmol l−1 to over 10 mmol l−1 to compensate for environmental hypercapnia (Gutowska et al., 2010); bivalves may also experience significant elevations in hemolymph [HCO3−] in response to ocean acidification (Lannig et al., 2010; Michaelidis et al., 2005b) and exposure to air (Michaelidis et al., 2005a). Although HCO3− accumulation in bivalves in response to hypercapnia is typically much more modest compared with that in active cephalopods, it can still double or in some cases quadruple. Exploring whether sAC plays any physiological role in these situations must start by establishing the sensitivity of mollusk sAC to HCO3−, pH and inhibitors, and by identifying the tissues where it is expressed.
sAC in corals
We have recently identified two isoforms of sAC in the genome of the coral Acropora digitifera (Barott et al., 2013), and sAC is present in at least two other coral species (A. yongei and Stylophora pistillata; M.T., K.L.B. and M.E.B., unpublished observations), as well as in the genome of the starlet sea anemone Nematostella vectensis. Total AC activity in coral tissue homogenates devoid of symbiotic zooxanthellae is among the highest observed from any organism. Indicative of sAC activity, cAMP production in homogenates is significantly stimulated by HCO3− with an apparent EC50 of ~10 mmol l−1, and the HCO3−-stimulated activity is inhibited by 50 μmol l−1 KH7 (Barott et al., 2013). The HCO3− EC50 and the KH7 dose–response of coral sAC need to be determined from kinetic studies using purified protein.
The biological role of sAC in corals remains an outstanding question, but multiple roles are possible, including the regulation of A/B homeostasis, photosynthesis and calcification. Because [HCO3−] in coral tissues ranges from ~4 mmol l−1 in the dark to over 100 mmol l−1 in the light (Furla et al., 2000), coral sAC is likely to be sensitive to physiologically relevant variations in [HCO3−] and most active in the light. Indeed, endogenous cAMP levels in corals in vivo are highest in the light (Barott et al., 2013), but whether this is due to sAC activity remains to be determined.
sAC in other aquatic animals
Table 1 contains a non-exhaustive list of sAC orthologs from other aquatic animals, including those from placozoa (Trichoplax adherens), sponge (Amphimedon queenslandica), acorn worm (Saccoglossus kowalevskii), amphioxus (Branchiostoma floridae) and sea squirt (Ciona intestinalis). Partial sAC sequences are also identifiable in WGS databases of appendicularia (Oikopleura dioica), green sea turtle (Chelonia mydas), painted turtle (Chrysemys picta) and American alligator (Alligator mississippiensis), as well as in snake (Python morulus) [which is not an aquatic animal but, like most reptiles and amphibians, undergoes a very pronounced post-feeding blood alkalosis (reviewed in Wang et al., 2001)]. Available sequences for sAC orthologs from marine mammals include those from orca whale (Orcinus orca), bottlenose dolphin (Tursiups truncatus), manatee (Trichechus manatus) and walrus (Odobenus rosmarus) (Table 1).
Need for complementary acid sensor(s)
While the sensory role of sAC during alkalosis is straightforward, its potential function during other A/B stress conditions is more complex. For example, both respiratory acidosis and metabolic alkalosis are characterized by elevated PCO2 and [HCO3−] and could result in sAC stimulation. However, the homeostatic A/B response to acidosis requires secretion of H+ and absorption of HCO3−, which is exactly the opposite of the response to alkalosis. To correct for A/B disturbances, cells and organisms must be able to tell the difference between the different types of stress. Both acid- and bicarbonate-secreting cells of shark gills and mammalian kidney express sAC, but how does sAC ‘know’ which response it should initiate? One potential mechanism involves the integration of inputs from HCO3−-responsive sAC and one or several H+ sensors (see Brown and Wagner, 2012; Tresguerres et al., 2010a), possibly in combination with differential cell membrane permeability to CO2 or HCO3−.
Conclusions
Because of the ubiquity of both HCO3− and cAMP in biological systems, sAC is poised to play multiple important physiological roles as a sensor of A/B stress. The existence of multiple sources of cAMP in different intracellular locations warrants a revisiting of the existing literature, as many of the functions currently ascribed to tmACs may actually depend on sAC.
Acknowledgements
We appreciate the useful comments by Dr Vic Vacquier (SIO-UCSD), Dr Jochen Buck and Dr Lonny R. Levin (Weill Cornell Medical College).
Footnotes
Competing interests
The authors declare no competing financial interests.
Funding
This work was supported by SIO funds to M.T., National Science Foundation grant EF-1220641 to M.T., National Science Foundation OCE postdoctoral fellowship 1226396 to K.L.B., Scripps Institution of Oceanography Regents and National Science Foundation graduate fellowships to M.E.B., and San Diego Fellowship to J.N.R., who is also partially supported by a National Institutes of Health Training Program in Marine Biotechnology grant. Deposited in PMC for release after 12 months.
References
- Acin-Perez R., Salazar E., Kamenetsky M., Buck J., Levin L. R., Manfredi G. (2009). Cyclic AMP produced inside mitochondria regulates oxidative phosphorylation. Cell Metab. 9, 265-276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appukuttan A., Kasseckert S. A., Micoogullari M., Flacke J.-P., Kumar S., Woste A., Abdallah Y., Pott L., Reusch H. P., Ladilov Y. (2012). Type 10 adenylyl cyclase mediates mitochondrial Bax translocation and apoptosis of adult rat cardiomyocytes under simulated ischaemia/reperfusion. Cardiovasc. Res. 93, 340-349 [DOI] [PubMed] [Google Scholar]
- Barott K. L., Helman Y., Haramaty L., Barron M. E., Hess K. C., Buck J., Levin L. R., Tresguerres M. (2013). High adenylyl cyclase activity and in vivo cAMP fluctuations in corals suggest central physiological role. Sci Rep 3, 1379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barron M. E., Roa J. N. B., Tresguerres M. (2012). Pacific oyster mantle, gill and hemocytes express the bicarbonate-sensing enzyme soluble adenylyl cyclase. FASEB J. 26, 1070.2 [Google Scholar]
- Beltrán C., Vacquier V. D., Moy G., Chen Y., Buck J., Levin L. R., Darszon A. (2007). Particulate and soluble adenylyl cyclases participate in the sperm acrosome reaction. Biochem. Biophys. Res. Commun. 358, 1128-1135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bettice J. A., Owens D., Riley S. (1984). The effects of hypocapnia on intracellular pH and bicarbonate. Respir. Physiol. 55, 121-130 [DOI] [PubMed] [Google Scholar]
- Bookbinder L. H., Moy G. W., Vacquier V. D. (1990). Identification of sea urchin sperm adenylate cyclase. J. Cell Biol. 111, 1859-1866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracho G. E., Fritch J. J., Tash J. S. (1998). Identification of flagellar proteins that initiate the activation of sperm motility in vivo. Biochem. Biophys. Res. Commun. 242, 231-237 [DOI] [PubMed] [Google Scholar]
- Braun T. (1974). Evidence for multiple, cell specific, distinctive adenylate cyclase systems in rat testis. Curr. Top. Mol. Endocrinol. 1, 243-264 [DOI] [PubMed] [Google Scholar]
- Braun T. (1975). The effect of divalent cations on bovine spermatozoal adenylate cyclase activity. J. Cyclic Nucleotide Res. 1, 271-281 [PubMed] [Google Scholar]
- Braun T. (1990). Inhibition of the soluble form of testis adenylate cyclase by catechol estrogens and other catechols. Proc. Soc. Exp. Biol. Med. 194, 58-63 [DOI] [PubMed] [Google Scholar]
- Braun T. (1991). Purification of soluble form of adenylyl cyclase from testes. Methods Enzymol. 195, 130-136 [DOI] [PubMed] [Google Scholar]
- Braun T., Dods R. F. (1975). Development of a Mn2+-sensitive, ‘soluble’ adenylate cyclase in rat testis. Proc. Natl. Acad. Sci. USA 72, 1097-1101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown D., Wagner C. A. (2012). Molecular mechanisms of acid-base sensing by the kidney. J. Am. Soc. Nephrol. 23, 774-780 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck J., Sinclair M. L., Schapal L., Cann M. J., Levin L. R. (1999). Cytosolic adenylyl cyclase defines a unique signaling molecule in mammals. Proc. Natl. Acad. Sci. USA 96, 79-84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne R. A., Dietz T. H. (2006). Ionic and acid-base consequences of exposure to increased salinity in the zebra mussel, Dreissena polymorpha. Biol. Bull. 211, 66-75 [DOI] [PubMed] [Google Scholar]
- Carvalho E. S., Gregório S. F., Power D. M., Canário A. V., Fuentes J. (2012). Water absorption and bicarbonate secretion in the intestine of the sea bream are regulated by transmembrane and soluble adenylyl cyclase stimulation. J. Comp. Physiol. B 182, 1069-1080 [DOI] [PubMed] [Google Scholar]
- Chaloupka J. A., Bullock S. A., Iourgenko V., Levin L. R., Buck J. (2006). Autoinhibitory regulation of soluble adenylyl cyclase. Mol. Reprod. Dev. 73, 361-368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Cann M. J., Litvin T. N., Iourgenko V., Sinclair M. L., Levin L. R., Buck J. (2000). Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science 289, 625-628 [DOI] [PubMed] [Google Scholar]
- Chen H., Guo J. H., Lu Y. C., Ding G. L., Yu M. K., Tsang L. L., Fok K. L., Liu X. M., Zhang X. H., Chung Y. W., et al. (2012a). Impaired CFTR-dependent amplification of FSH-stimulated estrogen production in cystic fibrosis and PCOS. J. Clin. Endocrinol. Metab. 97, 923-932 [DOI] [PubMed] [Google Scholar]
- Chen J., Levin L. R., Buck J. (2012b). Role of soluble adenylyl cyclase in the heart. Am. J. Physiol. 302, H538-H543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi H. B., Gordon G. R. J., Zhou N., Tai C., Rungta R. L., Martinez J., Milner T. A., Ryu J. K., McLarnon J. G., Tresguerres M., et al. (2012). Metabolic communication between astrocytes and neurons via bicarbonate-responsive soluble adenylyl cyclase. Neuron 75, 1094-1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper D. M. (2003). Regulation and organization of adenylyl cyclases and cAMP. Biochem. J. 375, 517-529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corredor R. G., Trakhtenberg E. F., Pita-Thomas W., Jin X., Hu Y., Goldberg J. L. (2012). Soluble adenylyl cyclase activity is necessary for retinal ganglion cell survival and axon growth. J. Neurosci. 32, 7734-7744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabbri E., Capuzzo A. (2010). Cyclic AMP signaling in bivalve molluscs: an overview. J. Exp. Zool. A 313, 179-200 [DOI] [PubMed] [Google Scholar]
- Farrell J., Ramos L., Tresguerres M., Kamenetsky M., Levin L. R., Buck J. (2008). Somatic ‘soluble’ adenylyl cyclase isoforms are unaffected in Sacytm1Lex/Sacytm1Lex ‘knockout’ mice. PLoS ONE 3, e3251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flacke J. P., Kempkes H., Appukuttan A., Palisaar R. J., Noldus J., Robinson B. D., Reusch H. P., Zippin J. H., Ladilov Y. (2013). Type 10 soluble adenylyl cyclase is overexpressed in prostate carcinoma and controls proliferation of prostate cancer cells. J. Biol. Chem. 288, 3126-3135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furla P., Galgani I., Durand I., Allemand D. (2000). Sources and mechanisms of inorganic carbon transport for coral calcification and photosynthesis. J. Exp. Biol. 203, 3445-3457 [DOI] [PubMed] [Google Scholar]
- Garbers D. L., Kopf G. S. (1980). The regulation of spermatozoa by calcium cyclic nucleotides. Adv. Cyclic Nucleotide Res. 13, 251-306 [PubMed] [Google Scholar]
- Geng W., Wang Z., Zhang J., Reed B. Y., Pak C. Y., Moe O. W. (2005). Cloning and characterization of the human soluble adenylyl cyclase. Am. J. Physiol. 288, C1305-C1316 [DOI] [PubMed] [Google Scholar]
- Gilmour K. M., Bayaa M., Kenney L., McNeill B., Perry S. F. (2007). Type IV carbonic anhydrase is present in the gills of spiny dogfish (Squalus acanthias). Am. J. Physiol. 292, R556-R567 [DOI] [PubMed] [Google Scholar]
- Gong F., Alzamora R., Smolak C., Li H., Naveed S., Neumann D., Hallows K. R., Pastor-Soler N. M. (2010). Vacuolar H+-ATPase apical accumulation in kidney intercalated cells is regulated by PKA and AMP-activated protein kinase. Am. J. Physiol. 298, F1162-F1169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosell M. (2011). Intestinal anion exchange in marine teleosts is involved in osmoregulation and contributes to the oceanic inorganic carbon cycle. Acta Physiol. (Oxf.) 202, 421-434 [DOI] [PubMed] [Google Scholar]
- Guillou J. L., Nakata H., Cooper D. M. (1999). Inhibition by calcium of mammalian adenylyl cyclases. J. Biol. Chem. 274, 35539-35545 [DOI] [PubMed] [Google Scholar]
- Gutowska M. A., Melzner F., Langenbuch M., Bock C., Claireaux G., Pörtner H. O. (2010). Acid-base regulatory ability of the cephalopod (Sepia officinalis) in response to environmental hypercapnia. J. Comp. Physiol. B 180, 323-335 [DOI] [PubMed] [Google Scholar]
- Hanoune J., Defer N. (2001). Regulation and role of adenylyl cyclase isoforms. Annu. Rev. Pharmacol. Toxicol. 41, 145-174 [DOI] [PubMed] [Google Scholar]
- Heisler N. (1988). Acid-base regulation. In Physiology of Elasmobranch Fishes (ed. Shuttleworth T. J.), pp. 215-252 Berlin: Springer:Verlag; [Google Scholar]
- Hess K. C., Jones B. H., Marquez B., Chen Y., Ord T. S., Kamenetsky M., Miyamoto C., Zippin J. H., Kopf G. S., Suarez S. S., et al. (2005). The ‘soluble’ adenylyl cyclase in sperm mediates multiple signaling events required for fertilization. Dev. Cell 9, 249-259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hōbe H., Wood C. M., Wheatly M. G. (1984). The mechanisms of acid-base and ionoregulation in the freshwater rainbow trout during environmental hyperoxia and subsequent normoxia. I. Extra- and intracellular acid-base status. Respir. Physiol. 55, 139-154 [DOI] [PubMed] [Google Scholar]
- Jaiswal B. S., Conti M. (2001). Identification and functional analysis of splice variants of the germ cell soluble adenylyl cyclase. J. Biol. Chem. 276, 31698-31708 [DOI] [PubMed] [Google Scholar]
- Jaiswal B. S., Conti M. (2003). Calcium regulation of the soluble adenylyl cyclase expressed in mammalian spermatozoa. Proc. Natl. Acad. Sci. USA 100, 10676-10681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamenetsky M., Middelhaufe S., Bank E. M., Levin L. R., Buck J., Steegborn C. (2006). Molecular details of cAMP generation in mammalian cells: a tale of two systems. J. Mol. Biol. 362, 623-639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodecik T. R., Shugrue C. A., Thrower E. C., Levin L. R., Buck J., Gorelick F. S. (2012). Activation of soluble adenylyl cyclase protects against secretagogue stimulated zymogen activation in rat pancreaic acinar cells. PLoS ONE 7, e41320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S., Kostin S., Flacke J. P., Reusch H. P., Ladilov Y. (2009). Soluble adenylyl cyclase controls mitochondria-dependent apoptosis in coronary endothelial cells. J. Biol. Chem. 284, 14760-14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lannig G., Eilers S., Pörtner H. O., Sokolova I. M., Bock C. (2010). Impact of ocean acidification on energy metabolism of oyster, Crassostrea gigas – changes in metabolic pathways and thermal response. Mar. Drugs 8, 2318-2339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecuona E., Sun H., Chen J., Trejo H. E., Baker M. A., Sznajder J. I. (2013). PKA Iα regulates Na,K-ATPase endocytosis in alveolar epithelial cells exposed to high CO2 levels. Am. J. Respir. Cell Mol. Biol. 48, 626-634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. S., Tresguerres M., Hess K., Marmorstein L. Y., Levin L. R., Buck J., Marmorstein A. D. (2011). Regulation of anterior chamber drainage by bicarbonate-sensitive soluble adenylyl cyclase in the ciliary body. J. Biol. Chem. 286, 41353-41358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J. U. (2006). Class III adenylyl cyclases: molecular mechanisms of catalysis and regulation. Cell. Mol. Life Sci. 63, 1736-1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linder J. U., Schultz J. E. (2008). Versatility of signal transduction encoded in dimeric adenylyl cyclases. Curr. Opin. Struct. Biol. 18, 667-672 [DOI] [PubMed] [Google Scholar]
- Litvin T. N., Kamenetsky M., Zarifyan A., Buck J., Levin L. R. (2003). Kinetic properties of ‘soluble’ adenylyl cyclase. Synergism between calcium and bicarbonate. J. Biol. Chem. 278, 15922-15926 [DOI] [PubMed] [Google Scholar]
- Lodish H. F. (1999). Molecular Cell Biology. New York, NY: Scientific American Books; [Google Scholar]
- Lomovatskaya L. A., Romanenko A. S., Filinova N. V., Salyaev R. K. (2008). Detection of soluble adenylyl cyclase isoforms in plants. Dokl. Biochem. Biophys. 420, 124-126 [DOI] [PubMed] [Google Scholar]
- Lopes-Lima M., Lopes A., Casaca P., Nogueira I., Checa A., Machado J. (2009). Seasonal variations of pH, pCO2, pO2, HCO3− and Ca2+ in the haemolymph: implications on the calcification physiology in Anodonta cygnea. J. Comp. Physiol. B 179, 279-286 [DOI] [PubMed] [Google Scholar]
- Magro C. M., Crowson A. N., Desman G., Zippin J. H. (2012a). Soluble adenylyl cyclase antibody profile as a diagnostic adjunct in the assessment of pigmented lesions. Arch. Dermatol. 148, 335-344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magro C. M., Yang S.-E., Zippin J. H., Zembowicz A. (2012b). Expression of soluble adenylyl cyclase in lentigo maligna: use of immunohistochemistry with anti-soluble adenylyl cyclase antibody (R21) in diagnosis of lentigo maligna and assessment of margins. Arch. Pathol. Lab. Med. 136, 1558-1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancebo M., Treviño M., Crespo C., Espinosa J. (1991). Adenylate cyclase activity in Mytilus galloprovincialis Lmk: characteristics of the enzyme from mantle tissue. J. Exp. Zool. A 258, 174-180 [Google Scholar]
- Matsuda Y., Nakajima K., Tachibana M. (2011). Recent progresses on the genetic basis of the regulation of CO2 acquisition systems in response to CO2 concentration. Photosynth. Res. 109, 191-203 [DOI] [PubMed] [Google Scholar]
- Michaelidis B., Haas D., Grieshaber M. K. (2005a). Extracellular and intracellular acid-base status with regard to the energy metabolism in the oyster Crassostrea gigas during exposure to air. Physiol. Biochem. Zool. 78, 373-383 [DOI] [PubMed] [Google Scholar]
- Michaelidis B., Ouzounis C., Paleras A., Portner H. O. (2005b). Effects of long-term moderate hypercapnia on acid-base balance and growth rate in marine mussels Mytilus galloprovincialis. Mar. Ecol. Prog. Ser. 293, 109-118 [Google Scholar]
- Middelhaufe S., Leipelt M., Levin L. R., Buck J., Steegborn C. (2012). Identification of a haem domain in human soluble adenylate cyclase. Biosci. Rep. 32, 491-499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore S. W., Lai Wing Sun K., Xie F., Barker P. A., Conti M., Kennedy T. E. (2008). Soluble adenylyl cyclase is not required for axon guidance to netrin-1. J. Neurosci. 28, 3920-3924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morisawa M., Ishida K. (1987). Short-term changes in levels of cyclic AMP, adenylate cyclase, and phosphodiesterase during the initiation of sperm motility in rainbow trout. J. Exp. Zool. 242, 199-204 [DOI] [PubMed] [Google Scholar]
- Morisawa S., Morisawa M. (1988). Induction of potential for sperm motility by bicarbonate and pH in rainbow trout and chum salmon. J. Exp. Biol. 136, 13-22 [DOI] [PubMed] [Google Scholar]
- Nomura M., Vacquier V. D. (2006). Proteins associated with soluble adenylyl cyclase in sea urchin sperm flagella. Cell Motil. Cytoskeleton 63, 582-590 [DOI] [PubMed] [Google Scholar]
- Nomura M., Beltrán C., Darszon A., Vacquier V. D. (2005). A soluble adenylyl cyclase from sea urchin spermatozoa. Gene 353, 231-238 [DOI] [PubMed] [Google Scholar]
- Pastor-Soler N., Beaulieu V., Litvin T. N., Da Silva N., Chen Y., Brown D., Buck J., Levin L. R., Breton S. (2003). Bicarbonate-regulated adenylyl cyclase (sAC) is a sensor that regulates pH-dependent V-ATPase recycling. J. Biol. Chem. 278, 49523-49529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastor-Soler N. M., Hallows K. R., Smolak C., Gong F., Brown D., Breton S. (2008). Alkaline pH- and cAMP-induced V-ATPase membrane accumulation is mediated by protein kinase A in epididymal clear cells. Am. J. Physiol. 294, C488-C494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paunescu T. G., Da Silva N., Russo L. M., McKee M., Lu H. A., Breton S., Brown D. (2008). Association of soluble adenylyl cyclase with the V-ATPase in renal epithelial cells. Am. J. Physiol. 294, F130-F138 [DOI] [PubMed] [Google Scholar]
- Piermarini P. M., Verlander J. W., Royaux I. E., Evans D. H. (2002). Pendrin immunoreactivity in the gill epithelium of a euryhaline elasmobranch. Am. J. Physiol. 283, R983-R992 [DOI] [PubMed] [Google Scholar]
- Ramos L. S., Zippin J. H., Kamenetsky M., Buck J., Levin L. R. (2008). Glucose and GLP-1 stimulate cAMP production via distinct adenylyl cyclases in INS-1E insulinoma cells. J. Gen. Physiol. 132, 329-338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards J. G., Heigenhauser G. J. F., Wood C. M. (2003). Exercise and recovery metabolism in the Pacific spiny dogfish (Squalus acanthias). J. Comp. Physiol. B 173, 463-474 [DOI] [PubMed] [Google Scholar]
- Roa J. N. B., Barron M. E., Tresguerres M. (2012). Bicarbonate-sensing soluble adenylyl cyclase in elasmobranch and teleost fishes. FASEB J. 26, 442-447 [Google Scholar]
- Roelofs J., Van Haastert P. J. (2002). Deducing the origin of soluble adenylyl cyclase, a gene lost in multiple lineages. Mol. Biol. Evol. 19, 2239-2246 [DOI] [PubMed] [Google Scholar]
- Romanenko A. S., Lomovatskaya L. A., Filinova N. V., Kopytchuk V. N., Salyaev R. K. (2008). Intracellular localization of ‘soluble’ adenylate cyclase in potato plants. Doklady Biological Sciences 422, 328-329 [DOI] [PubMed] [Google Scholar]
- Schmid A., Sutto Z., Schmid N., Novak L., Ivonnet P., Horvath G., Conner G., Fregien N., Salathe M. (2010). Decreased soluble adenylyl cyclase activity in cystic fibrosis is related to defective apical bicarbonate exchange and affects ciliary beat frequency regulation. J. Biol. Chem. 285, 29998-30007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamon K. B., Daly J. W. (1981). Forskolin: a unique diterpene activator of cyclic AMP-generating systems. J. Cyclic Nucleotide Res. 7, 201-224 [PubMed] [Google Scholar]
- Seamon K. B., Padgett W., Daly J. W. (1981). Forskolin: unique diterpene activator of adenylate cyclase in membranes and in intact cells. Proc. Natl. Acad. Sci. USA 78, 3363-3367 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuttleworth T. J., Thompson J., Munger R. S., Wood C. M. (2006). A critical analysis of carbonic anhydrase function, respiratory gas exchange, and the acid-base control of secretion in the rectal gland of Squalus acanthias. J. Exp. Biol. 209, 4701-4716 [DOI] [PubMed] [Google Scholar]
- Steegborn C., Litvin T. N., Hess K. C., Capper A. B., Taussig R., Buck J., Levin L. R., Wu H. (2005a). A novel mechanism for adenylyl cyclase inhibition from the crystal structure of its complex with catechol estrogen. J. Biol. Chem. 280, 31754-31759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steegborn C., Litvin T. N., Levin L. R., Buck J., Wu H. (2005b). Bicarbonate activation of adenylyl cyclase via promotion of catalytic active site closure and metal recruitment. Nat. Struct. Mol. Biol. 12, 32-37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strobel A., Bennecke S., Leo E., Mintenbeck K., Pörtner H. O., Mark F. C. (2012). Metabolic shifts in the Antarctic fish Notothenia rossii in response to rising temperature and PCO2. Front. Zool. 9, 28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M., Katoh F., Fenton H., Jasinska E., Goss G. G. (2005). Regulation of branchial V-H+-ATPase, Na+/K+-ATPase and NHE2 in response to acid and base infusions in the Pacific spiny dogfish (Squalus acanthias). J. Exp. Biol. 208, 345-354 [DOI] [PubMed] [Google Scholar]
- Tresguerres M., Katoh F., Orr E., Parks S. K., Goss G. G. (2006a). Chloride uptake and base secretion in freshwater fish: a transepithelial ion-transport metabolon? Physiol. Biochem. Zool. 79, 981-996 [DOI] [PubMed] [Google Scholar]
- Tresguerres M., Parks S. K., Katoh F., Goss G. G. (2006b). Microtubule-dependent relocation of branchial V-H+-ATPase to the basolateral membrane in the Pacific spiny dogfish (Squalus acanthias): a role in base secretion. J. Exp. Biol. 209, 599-609 [DOI] [PubMed] [Google Scholar]
- Tresguerres M., Parks S. K., Wood C. M., Goss G. G. (2007). V-H+-ATPase translocation during blood alkalosis in dogfish gills: interaction with carbonic anhydrase and involvement in the postfeeding alkaline tide. Am. J. Physiol. 292, R2012-R2019 [DOI] [PubMed] [Google Scholar]
- Tresguerres M., Buck J., Levin L. (2010a). Physiological carbon dioxide, bicarbonate, and pH sensing. Pflügers Arch. 460, 953-964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M., Levin L. R., Buck J., Grosell M. (2010b). Modulation of NaCl absorption by [HCO3−] in the marine teleost intestine is mediated by soluble adenylyl cyclase. Am. J. Physiol. 299, R62-R71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M., Parks S. K., Salazar E., Levin L. R., Goss G. G., Buck J. (2010c). Bicarbonate-sensing soluble adenylyl cyclase is an essential sensor for acid/base homeostasis. Proc. Natl. Acad. Sci. USA 107, 442-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tresguerres M., Levin L. R., Buck J. (2011). Intracellular cAMP signaling by soluble adenylyl cyclase. Kidney Int. 79, 1277-1288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valbonesi P., Caselli F., Capuzzo A., Fabbri E. (2004). Modulation of adenyl cyclase activity in the gills of Tapes philippinarum. J. Exp. Zool. A 301, 952-960 [DOI] [PubMed] [Google Scholar]
- Walsh P. J., Blackwelder P., Gill K. A., Danulat E., Mommsen T. P. (1991). Carbonate deposits in marine fish intestines: a new source of biomineralization. Limnol. Oceanogr. 36, 1227-1232 [Google Scholar]
- Walsh P. J., Kajimura M., Mommsen T. P., Wood C. M. (2006). Metabolic organization and effects of feeding on enzyme activities of the dogfish shark (Squalus acanthias) rectal gland. J. Exp. Biol. 209, 2929-2938 [DOI] [PubMed] [Google Scholar]
- Wang H., Storm D. R. (2003). Calmodulin-regulated adenylyl cyclases: cross-talk and plasticity in the central nervous system. Mol. Pharmacol. 63, 463-468 [DOI] [PubMed] [Google Scholar]
- Wang T., Busk M., Overgaard J. (2001). The respiratory consequences of feeding in amphibians and reptiles. Comp. Biochem. Physiol. 128A, 533-547 [PubMed] [Google Scholar]
- Weiss S., Drummond G. I. (1985). Biochemical properties of adenylate cyclase in the gill of Aplysia californica. Comp. Biochem. Physiol. 80B, 251-255 [Google Scholar]
- Willoughby D., Cooper D. M. (2007). Organization and Ca2+ regulation of adenylyl cyclases in cAMP microdomains. Physiol. Rev. 87, 965-1010 [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Wilson J. M., Grosell M. (2002). Intestinal bicarbonate secretion by marine teleost fish – why and how? Biochim. Biophys. Acta 1566, 182-193 [DOI] [PubMed] [Google Scholar]
- Wilson R. W., Millero F. J., Taylor J. R., Walsh P. J., Christensen V., Jennings S., Grosell M. (2009). Contribution of fish to the marine inorganic carbon cycle. Science 323, 359-362 [DOI] [PubMed] [Google Scholar]
- Wong W., Scott J. D. (2004). AKAP signalling complexes: focal points in space and time. Nat. Rev. Mol. Cell Biol. 5, 959-970 [DOI] [PubMed] [Google Scholar]
- Wood C. M., Turner J. D., Munger R. S., Graham M. S. (1990). Control of ventilation in the hypercapnic skate Raja ocellata: II. Cerebrospinal fluid and intracellular pH in the brain and other tissues. Respir. Physiol. 80, 279-297 [DOI] [PubMed] [Google Scholar]
- Wood C. M., Kajimura M., Mommsen T. P., Walsh P. J. (2005). Alkaline tide and nitrogen conservation after feeding in an elasmobranch (Squalus acanthias). J. Exp. Biol. 208, 2693-2705 [DOI] [PubMed] [Google Scholar]
- Wood C. M., Bucking C., Fitzpatrick J., Nadella S. (2007a). The alkaline tide goes out and the nitrogen stays in after feeding in the dogfish shark, Squalus acanthias. Respir. Physiol. Neurobiol. 159, 163-170 [DOI] [PubMed] [Google Scholar]
- Wood C. M., Munger R. S., Thompson J., Shuttleworth T. J. (2007b). Control of rectal gland secretion by blood acid-base status in the intact dogfish shark (Squalus acanthias). Respir. Physiol. Neurobiol. 156, 220-228 [DOI] [PubMed] [Google Scholar]
- Wood C. M., Kajimura M., Mommsen T. P., Walsh P. J. (2008). Is the alkaline tide a signal to activate metabolic or ionoregulatory enzymes in the dogfish shark (Squalus acanthias)? Physiol. Biochem. Zool. 81, 278-287 [DOI] [PubMed] [Google Scholar]
- Wood C. M., Schultz A. G., Munger R. S., Walsh P. J. (2009). Using omeprazole to link the components of the post-prandial alkaline tide in the spiny dogfish, Squalus acanthias. J. Exp. Biol. 212, 684-692 [DOI] [PubMed] [Google Scholar]
- Zaccolo M. (2009). cAMP signal transduction in the heart: understanding spatial control for the development of novel therapeutic strategies. Br. J. Pharmacol. 158, 50-60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaccolo M., Di Benedetto G., Lissandron V., Mancuso L., Terrin A., Zamparo I. (2006). Restricted diffusion of a freely diffusible second messenger: mechanisms underlying compartmentalized cAMP signalling. Biochem. Soc. Trans. 34, 495-497 [DOI] [PubMed] [Google Scholar]
- Zhu X., Mahairas G., Illies M., Cameron R. A., Davidson E. H., Ettensohn C. A. (2001). A large-scale analysis of mRNAs expressed by primary mesenchyme cells of the sea urchin embryo. Development 128, 2615-2627 [DOI] [PubMed] [Google Scholar]
- Zilli L., Schiavone R., Storelli C., Vilella S. (2008). Molecular mechanisms determining sperm motility initiation in two sparids (Sparus aurata and Lithognathus mormyrus). Biol. Reprod. 79, 356-366 [DOI] [PubMed] [Google Scholar]
- Zippin J. H., Chen Y., Nahirney P., Kamenetsky M., Wuttke M. S., Fischman D. A., Levin L. R., Buck J. (2003). Compartmentalization of bicarbonate-sensitive adenylyl cyclase in distinct signaling microdomains. FASEB J. 17, 82-84 [DOI] [PubMed] [Google Scholar]
- Zippin J. H., Farrell J., Huron D., Kamenetsky M., Hess K. C., Fischman D. A., Levin L. R., Buck J. (2004). Bicarbonate-responsive ‘soluble’ adenylyl cyclase defines a nuclear cAMP microdomain. J. Cell Biol. 164, 527-534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zippin J. H., Chadwick P. A., Levin L. R., Buck J., Magro C. M. (2010). Soluble adenylyl cyclase defines a nuclear cAMP microdomain in keratinocyte hyperproliferative skin diseases. J. Invest. Dermatol. 130, 1279-1287 [DOI] [PMC free article] [PubMed] [Google Scholar]