Abstract
Background
In the past, successful use of decellularized xenogenic tissue was shown in the pulmonary circulation. This study, however, evaluates a newly developed decellularized equine pericardial patch under high pressure circumstances.
Material/Methods
Seven decellularized equine pericardial scaffolds were implanted into the descending aorta of the juvenile sheep. The implanted patches were oversized to evaluate the durability of the decellularized tissue under high surface tension (Law of Laplace). After 4 months of implantation, all decellularized patches were inspected by gross examination, light microscopy (H&E, Serius red, Gomori, Weigert, and von Kossa straining), and immunohistochemical staining.
Results
The juvenile sheep showed fast recovery after surgery. There was no mortality during follow-up. At explantation, only limited adhesion was seen at the surgical site. Gross examination showed a smooth and pliable surface without degeneration, as well as absence of aneurysmatic dilatation. Light microscopy showed a well preserved extracellular scaffold with a monolayer of endothelial cells covering the luminal side of the patch. On the outside part of the patch, a well developed neo-vascularization was seen. Host fibroblasts were seen in all layers of the scaffolds. There was no evidence for structural deterioration or calcification of the decellularized equine pericardial scaffolds.
Conclusions
In the juvenile sheep, decellularized equine tissue showed no structural deterioration, but regeneration and remodeling processes at systemic circulation.
Keywords: tissue engineering, juvenile sheep, equine patch, xenografts, decellularization, remodelling potential
Background
Patch reconstruction for tissue deficiencies due to congenital diseases is a common problem in cardiovascular surgery [1,2]. Besides autologous pericardium, which is often unavailable due to previous operations, implants used are made of synthetic materials such as Dacron (based on Polyethylenterephtalate) or Gore-Tex (based on polytetrafluoroethylene) and biological materials like glutaraldehyde-fixed bovine pericardium [3,4]. A limitation of these implants is that they are permanent foreign bodies with the potential for infection and the absence of remodeling, regeneration, and growth potential [5,6]. Furthermore, the glutaraldehyde-treated material shows early deterioration and should ideally be avoided in young patients [7].
Tissue engineering could overcome these limitations, as an interdisciplinary field which applies the principles of biology and engineering to develop functional substitutes for damaged tissue [8]. Materials, however, should be ideally available from the shelf, and recellularize and remodel in the host to overcome a waiting time for the patient and to avoid additional surgery [9] or intervention [10] to harvest autologous tissue materials. Furthermore these structures would also have growth potential, which is of enormous interest in treating congenital heart diseases. Limited clinical data are available of tissue engineered materials for pericardial reconstruction [11], pediatric patients with congenital cardiac anomalies [12], or aortic annulus enlargement [13].
This study was performed to evaluate the possibilities of a decellularized equine pericardial patch in the high pressure system and the mechanism of recellularization during these circumstances.
Material and Methods
All experiments were performed in accordance with the “Principles of Laboratory Animal Care” prepared by the National Society of Medical Research, and the “Guide for the Care and Use of Laboratory Animals” prepared by the Institute of Laboratory Animal Resources and published by the National Institutes of Health (NIH publication, revised 1996). The study was approved by the ethics committee of the Pontificia Universidade Catolica de Parana. We performed implantation of a decellularized equine pericardial patch in 7 juvenile sheep. Equine pericardium was chosen due to the similar strength as in bovine material, which is significantly higher compared to porcine pericardium [14]. We did not prefer to use bovine material due to previous reports of limited risk for contamination [15], latent general transmission of other diseases [16] and, eventually, immune response [17]. The decellularization procedure has been described previously [18]. In brief, after preparing the equine pericardium, it was stored in a physiological solution, adding antibiotics (penicillin, streptomycin, and amphotericin B) until decellularization was performed with deoxycholic acid (Sigma Chemical Co, St. Louis, MO) followed by an ethanol treatment. Additional extensive raising with physiologic solutions was performed. The decellularized equine pericardial patches were stored in an antibiotic solution until sterility was proven.
Surgery
All juvenile sheep were female, mean age of 14±2 weeks, with a mean body weight of 28.8±7.0 kg. Each animal was hospitalized 4 weeks prior to the procedure in the Veterinary Hospital of the Catholic Pontifical University of Parana State, to be certified for freedom of pulmonary diseases, vaccinated for clostridiosis and, additionally, all animals received anti-helmintics and systematic anti-parasitologic treatment. Hematocrit examinations were done weekly. Animals were fasted prior to the surgical procedure.
Pre-medication was performed with 0.5 mg/kg diazepam i.v. (Valium®, Roche Quim. e Farm. S/A, São Paulo – Brazil) and 0.4 mg/kg i.v. butorphanol tartrate (Torbugesic®, Fort Dodge Saúde Animal Ltda., Campinas – Brazil). After induction of anesthesia with 10 mg/kg of ketamine chloridrate i.v. (Vetaset®, Fort Dodge Saúde Animal, Campinas – Brazil), 3% halothane (Halothano, Cristália Produtos Químicos Farmacêuticos Ltda., São Paulo – Brazil) with an oxygen mixture was given. Afterwards, animals were intubated with a 7.0 size endotracheal tube and ventilated with an OXIGEL 1722 (Oxigel Mats Hosps Ind. E Com. Ltda, São Paulo - Brazil) respirator. Oxygen saturation, ETCO2, blood pressure, heart rate, 3-lead electrocardiogram, and rectal and nasopharyngeal temperature were monitored by the cardioscopy DIXTAL DX-2010 (Dixtal Biomedica Ind. E Com. Ltda., Manaus – Brazil). Blood gas values were measured by the AGS12 DRAKE Gasometer (Drake Electronic Com. Ltda, São Paulo – Brazil). The values were adjusted within normal ranges. The sheep were placed in a lateral decubitus position. Invasive arterial blood pressure measurement was obtained by cannulation of the left central ear artery. A left mini-thoracotomy was performed at the second intercostal space. The descending aorta was carefully dissected free, partially clamped, and incised. The decellularized equine pericardial patch was implanted using a running 6-0 polypropylene suture lines (Figure 1). Hemostasis was controlled, a chest drain was inserted, and the chest was closed in layers.
Figure 1.
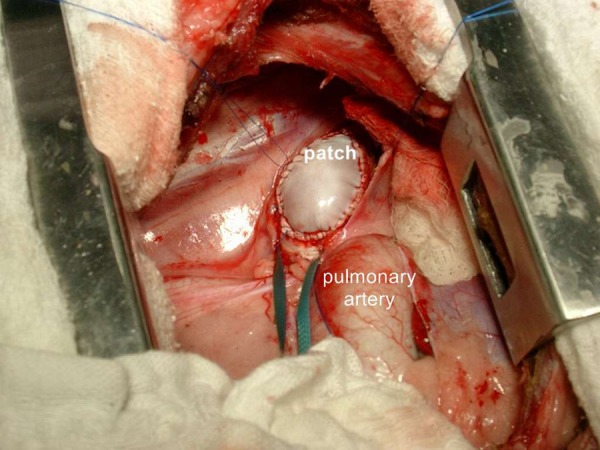
This representative sample shows an implanted decellularized equine pericardial patch into the descending aorta.
At the time of skin closure, 1% bupivacaine chloridrate (Marcaína® Astra Quím. E Farm. Ltda., São Paulo – Brazil) was given in the wound as local pain medication. The chest drain was removed approximately 30 minutes after surgery in all cases. Two mg/kg of Flunixin meglumine (Excenel RTU Pharmacia Brazil Ltda., São Paulo – Brazil) was administered intramuscularly given as pain medication. Antibiotic therapy was performed by administering 4 mg/kg of gentamicin (Schering-Plough S/A, São Paulo – Brazil) and 1 mg/kg of ceftiofur (Excenel RTU Pharmacia Brazil Ltda., São Paulo – Brazil) during the first 5 postoperative days.
After 1 day, the animals were transported back to the farm. Clinical examinations were performed daily by a veterinarian.
Explants and analysis
The animals were observed for external abnormalities, including palpable masses and skin scarring. After the animals were pre-medicated and intubated, 3 mg/kg of heparin was administered intravenously. The descending aorta were carefully prepared and the experiments terminated by administering an overdose of potassium. When there was no heart activity seen on the ECG, the patches were dissected and removed. All animals were exsanguinated at 4 months (n=7) based on previous experience, since recellularization in the systemic circulation was seen to be completed after 4 months [19], confirmed by clinical data of Scholl et al. [20].
Gross examination
The explanted decellularized patches were inspected and color photographs were taken before fixation. Special attention was given to identify vegetations, hematomas, thrombotic material, and signs of mechanical or biological degradation (tears or perforations, fibrous tissue overgrowth, aneurysmatic dilatation, calcific deposits, deformations, retractions or hardness).
Histology
Histological examination was performed to observe the in vivo repopulation of the decellularized equine pericardial patches. Representative samples were taken from different areas of the patches.
Light microscopy
Longitudinal sections were imbedded in paraffin and four-micrometer thick sections were stained. Hematoxylin-eosin staining was performed to allow general evaluation of the samples. Gomori staining showed collagen fibers in general. Sirius red staining was used to visualize the difference between mature and immature collagen fibers. Weigert staining expressed elastin fibers. Von Kossa staining identified valve area’s with calcification if present.
Immunohistochemistry
Endothelial cells were characterized by the presence of factor VIII-related antigen (DAKO, Hamburg, Germany) as well as CD 31 and CD 34 (DAKO, Hamburg, Germany). Fibroblasts were identified by anti-fibroblast factor (DAKO, Hamburg, Germany).
Results
During follow-up, no events were observed. All animals survived the follow-up period of 4 months.
Gross examination
Explantations were undertaken without any problem, as identification of the implanted decellularized equine patches showed only minimal adhesions. The decellularized equine patches were pliable comparable to pre-implantation, without loosing wall thickness (Figure 2). No hematomas, vegetations, or thrombotic materials were seen at the inner surfaces of the patch. Furthermore, there was no evidence of aneurysmatic dilatation or tissue deterioration, although the patches were largely oversized. No calcific spots were visible on the decellularized patches.
Figure 2.
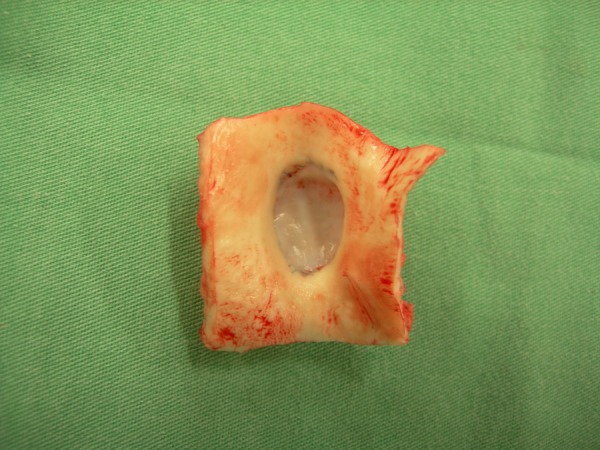
This representative sample is a decellularized equine pericardial patch at explantation after 4 months of implantation.
Histology
Light microscopy
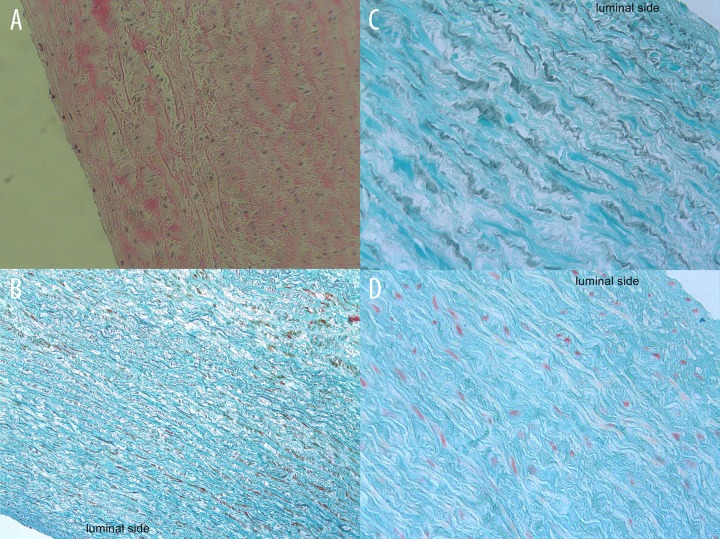
Hematoxylin-eosin staining showed in all implanted patches complete repopulation of the decellularized scaffolds (Figure 3A). The inner surface showed a complete intact monolayer of endothelial-like cells. The orientation and integrity of the extracellular matrix showing a well preserved collagen and elastin. In all areas of the decellularized scaffolds, there was a complete infiltration of interstitial cells. These included fibroblast-like and myofibroblast-like cells showed partially large nucleus, characteristic for young fibroblasts.
Figure 3A–D.
(A) Histological examination of a piece of the explanted decellularized pericardial patch shows a normal extracellular matrix with a monolayer of endothelial-like cells (H&E staining). (B) Goldner stained pericardial patch showed the well preserved collagen structures of the pericardial patch confirmed with Gomori staining (C). (D) Pentachrome staining reflects an intact extracellular matrix.
Goldner and Gomori staining supported the findings show at the hematoxylin-eosin staining by a normal pattern of collagen in all layers of the extracellular matrix. The decellularized patches were not affected by high pressure and showed a well preserved integrity of the extracellular matrix (Figure 3B, 3C).
Pentachrome staining showed a similar pattern for the preserved elastin fibers, without structural deterioration as well as the collagen fibers (Figure 3D).
Serius red staining showed mature and immature collagen structures of the extracellular scaffold as well as the well preservation of the extracellular matrix at the systemic circulation.
Von Kossa staining was able to support the findings that there is no calcification at any part of the decellularized patches (Figure 3E).
Figure 3E–I.
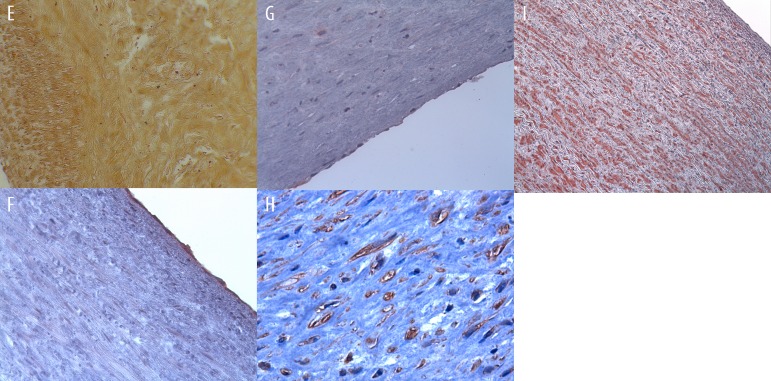
(E) Absence of tissue calcification is shown in this representative sample at 4 months (Von Kossa staining). (F) von Willebrand Factor shows a confluent monolayer of endothelial cells as well as CD-31 (G). (H) Anti-fibroblast factor positive cells are noticed in all layers of the decellularized pericardial patches, including myofibroblasts (I).
Immunohistochemistry
With immunohistochemic analyses it was possible to identify that the inner surface was covered by a monolayer of endothelial cells (Figure 3F). This was confirmed by CD-31 (Figure 3G) and CD-34 staining. The interstitial cells were positive stained by a specific anti-fibroblast marker, showing that these interstitial cells were fibroblasts (Figure 3H) and also myofibroblast (Figure 3I).
Discussion
Commonly used patch materials
Pediatric cardiac surgeons are faced with less satisfying patch materials for tissue repair after several previous operations; autologous pericardium is often unavailable or of poor quality. Any prosthetic patch material is difficult to handle, prone to infection, and stiffened after some time in the blood circulation [21,22]. Recurrent coarctation repair is exceptional high; in as high as 30% in tube graft insertion, compared with 12% and 11% in patch aortoplasty and end-to-end anastomosis, respectively [23].
In a study including 317 patients with patch aortoplasty, Hehrlein et al. [24] showed that 18 patients (5.7%) developed an aneurysm. Twelve patients (66.7%) underwent extensive resection of the intimal layer, the so-called fibrous coarctation membrane, which was the only factor predictive of developing an aneurysm. Experimental data of DeSanto et al. [25], in which aneurysm formation was found in 8 out of 12 animals (66.7%), support these findings.
Other studies showed the impact of using synthetic materials and explained the formation of an aneurysm by the development of a hemodynamic pulsatile wave, which is completely absorbed by the posterior native wall due to the rigid patch that is anteriorly placed [26,27]. Further differences have been found in the use of various materials, including knitted grafts, which showed a significant higher rate of aneurysm compared to woven Dacron grafts [28,29]. Backer et al. [30] showed, by using polytetrafluoroethylene grafts versus Dacron, a statistically significant reduction of aneurysms; 0.3% (1/326 patients) and 11.9%, respectively (70/586 patients) (P<0.001).
Bovine, porcine, or equine bioprosthetic patches classically are tanned with glutaraldehyde and thus undergo stiffening and calcification over time, especially in young patients [31–33]. Surgical handling and resistance to infection of pericardial tissue is much better than prosthetic material; however, Roussin et al. [34] showed that 3 out of 4 glutaraldehyde-fixed xenopericardial patches needed to be explanted after aortic arch reconstruction within 1 year, and 3 out of 4 pulmonary homograft patches were explanted within 18 months of follow-up. Mirsadraee et al. [35] was able to show significantly lower calcium levels in fresh/frozen pericardia compared with glutaraldehyde-fixed pericardia, 117 mg/g versus 332 μg/g Ca2+, respectively, of tissue dry weight (p<0.001). Other studies showed the fate of using autogenic pericardia, even in the systemic circulation [36,37].
In an experimental study, Cheung et al. [38] reported early postoperative fibrosis as well as tissue retraction of the autologous pericardium, probably due to high serum levels. Increase cellular accumulation within parts of the tissue could not be explained.
All of the currently available patch materials, however, show no regeneration, remodeling, or growth potential, which is a serious drawback in the setting of cardiac repair in infants and children, which results in reoperations. These problems could be overcome by tissue with regeneration, remodeling, and growth potential, especially in the high-pressure circulation such as enlargements of the aorta in the Norwood I procedure, mitral or aortic valve repair, or complex repair of coarctation of the thoracic aorta.
Several experimental and clinical studies were performed to evaluate tissue-engineered materials, however, these were mainly within the low-pressure system [39,40]. Limited studies show results of decellularized heart valves implanted into the high-pressure system [41,42].
Alternative tissue-engineered patch materials in clinics
Recent studies present a few new tissue-engineered patches available to be used in congenital heart diseases. One commercially available patch is the so-called CorMatrix (CorMatrix Cardiovascular, Alpharetta, GA, USA). This is a decellularized porcine small intestinal submucosa extracellular matrix (SIS-ECM) including elastin, glycosaminoglycans, proteoglycans, and adhesion glycoproteins.
In an initial study, Boyd et al. [11] demonstrated the effect of pericardial closure with SIS-ECM in adult patients. In this study there was a statistically significant reduction seen of postoperative atrial fibrillation in coronary artery bypass patients versus the controls. Scholl et al. [20] used this new acellular xenograft patch in 43 congenital heart diseased patients. In 16 patients, the pericardium was closed with the SIS-ECM, which in 37 SIS-ECM was used for cardiac or great vessel repair. However, the SIS-ECM generally was used in the low-pressure system. In 1 patient, SIS-ECM was used for tricuspid valve anterior leaflet augmentation and 4 months after surgery a catheter-based valve replacement was performed due to severe regurgitation. Stelly et al. [43] reported that more than 50,000 of these implants have been performed. In this reoperation case study, limited adhesion was found if SIS-ECM was used for pericardial closure. Quari et al. [44] reported the use of SIS-ECM in 26 patients, including 5 aortic valves, 1 mitral valve repair, and 7 vascular repairs (including 4 ascending aorta and 3 aortic arch repairs). Early results of this study look promising. Schomisch et al. [45], however, reported that the use 3 different extracellular matrices (small intestinal mucosa (SIS), acellular dermal matrix, and urinary bladder matrix) could not prevent stricture after esophageal mucosectomy. Another caution was reported by Weber et al. [46], presenting early asymptomatic pseudoaneurysm degeneration by using SIS for carotid repair.
Lofland et al. [47] used a decellularized allograft pulmonary artery patch (MatrACELL, LifeNet Health, Virginia Beach, VA, USA) to reconstruct right ventricular outflow tract reconstruction. During complementation of the surgical procedure, a right ventricle-to-pulmonary artery conduit was implanted, histological examination was performed from a small biopsy of the patch, showing recellularization with absence of inflammation.
Neethling et al. [12] evaluated a tissue-engineered bovine pericardial patch treated with ADAPT. In total, 30 patches of this type have been implanted with (CardioCel, Celxcel Pty Ltd., Perth, Western Australia). At least 6 vascular reconstructions were performed, which needs to withstand the circulatory pressure. During 18–36 months of follow-up, 19 out of 30 patients underwent echocardiographic examination with absence of device calcification, infection, thromboembolic events, or device failure.
These early result show the potential of tissue-engineered patches to be used for correction of congenital cardiac defect. The SIS-ECM, however, needs further evaluation, especially if this material is to be able to withstand the systemic circulation. Therefore, we evaluated equine pericardium implanted into the system circulation.
Evaluation different pericardial patches
Gauvin et al. [14] showed that bovine pericardium has superior biomechanical strength, ultimate tensile strength, and linear modulus, compared with porcine pericardium. Furthermore, this study showed that the fibrocollagenous surfaces of bovine and porcine tissue were not equal, showing that the serous side of porcine pericardium retained more platelets when compared with bovine material, with increase risk of acute thrombogenicity and building of a biofilm.
On the other hand, Umashankar et al. [48] studied the inflammation and immune response on regeneration induced by decellularized bovine pericardium. Therefore, these un-cross-linked decellularized bovine pericardia were compared with mild cross-linked decellularized bovine pericardia, showing a reduction of inflammatory response for plasma cells and macrophages of 2.0±0.9 cells/hpf versus 1.1±0.2 cells/hpf and 2.89±0.33 cells/hpf versus 2.47±0.74 cells/hpf, respectively, (p<0.05). The adaptive immune response at 90 days showed statistically significant higher antibody response in un-cross-linked decellularized bovine pericardium as compared with mild cross-linked decellularized bovine pericardium (p=0.006).
Since bovine pericardium seems to be stronger, limited reports have been presented that bovine pericardium can be contaminated, which is a serious problem, especially in non-cross-linked bovine pericardium [15]. If there is a risk of Onchocerca armillata availability in bovine pericardium, a potential risk will arise in unfixed tissue, resulting in acute aortitis with necrosis, which in turn results in aneurysm formation and eventual rupture.
The present study with a decellularized equine pericardial patch implanted into the aortic wall proved, macroscopically, the strength of the material. Although the patches were not seeded, no thrombotic material was noticed at the inner surface of the patch, and there was an absence of biofilm resulting in tissue infection. This could be another advantage over porcine and eventually also unfixed bovine pericardium.
Histologically, an intact extracellular matrix was seen with ingrowth of interstitial cells and absence of tissue retraction. A monolayer of endothelial cells had formed at the inner surface, providing the development of a pseudo-intima. The reason for this controlled process of repopulation may be the tissue-conserving decellularization process, which results in an intact functional extracellular matrix [41]. This has been shown previously by our group for decellularized valves in the pulmonary position [8–10,39,40].
Conclusions
The decellularized equine pericardial patch showed excellent hemodynamic performance at systemic pressures, was easy to handle, free from calcification and tissue degeneration, and, as it has growth potential, it may become an ideal source for cardiac repair.
Acknowledgements
We would like to thank Mrs. Krüger and Mrs. Noronha for assistance on preparing and staining the histological sections.
Footnotes
Source of support: Departmental sources
References
- 1.Ozkara A, Mert M, Cetin G, et al. Right ventricular outflow tract reconstruction for tetralogy of Fallot with abnormal coronary artery: Experience with 35 patients. J Card Surg. 2006;21(2):131–36. doi: 10.1111/j.1540-8191.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 2.Jonas RA, Freed MD, Mayer JE, Jr, Castaneda AR. Long-term follow-up of patients with synthetic right heart conduits. Circulation. 1985;72(3Pt2):II77–83. [PubMed] [Google Scholar]
- 3.Mayer JE., Jr Uses of homograft conduits for right ventricle to pulmonary artery connections in the neonatal period. Semin Thorac Cardiovasc Surg. 1995;7(3):130–32. [PubMed] [Google Scholar]
- 4.Smaill BH, McGiffin DC, Legrice IJ, et al. The effect of synthetic patch repair of coarctation on regional deformation of the aortic wall. J Thorac Cardiovasc Surg. 2000;120(6):1053–63. doi: 10.1067/mtc.2000.110187. [DOI] [PubMed] [Google Scholar]
- 5.Kirklin JK, Kirklin JW, Blackstone EH, et al. Effect of transannular patching on outcome after repair of tetralogy of Fallot. Ann Thorac Surg. 1989;48(6):783–91. doi: 10.1016/0003-4975(89)90671-1. [DOI] [PubMed] [Google Scholar]
- 6.Valente M, Laborde F, Thiene G, et al. Glutaraldehyde-fixed bovine iliac veins used as bioprothetic conduits: an experiemental animal study. J Card Surg. 1992;7(2):156–62. doi: 10.1111/j.1540-8191.1992.tb00791.x. [DOI] [PubMed] [Google Scholar]
- 7.Fiddler GI, Gerlis LM, Walker DR, et al. Calcification of glutaraldehyde-preserved porcine and bovine xenograft valves in young children. Ann Thorac Surg. 1983;35(3):257–61. doi: 10.1016/s0003-4975(10)61554-8. [DOI] [PubMed] [Google Scholar]
- 8.Langer R, Vacanti JP. Tissue Engineering. Science. 1993;260(5110):920–26. doi: 10.1126/science.8493529. [DOI] [PubMed] [Google Scholar]
- 9.Dohmen PM, Lembcke A, Holinski S, et al. Midterm results of right ventricular outflow tract reconstruction with a tissue engineered pulmonary valve in eleven consecutive patients. Ann Thorac Surg. 2007;84(3):729–36. doi: 10.1016/j.athoracsur.2007.04.072. [DOI] [PubMed] [Google Scholar]
- 10.Dohmen PM, da Costa F, Yoschi S, et al. Histological evaluation of tissue engineered heart valves implanted in the juvenile sheep model: is there a need for in-vitro seeding? J Heart Valve Dis. 2006;15(6):823–29. [PubMed] [Google Scholar]
- 11.Boyd WD, Johnson WE, III, Sultan PK, et al. Pericardial reconstruction using an extracellular matrix implant correlates with reduced risk of postoperative atrial fibrillation in coronary artery bypass surgery patients. Heart Surg Forum. 2010;13(5):E311–16. doi: 10.1532/HSF98.20091184. [DOI] [PubMed] [Google Scholar]
- 12.Neethling WML, Strange G, Firth L, Smit FE. Evaluation of a tissue-engineered bovine pericardial patch in paediatric patients with congenital cardiac anomalies: initial experience with the ADAPT-treated CardioCel patch. Interact Cardiovasc Thorac Surg. 2013;17(4):698–702. doi: 10.1093/icvts/ivt268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerdisch MW, Akinwande AO, Matheny RG. Use of a novel acellular xenograft as a patch for aortic annular enlargement during aortic valve replacement. Innovations. 2010;5:60–62. doi: 10.1097/IMI.0b013e3181cbb421. [DOI] [PubMed] [Google Scholar]
- 14.Gauvin R, Marinov G, Mehri Y, et al. A comparative study of bovine and porcine pericardium to highlight their potential advantages to manufacture percutaneous cardiovascular implants. J Biomater Appl. 2013;28(4):552–65. doi: 10.1177/0885328212465482. [DOI] [PubMed] [Google Scholar]
- 15.Mather C, Treuting P. Onchocerca armillata contamination of a bovine pericardial xenograft in a human patient with repaired tetralogy of Fallot. Cardiovasc Pathol. 2012;12(3):e35–38. doi: 10.1016/j.carpath.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Martin M, Trouvin JH. Risk of transmission of Creutzfeldt-Jakob disease via blood and blood products. The French risk-analysis over the last 15 years. Transfus Clin Biol. 2013;20(11):398–404. doi: 10.1016/j.tracli.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 17.Bloch O, Golde P, Dohmen PM, et al. Immune response in patients receiving a bioprosthetic heart valve: lack of response with decellularized valves. Tissue Eng Part A. 2011;17(19–20):2399–405. doi: 10.1089/ten.TEA.2011.0046. [DOI] [PubMed] [Google Scholar]
- 18.Dohmen PM, da Costa F, Lopes SV, et al. Is there a possibility for a Glutaraldehyd-free porcine heart valve to grow ? Eur Surg Res. 2006;38(1):54–61. doi: 10.1159/000091597. [DOI] [PubMed] [Google Scholar]
- 19.Dohmen PM, da Costa F, Yoshi S, et al. An experimental study of decellularized xenografts implanted into the aortic position with 4 months of follow-up. J Clin Experiment Cardiol. 2012;S4:004. [Google Scholar]
- 20.Scholl FG, Boucek MM, Chan KC, et al. Preliminary experience with cardiac reconstruction using decellularized porcine extracellular matrix scaffold: human applications in congenital heart disease. World J Pediatr Congenit Heart Surg. 2010;1(1):132–36. doi: 10.1177/2150135110362092. [DOI] [PubMed] [Google Scholar]
- 21.Tremblay D, Zigras T, Cartier R, et al. A comparison of mechanical properties of materials used in aortic arch reconstruction. Ann Thorac Surg. 2009;88(5):1481–91. doi: 10.1016/j.athoracsur.2009.07.023. [DOI] [PubMed] [Google Scholar]
- 22.Hesslein PS, McNamara DG, Morriss MJH, et al. Comparison of resection versus patch aortoplasty for repair of coarctation in infants and children. Circulation. 1981;64(1):164–68. doi: 10.1161/01.cir.64.1.164. [DOI] [PubMed] [Google Scholar]
- 23.Brown JW, Ruzmetov M, Hoyer MH, et al. Recurrent coarctation: Is surgical repair of recurrent coartation of the aorta safe and effective? Ann Thorac Surg. 2009;88(6):1923–31. doi: 10.1016/j.athoracsur.2009.07.024. [DOI] [PubMed] [Google Scholar]
- 24.Hehrlein FW, Mulch J, Rautenburg HW, et al. Incidence and pathogenesis of late aneurysm after patch graft aortoplasty for coarctation. J Thoracic Cardiovasc Surg. 1986;92(2):226–30. [PubMed] [Google Scholar]
- 25.DeSanto A, Bills RG, King H, et al. Pathogenesis of aneurysm formation opposite prosthetic patches used for coarctation repair. An experimental study. J Thorac Cardiovasc Surg. 1987;94(5):720–23. [PubMed] [Google Scholar]
- 26.Bromberg BI, Beekman RH, Rocchini AP, et al. Aortic aneurysm after patch aortoplasty repair of coarctation: a prospective analysis of prevalence, screening test and risks. J Am Coll Cardiol. 1989;14(3):734–41. doi: 10.1016/0735-1097(89)90119-8. [DOI] [PubMed] [Google Scholar]
- 27.Dietl CA, Torres AR, Favaloro RG, et al. Risk of recoactation in neonates and infants after repair with patch aortoplasty, subclavian flap, and the combined resection-flap procedure. J Thoracic Cardiovasc Surg. 1992;103(4):724–32. [PubMed] [Google Scholar]
- 28.Monaghan RA, Meban S. Expanded polytetrafluoroethylene patch in hernia repair: a review of clinical experience. Can J Surg. 1991;34(5):502–5. [PubMed] [Google Scholar]
- 29.Wilson SE, Krug R, Mueller G, Wilson L. Late disruption of Dacron aortic grafts. Ann Vasc Surg. 1997;11(4):383–86. doi: 10.1007/s100169900065. [DOI] [PubMed] [Google Scholar]
- 30.Backer CL, Paape K, Zales VR, et al. Coarctation of the aorta. Repair with polytetrafluoroethylene patch aortoplasty. Circulation. 1995;92(9 Suppl):II132–36. doi: 10.1161/01.cir.92.9.132. [DOI] [PubMed] [Google Scholar]
- 31.Moulton AL, De Leval MR, Macartney FJ, et al. Rastelli procedure for transposition of the great arteries, ventricular septal defect, and left ventricular outflow tract obstruction. Early and late results in 41 patients 1971 to 1978. Br Heart J. 1981;45(1):20–28. doi: 10.1136/hrt.45.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams DB, Danielson GK, McGoon DC, et al. Porcine heterograft valve replacement in children. J Thorac Cardiovasc Surg. 1982;84(3):446–50. [PubMed] [Google Scholar]
- 33.Lloyd TR, Marvin WJ, Jr, Mahoney LT, Lauer RM. Balloon dilation valvuloplasty of bioprothetic valves in extracardiac conduits. Am Heart J. 1987;114:268–74. doi: 10.1016/0002-8703(87)90489-3. [DOI] [PubMed] [Google Scholar]
- 34.Roussin R, Belli E, Lacour-Gayet F, et al. Aortic arch reconstruction with pulmonary autograft patch aortoplasty. J Thoracic Cardiovasc Surg. 2002;123(3):443–48. doi: 10.1067/mtc.2002.120733. [DOI] [PubMed] [Google Scholar]
- 35.Mirsadraee S, Wilcox HE, Watterson KG, et al. Biocompatibility of acellular human pericardium. J Surg Res. 2007;143(3):407–14. doi: 10.1016/j.jss.2007.01.026. [DOI] [PubMed] [Google Scholar]
- 36.Schulte HD, Bircks W, Frenzel H, et al. Patch-graft enlargement of the aortic root using autologous pericardium (long-term results) Thorac Cardiovasc Surg. 1983;31(4):219–23. doi: 10.1055/s-2007-1021983. [DOI] [PubMed] [Google Scholar]
- 37.Dalichau H, Hannekum A, Niehues B, et al. Hemodynamic and angiographic late results following enlargement of narrow aortic root using autologous pericardium in prosthetic aortic valve replacement. Thorac Cardiovasc Surg. 1985;33(5):288–95. doi: 10.1055/s-2007-1014143. [DOI] [PubMed] [Google Scholar]
- 38.Cheung DT, Choo SJ, Grobe AC, et al. Behavior of vital and killed autologous pericardium in the descending aorta of sheep. J Thoracic Cardiovasc Surg. 1999;118(6):998–1005. doi: 10.1016/S0022-5223(99)70093-0. [DOI] [PubMed] [Google Scholar]
- 39.Dohmen PM, Lembcke A, Holinski S, Pruss A, Konertz W. Ten years of clinical results with a tissue-engineered pulmonary valve. Ann Thorac Surg. 2011;92(4):1308–14. doi: 10.1016/j.athoracsur.2011.06.009. [DOI] [PubMed] [Google Scholar]
- 40.Konertz W, Angeli E, Tarusinov G, et al. Right ventricular outflow tract reconstruction with decellularized porcine xenografts in patients with congenital heart disease. J Heart Valve Dis. 2010;20(3):341–47. [PubMed] [Google Scholar]
- 41.Honge JL, Funder J, Hansen E, et al. Recellularization of aortic valve in pigs. Eur J Cardiothor Surg. 2011;39(6):829–34. doi: 10.1016/j.ejcts.2010.08.054. [DOI] [PubMed] [Google Scholar]
- 42.Dohmen PM. Tissue engineered aortic valve. HSR Proc Intensive Care Cardiovasc Anesth. 2012;4(2):89–93. [PMC free article] [PubMed] [Google Scholar]
- 43.Stelly M, Stelly TC. Histology of CorMatrix bioscaffold 5 years after pericardial closure. Ann Thorac Surg. 2013;96:e127–29. doi: 10.1016/j.athoracsur.2013.06.114. [DOI] [PubMed] [Google Scholar]
- 44.Quarti A, Nardone S, Colaneri M, et al. Preliminary experience in the use o fan extracellular matrix to repair congenital heart diseases. Interact Cardiovasc Thorac Surg. 2011;13(6):569–72. doi: 10.1510/icvts.2011.280016. [DOI] [PubMed] [Google Scholar]
- 45.Schomisch SJ, Yu Y, Pauli EM, et al. Commercially available biological mesh does not prevent stricture after esophageal mucosectomy. Endoscopy. 2013 Nov 11; doi: 10.1055/s-0033-1344997. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber SS, Annenberg AJ, Wright CB, et al. Early pseudoaneurysm degeneration in biologic extracellular matrix patch for carotid repair. J Vasc Surg. 2013 doi: 10.1016/j.jvs.2013.05.012. pii: S0741-5214(13)00949-X. [DOI] [PubMed] [Google Scholar]
- 47.Lofland GK, O’Brien JE, Jr, Gandy KL, et al. Initial pediatric cardiac experience with decellularized allograft patches. Ann Thorac Surg. 2012;93(3):968–71. doi: 10.1016/j.athoracsur.2011.09.039. [DOI] [PubMed] [Google Scholar]
- 48.Umashankar PR, Arun T, Kumary TV. Effect of chronic inflammation and immune response on regeneration induced by decellularized bovine pericardium. J Biomed Mater Res Part A. 2013;101A:2202–9. doi: 10.1002/jbm.a.34535. [DOI] [PubMed] [Google Scholar]
- 49.Dohmen PM, Konertz W. Tissue-engineered heart valve scaffolds. Ann Thorac Cardiovasc Surg. 2009;15(6):362–67. [PubMed] [Google Scholar]