Abstract
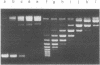
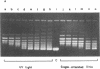
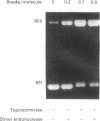
The activity of Micrococcus luteus DNA topoisomerase I on UV-irradiated supercoiled DNA was studied under either processive or distributive reaction conditions. Changes in DNA structure caused by UV irradiation reduce the rate of DNA relaxation at very low concentration of photoproducts. Under processive conditions the inhibition of the topoisomerase I by photoproducts can be quantitated by measuring the amount of substrate left in the replicative form I band. The mode of action of DNA topoisomerase I was affected by the presence of photoproducts in the DNA substrate, although the ability of the enzyme to form a covalent complex with UV-irradiated supercoiled DNA was not changed. The inhibition of topoisomerase I by UV photoproducts has been compared to the effects of single-stranded DNA and UV-irradiated duplex linear DNA on the enzyme, and the results suggest that the inhibition by photoproducts is caused by changes in the conformation of the supercoil. Our findings indicate the possibility that DNA topoisomerase I plays a role in repair.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi G., Pedrini A. M. Determination of pyrimidine dimer unwinding angle by measurement of DNA electrophoretic mobility. J Mol Biol. 1982 Feb 25;155(2):177–183. doi: 10.1016/0022-2836(82)90445-4. [DOI] [PubMed] [Google Scholar]
- Ciarrocchi G., Sutherland B. M., Pedrini A. M. Photoreversal of DNA unwinding caused by pyrimidine dimers. Biochimie. 1982 Aug-Sep;64(8-9):665–668. doi: 10.1016/s0300-9084(82)80107-7. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R. DNA topoisomerases. Cell. 1980 Nov;22(2 Pt 2):327–328. doi: 10.1016/0092-8674(80)90341-4. [DOI] [PubMed] [Google Scholar]
- Crumplin G. C. The involvement of DNA topoisomerases in DNA repair and mutagenesis. Carcinogenesis. 1981;2(2):157–160. doi: 10.1093/carcin/2.2.157. [DOI] [PubMed] [Google Scholar]
- Depew R. E., Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli protein omega. Formation of a complex between single-stranded DNA and omega protein. J Biol Chem. 1978 Jan 25;253(2):511–518. [PubMed] [Google Scholar]
- Fidanián H. M., Ray D. S. Replication of bacteriophage M13. 8. Differential effects of rifampicin and nalidixic acid on the synthesis of the two strands of M13 duplex DNA. J Mol Biol. 1974 Feb 15;83(1):63–82. doi: 10.1016/0022-2836(74)90424-0. [DOI] [PubMed] [Google Scholar]
- Gellert M. DNA topoisomerases. Annu Rev Biochem. 1981;50:879–910. doi: 10.1146/annurev.bi.50.070181.004311. [DOI] [PubMed] [Google Scholar]
- Gordon L. K., Haseltine W. A. Comparison of the cleavage of pyrimidine dimers by the bacteriophage T4 and Micrococcus luteus UV-specific endonucleases. J Biol Chem. 1980 Dec 25;255(24):12047–12050. [PubMed] [Google Scholar]
- Hariharan P. V., Cerutti P. A. Formation of products of the 5,6-dihydroxydihydrothymine type by ultraviolet light in HeLa cells. Biochemistry. 1977 Jun 14;16(12):2791–2795. doi: 10.1021/bi00631a032. [DOI] [PubMed] [Google Scholar]
- Haseltine W. A., Gordon L. K., Lindan C. P., Grafstrom R. H., Shaper N. L., Grossman L. Cleavage of pyrimidine dimers in specific DNA sequences by a pyrimidine dimer DNA-glycosylase of M. luteus. Nature. 1980 Jun 26;285(5767):634–641. doi: 10.1038/285634a0. [DOI] [PubMed] [Google Scholar]
- Hayes F. N., Williams D. L., Ratlift R. L., Varghese A. J., Rupert C. S. Effect of a single thymine photodimer on the oligodeoxythymidylate-polydeoxyadenylate interaction. J Am Chem Soc. 1971 Sep;93(19):4940–4942. doi: 10.1021/ja00748a065. [DOI] [PubMed] [Google Scholar]
- Hays J. B., Boehmer S. Antagonists of DNA gyrase inhibit repair and recombination of UV-irradiated phage lambda. Proc Natl Acad Sci U S A. 1978 Sep;75(9):4125–4129. doi: 10.1073/pnas.75.9.4125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helland D., Nes I. F., Kleppe K. Mammalian DNA-repair endonuclease acts only on supercoiled DNA. FEBS Lett. 1982 Jun 1;142(1):121–124. doi: 10.1016/0014-5793(82)80233-0. [DOI] [PubMed] [Google Scholar]
- Kahn M. The effect of thymine dimers on DNA:DNA hybridization. Biopolymers. 1974 Apr;13(4):669–675. doi: 10.1002/bip.1974.360130403. [DOI] [PubMed] [Google Scholar]
- Kung V. T., Wang J. C. Purification and characterization of an omega protein from Micrococcus luteus. J Biol Chem. 1977 Aug 10;252(15):5398–5402. [PubMed] [Google Scholar]
- Liu L. F., Wang J. C. Interaction between DNA and Escherichia coli DNA topoisomerase I. Formation of complexes between the protein and superhelical and nonsuperhelical duplex DNAs. J Biol Chem. 1979 Nov 10;254(21):11082–11088. [PubMed] [Google Scholar]
- McConaughy B. L., Young L. S., Champoux J. J. The effect of salt on the binding of the eucaryotic DNA nicking-closing enzyme to DNA and chromatin. Biochim Biophys Acta. 1981 Aug 27;655(1):1–8. doi: 10.1016/0005-2787(81)90059-9. [DOI] [PubMed] [Google Scholar]
- Overbye K. M., Margolin P. Role of the supX gene in ultraviolet light-induced mutagenesis in Salmonella typhimurium. J Bacteriol. 1981 Apr;146(1):170–178. doi: 10.1128/jb.146.1.170-178.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purdy M. A., Yeilding K. L. Nalidixic acid inhibition of post-ultraviolet recovery in Escherichia coli K-12: requirement for recBC function. Antimicrob Agents Chemother. 1976 Jul;10(1):182–184. doi: 10.1128/aac.10.1.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryan M. J. Coumermycin A1: A preferential inhibitor of replicative DNA synthesis in Escherichia coli. I. In vivo characterization. Biochemistry. 1976 Aug 24;15(17):3769–3777. doi: 10.1021/bi00662a020. [DOI] [PubMed] [Google Scholar]
- Schneck P. K., Staudenbauer W. L. Escherichia coli DNA synthesis in vitro: insensitivity of ATP-dependent DNA repair to inhibition by novobiocin. Nucleic Acids Res. 1977 Jun;4(6):2057–2064. doi: 10.1093/nar/4.6.2057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafranovskaya N. N., Trifonov E. N., Lazurkin Y. S., Frank-Kamenetskii M. D. Clustering of thymine dimers in ultraviolet irradiated DNA and the long-range transfer of electronic excitation along the molecule. Nat New Biol. 1973 Jan 10;241(106):58–60. doi: 10.1038/newbio241058a0. [DOI] [PubMed] [Google Scholar]
- Simon T. J., Masker W. E., Hanawalt P. C. Selective inhibition of semiconservative DNA synthesis by nalidixic acid in permeabilized bacteria. Biochim Biophys Acta. 1974 May 17;349(2):271–274. doi: 10.1016/0005-2787(74)90089-6. [DOI] [PubMed] [Google Scholar]
- Sternglanz R., DiNardo S., Voelkel K. A., Nishimura Y., Hirota Y., Becherer K., Zumstein L., Wang J. C. Mutations in the gene coding for Escherichia coli DNA topoisomerase I affect transcription and transposition. Proc Natl Acad Sci U S A. 1981 May;78(5):2747–2751. doi: 10.1073/pnas.78.5.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel H., Reinert K. E., Bär H., Lang H. Structural changes of ultraviolet-irradiated DNA derived from hydrodynamic measurements. Biochim Biophys Acta. 1979 Jan 26;561(1):59–68. doi: 10.1016/0005-2787(79)90490-8. [DOI] [PubMed] [Google Scholar]
- Twigg A. J., Sherratt D. Trans-complementable copy-number mutants of plasmid ColE1. Nature. 1980 Jan 10;283(5743):216–218. doi: 10.1038/283216a0. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Palecek E. Changes in properties of DNA caused by gamma and ultraviolet radiation. Dependence of conformational changes on the chemical nature of the damage. Biochim Biophys Acta. 1978 Feb 16;517(2):308–318. [PubMed] [Google Scholar]
- von Wright A., Bridges B. A. Effect of gyrB-mediated changes in chromosome structure on killing of Escherichia coli by ultraviolet light: experiments with strains differing in deoxyribonucleic acid repair capacity. J Bacteriol. 1981 Apr;146(1):18–23. doi: 10.1128/jb.146.1.18-23.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]