Abstract
Patient: Female, 56
Final Diagnosis: Breast cancer
Symptoms: Solid mass in the right breast
Medication: Exemestane
Clinical Procedure: —
Specialty: Oncology
Objective:
Unusual clinical course
Background:
The efficacy of third-generation aromatase inhibitors for hormone receptor-positive postmenopausal metastatic breast cancer is well established. Although several clinical trials have reported incomplete cross-resistance between different aromatase inhibitors, few cases of complete responses of recurrent metastatic breast cancer occurring after substituting a second aromatase inhibitor have been reported. We here present a rare case of non-steroidal aromatase inhibitor-tolerant metastatic breast cancer with long-term complete remission following substitution of a steroidal aromatase inhibitor.
Case Report:
We present the case of a 56-year-old Japanese woman who underwent right breast-conserving surgery for breast cancer, TNM staging T1, N0, M0, Stage I. She received adjuvant chemotherapy with 6 cycles of FEC100 and radiation therapy, and then began hormonal therapy with anastrozole. Twelve months postoperatively, computed tomography (CT) revealed multiple lung metastases. Exemestane was substituted for anastrozole. After 3 months of exemestane, CT showed that all lung metastases had completely resolved. Her complete response was maintained for 5 years: she died during a tsunami 6 years after the initial surgery.
Conclusions:
Substitution of a steroidal for a non-steroidal aromatase inhibitor produced a sustained complete remission in a patient with hormonal receptor-positive postmenopausal recurrent breast cancer.
Achieving complete response after switching from a non-steroidal to a steroidal aromatase inhibitor in a hormonal receptor-positive postmenopausal recurrent breast cancer contributed to a higher quality of life for the patient. Further investigation is needed to identify the predictors of long-term remission following such a switch.
MeSH Keywords: Neoplasm Metastasis, Breast Neoplasms – therapy, Aromatase Inhibitors
Background
Most patients with metastatic breast cancer treated with systemic therapies have only temporary responses to treatment [1]. Metastatic breast cancer is a nearly incurable disease; the main priorities of treatment are restoration of quality of life, reduction of tumor-related symptoms, maintenance of the patient’s social environment, and prolonging survival. Endocrine therapy, which is the standard treatment for hormone receptor-positive metastatic breast cancer that is not at a life-threatening stage, is effective and well tolerated [2]. Aromatase inhibitors (AIs) currently used include anastrozole and letrozole, which are non-steroidal AIs (NSAIs) and exemestane, which is a steroidal AI (SAI);these block estrogen synthesis competitively and irreversibly,respectively, to aromatase.
However, these different mechanisms may mean there is no cross-resistance between NSAIs and the SAI exemestane, making the latter a reasonable option for advanced or recurrent breast cancer treatment after treatment with the former [3–12]; complete response (CR), particularly a sustained CR, would probably be very rare. Herein, we report a case of metastatic breast cancer in which substitution of exemestane after early relapse during adjuvant endocrine therapy with anastrozole resulted in long-term complete remission.
Case Report
We here describe the case of a 56-year-old Japanese post-menopausal woman who presented with right breast cancer in April 2005. She underwent right breast-conserving surgery in May 2005. The primary tumor was 17×12 mm in diameter. The histological diagnosis was invasive ductal carcinoma of the right breast with no metastasis in the sentinel lymph node. Immunohistochemical (IHC) examination of the tumor cells was positive for estrogen receptors, negative for progesterone receptors, and showed moderate membrane staining for human epidermal growth factor receptor 2 (HER-2) (2+ score) (Figure 1). On IHC examination the Ki-67 labeling index was 5%. Fluorescence in situ hybridization analysis showed HER-2 gene amplification (HER2/CEP17 ratio >2.0). TNM staging was T1, N0, M0, Stage I. The patient received postoperative adjuvant chemotherapy with 6 cycles of FEC100 (5-fluorouracil 500 mg/m2, epirubicin 100 mg/m2, and cyclophosphamide 500 mg/m2), completing this in November 2005. She also underwent right whole breast irradiation (total 46 Gy), followed by a boost to the tumor bed (9 Gy). After completion of chemotherapy, the patient began adjuvant endocrine therapy with the third-generation aromatase inhibitor, anastrozole. Twelve months postoperatively, in May 2006, she was asymptomatic, yet screening computed tomography (CT) revealed multiple nodular shadows in both lungs (Figure 2A), which were diagnosed as metastases to the lungs with a 12-month disease-free interval. Because her condition was not life-threatening, her endocrine therapy was switched from anastrozole to exemestane. Three months after this treatment change, a CT scan showed a CR of her lung metastases with no development of any new lesions (Figure 2B). She was diagnosed as having a clinical CR according to the Response Evaluation Criteria in Solid Tumors (RECIST, version 1.1); this CR was maintained for 5 years. Radiological examinations revealed no new metastatic lesion and there were no increases in serum concentrations of tumor markers (carcinoembryonic antigen, carcinoma antigen 15-3, breast cancer antigen 225, and NCC-ST-439) throughout her clinical course. The patient remained in good health, led an active life, and experienced no adverse events. She died during a tsunami 6 years postoperatively.
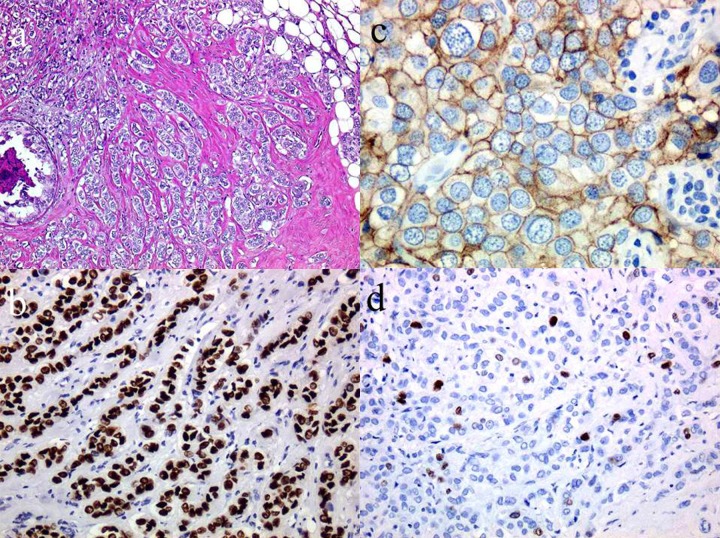
Figure 1.
Histological and immunohistochemical findings. (A) Micrograph of hematoxylin and eosin stained section of surgical specimen showing invasive ductal carcinoma proliferating in a trabecular manner (×40). (B) Micrograph of immunohistochemical stained section of surgical specimen showing the tumor cells have strongly positive nuclear staining for estrogen receptors (×100). (C) Micrograph of immunohistochemical stained section of surgical specimen showing the tumor cells have strong membrane staining for human epidermal growth factor receptor 2 (2+ score) (×100)]. (D) The Ki-67 (MIB-1, DAKO, 1:100) labeling index is 5%.
Figure 2.
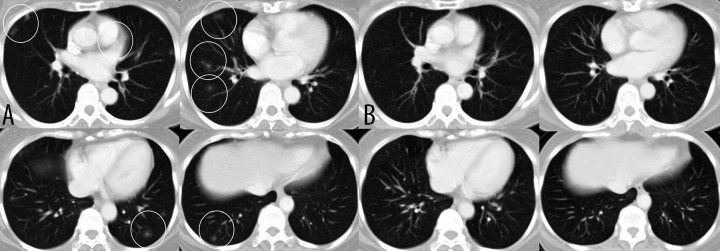
(A) CT image obtained 12 months postoperatively showing multiple nodular shadows in both lungs (white circle). (B) CT image obtained 3 months after commencing exemestane showing the lung metastases have resolved completely with no development of new lesions.
Discussion
We here present a patient in whom SAI induced long-term complete remission following relapse with non-life-threatening, measurable lung metastases on adjuvant therapy with an NSAI. In our institution, we regard treatment with another AI as a reasonable option, especially for hormone receptor-positive postmenopausal breast cancer patients who have relapsed with non-life-threatening metastases while receiving an NSAI, reserving use of a selective estrogen receptor down-regulator or selective estrogen receptor modulators, as recommended by National Comprehensive Cancer Network guidelines. As reported by Lønning [3] and others (Table 1), the efficacy of switching from NSAIs to an SAI for treatment of metastases is not negligible; CRs, partial responses, and clinical benefits (including stable disease) rates of 1.8–6.5%, 4.5–16.6%, and 24–54.8%, respectively, have been reported [4–12]. This incomplete cross-resistance is likely not a result of differences between the AIs in the degree of aromatase inhibition [10], but more probably is attributable to the weak androgen-antagonistic activity of exemestane through its 17-hydro metabolite [13]. Indeed, exemestane is a standard arm of the Breast Cancer Trials of Oral Everolimus-2 (BOLERO-2) trial, which is a phase III study comparing exemestane alone with exemestane plus the mTOR inhibitor everolimus after NSAI treatment failure [14].
Table 1.
Previous studies of results of switching from third-generation NSAIs to the SAI exemestane.
| Reference | Year | The first AI | The second AI | Total number of patients | Complete response, n (%) | Partial response, n (%) | Stable disease (>24 weeks), n (%) | * Objective response, n (%) | ** Clinical benefit, n (%) |
|---|---|---|---|---|---|---|---|---|---|
| Bertelli et al. [4] | 2005 | Anastrozole (1 mg) Letrozole (2.5 mg) |
Exemestane (25 mg) | 23 | 0 | 2 (8.7) | 8 (34.8) | 2 (8.7) | 10 (43.5) |
| Laffaioli et al. [5] | 2005 | Anastrozole (1 mg) | Exemestane (25 mg) | 50 | 1 (2.0) | 3 (6.0) | 18 (36.0) | 4 (8) | 22 (24.0) |
| Gennetas et al. [6] | 2006 | Anastrozole (1 mg) Letrozole (2.5 mg) |
Exemestane (25 mg) | 60 | 2 (3.3) | 10 (16.6) | 11 (18.3) | 12 (20) | 23 (38.3) |
| Steele [7] | 2006 | The third-generation NSAIs |
Exemestane (25 mg) | 108 | 6 (5.6) | 50 (46.3) | |||
| Carlini et al. [8] | 2007 | Anastrozole (1 mg) Letrozole (2.5 mg) |
Exemestane (25 mg) | 30 | 0 | 0 | 14 (46.6) | 0 | 14 (46.6) |
| Chin et al. [9] | 2007 | Anastrozole (1 mg) Letrozole (2.5 mg) |
Exemestane (25 mg) | 31 | 2 (6.5) | 4 (12.9) | 11 (35.5) | 6 (19.4) | 17 (54.8) |
| Chia et al. [10] | 2008 | NSAIs | 342 | 23 (6.7) | 108 (31.6) | ||||
| Lim et al. [11] | 2012 | Anastrozole (1 mg) Letrozole (2.5 mg) |
Exemestane (25 mg) | 88 | 0 | 0 | 27 (30.7) | 0 | 27 (30.7) |
| Lee et al. [12] | 2013 | The third-generation NSAIs |
Exemestane (25 mg) | 110 | 2 (1.8) | 5 (4.5) | 44 (39.2) | 7 (6.4) | 51 (46.4) |
Objective responses include both complete and partial responses.
Clinical responses include complete and partial responses and stable disease. AI – aromatase inhibitor; NSAI – non-steroidal aromatase inhibitors; SAI – steroidal aromatase inhibitor.
As evidenced by the duration of the CR, our patient’s cancer retained non-cross-resistance between the prior NSAI and the SAI for 6 years. We presume that the patient’s small, non-life-threatening metastases contributed to the success of her second-line hormonal therapy. In addition, Lee et al. [12] reported that patients with asymptomatic viscera-dominant disease have better outcomes following exemestane treatment than those with non-visceral disease; indeed, hormonal therapy with exemestane was effective for asymptomatic visceral metastases in our patient.
One limitation of this case report is that the lung tumors were diagnosed as metastases on the basis of CT, not histological, findings. The lung tumors were too small for needle biopsy or detection by positron emission tomography scan. We believe that the disappearance of the lesions after institution of exemestane therapy and the absence of evidence of inflammation or other pulmonary neoplasms support our contention that they were indeed metastases from this patient’s breast cancer.
Conclusions
We present here a case in which long-term CR of NSAI-tolerant metastatic breast cancer was achieved by switching to the SAI exemestane. We consider that, provided the patient’s condition is not life-threatening and the tumor burden is small, switching from an NSAI to this SAI is an appropriate option for treating metastatic breast cancer. Further investigation is needed to identify the predictors for achieving long-term remission by switching AIs.
References:
- 1.Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. J Clin Oncol. 1996;14:2197–205. doi: 10.1200/JCO.1996.14.8.2197. [DOI] [PubMed] [Google Scholar]
- 2.Mauri D, Pavlidis N, Polyzos NP, Ioannidis JP. Survival with aromatase inhibitors and inactivators versus standard hormonal therapy in advanced breast cancer: meta-analysis. J Natl Cancer Inst. 2006;98:1285–91. doi: 10.1093/jnci/djj357. [DOI] [PubMed] [Google Scholar]
- 3.Lønning PE, Bajetta E, Murray R, et al. Activity of exemestane in metastatic breast cancer after failure of nonsteroidal aromatase inhibitors: a phase II trial. J Clin Oncol. 2000;18:2234–44. doi: 10.1200/JCO.2000.18.11.2234. [DOI] [PubMed] [Google Scholar]
- 4.Bertelli G, Garrone O, Merlano M, et al. Sequential treatment with exemestane and non-steroidal aromatase inhibitors in advanced breast cancer. Oncology. 2005;69:471–77. doi: 10.1159/000090985. [DOI] [PubMed] [Google Scholar]
- 5.Iaffaioli RV, Formato R, Tortoriello A, et al. Phase II study of sequential hormonal therapy with anastrozole/exemestane in advanced and metastatic breast cancer. Br J Cancer. 2005;92:1621–25. doi: 10.1038/sj.bjc.6602579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gennatas C, Michalaki V, Carvounis E, et al. Third-line hormonal treatment with exemestane in postmenopausal patients with advanced breast cancer progressing on letrozole or anastrozole: a phase II trial conducted by the Hellenic Group of Oncology (HELGO) Tumori. 2006;92:13–17. doi: 10.1177/030089160609200103. [DOI] [PubMed] [Google Scholar]
- 7.Steele N, Zekri J, Coleman R, et al. Exemestane in metastatic breast cancer: effective therapy after third-generation non-steroidal aromatase inhibitor failure. Breast. 2006;15:430–36. doi: 10.1016/j.breast.2005.08.032. [DOI] [PubMed] [Google Scholar]
- 8.Carlini P, Michelotti A, Ferretti G, et al. Clinical evaluation of the use of exemestane as further hormonal therapy after nonsteroidal aromatase inhibitors in postmenopausal metastatic breast cancer patients. Cancer Invest. 2007;25:102–5. doi: 10.1080/07357900701224789. [DOI] [PubMed] [Google Scholar]
- 9.Chin YS, Beresford MJ, Ravichandran D, Makris A. Exemestane after nonsteroidal aromatase inhibitors for post-menopausal women with advanced breast cancer. Breast. 2007;16:436–39. doi: 10.1016/j.breast.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70. doi: 10.1200/JCO.2007.13.5822. [DOI] [PubMed] [Google Scholar]
- 11.Kim SH, Park IH, Lee H, et al. Efficacy of exemestane after nonsteroidal aromatase inhibitor use in metastatic breast cancer patients. Asian Pac J Cancer Prev. 2012;13:979–83. doi: 10.7314/apjcp.2012.13.3.979. [DOI] [PubMed] [Google Scholar]
- 12.Lee JK, Im SA, Lee D, et al. Efficacy of exemestane in korean patients with metastatic breast cancer after failure of nonsteroidal aromatase inhibitors. J Breast Cancer. 2013;16:66–71. doi: 10.4048/jbc.2013.16.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lønning PE, Geisler J. Experience with exemestane in the treatment of early and advanced breast cancer. Expert Opin Drug Metab Toxicol. 2008;4:987–97. doi: 10.1517/17425255.4.7.987. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–29. doi: 10.1056/NEJMoa1109653. [DOI] [PMC free article] [PubMed] [Google Scholar]