Abstract
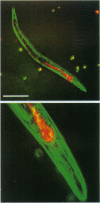
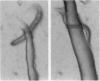
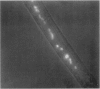
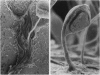
We have found a predator-prey association between the social amoeba Dictyostelium discoideum and the free soil living nematode Caenorhabditis elegans. C. elegans feeds on the amoebae and multiplies indefinitely when amoebae are the sole food source. In an environment created from soil, D. discoideum grows and develops, but not in the presence of C. elegans. During development, C. elegans feeds on amoebae until they aggregate and synthesize an extracellular matrix called the slime sheath. After the sheath forms, the aggregate and slug are protected. Adult nematodes ingest Dictyostelium spores, which pass through the gut of the worm without loss of structure and remain viable. Nematodes kill the amoebae but disperse the spores. The sheath that is constructed when the social amoebae aggregate and the spore coats of the individual cells may protect against this predator. Individual amoebae may also protect themselves by secreting compounds that repel nematodes.
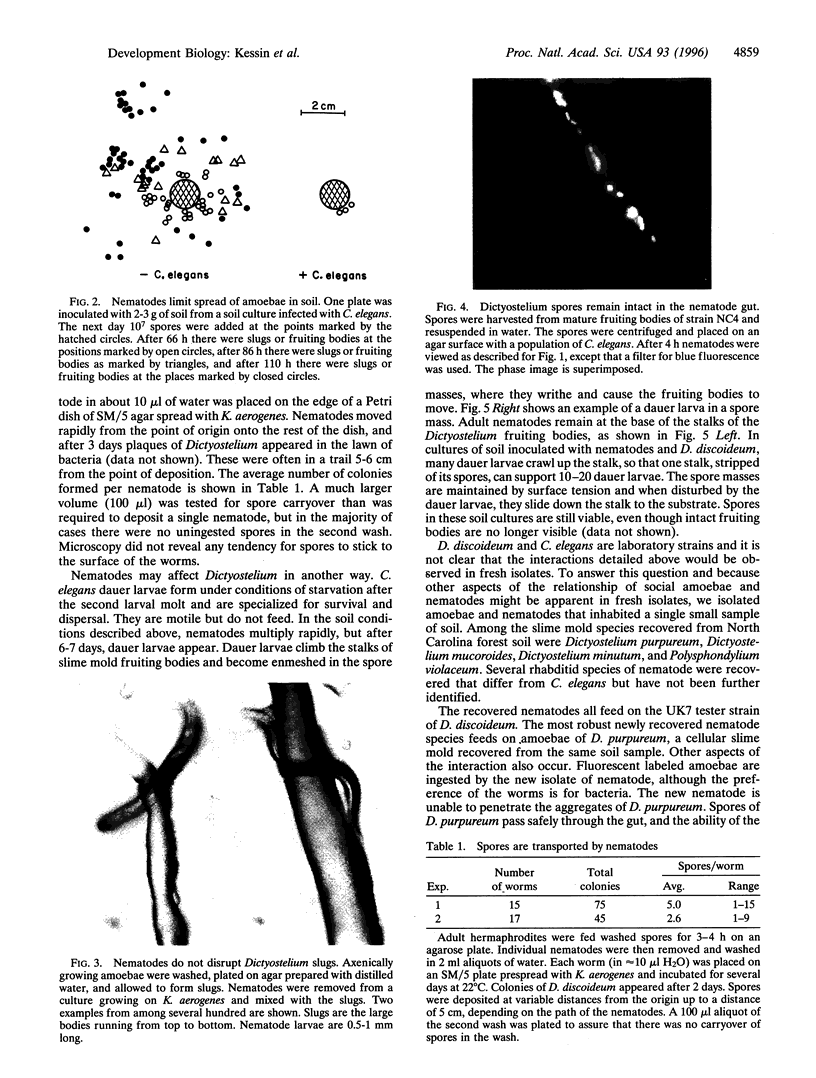
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CAVENDER J. C., RAPER K. B. THE ACRASIEAE IN NATURE. I. ISOLATION. Am J Bot. 1965 Mar;52:294–296. [PubMed] [Google Scholar]
- Ekelund F., Rønn R. Notes on protozoa in agricultural soil with emphasis on heterotrophic flagellates and naked amoebae and their ecology. FEMS Microbiol Rev. 1994 Dec;15(4):321–353. doi: 10.1111/j.1574-6976.1994.tb00144.x. [DOI] [PubMed] [Google Scholar]
- Fosnaugh K., Fuller D., Loomis W. F. Structural roles of the spore coat proteins in Dictyostelium discoideum. Dev Biol. 1994 Dec;166(2):823–825. doi: 10.1006/dbio.1994.1362. [DOI] [PubMed] [Google Scholar]
- Franke J., Kessin R. A defined minimal medium for axenic strains of Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1977 May;74(5):2157–2161. doi: 10.1073/pnas.74.5.2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeze H., Loomis W. F. Isolation and characterization of a component of the surface sheath of Dictyostelium discoideum. J Biol Chem. 1977 Feb 10;252(3):820–824. [PubMed] [Google Scholar]
- Freeze H., Loomis W. F. The role of the fibrillar component of the surface sheath in the morphogenesis of Dictyostelium discoideum. Dev Biol. 1977 Mar;56(1):184–194. doi: 10.1016/0012-1606(77)90161-0. [DOI] [PubMed] [Google Scholar]
- Harrington B. J., Raper K. B. Use of a fluorescent brightener to demonstrate cellulose in the cellular slime molds. Appl Microbiol. 1968 Jan;16(1):106–113. doi: 10.1128/am.16.1.106-113.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomis W. F., Jr Role of the surface sheath in the control of morphogenesis in Dictyostelium discoideum. Nat New Biol. 1972 Nov 1;240(96):6–9. doi: 10.1038/newbio240006a0. [DOI] [PubMed] [Google Scholar]
- Nakao H., Yamamoto A., Takeuchi I., Tasaka M. Dictyostelium prespore-specific gene Dp87 encodes a sorus matrix protein. J Cell Sci. 1994 Mar;107(Pt 3):397–403. doi: 10.1242/jcs.107.3.397. [DOI] [PubMed] [Google Scholar]
- Sussman M. Cultivation and synchronous morphogenesis of Dictyostelium under controlled experimental conditions. Methods Cell Biol. 1987;28:9–29. doi: 10.1016/s0091-679x(08)61635-0. [DOI] [PubMed] [Google Scholar]
- Toama M. A., Raper K. B. Microcysts of the cellular slime mold Polysphondylium pallidum. II. Chemistry of the microcyst walls. J Bacteriol. 1967 Oct;94(4):1150–1153. doi: 10.1128/jb.94.4.1150-1153.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward S. Chemotaxis by the nematode Caenorhabditis elegans: identification of attractants and analysis of the response by use of mutants. Proc Natl Acad Sci U S A. 1973 Mar;70(3):817–821. doi: 10.1073/pnas.70.3.817. [DOI] [PMC free article] [PubMed] [Google Scholar]