Summary
Romantic relationships can have a profound effect on adults’ health and well-being whereas the inability to maintain intimate bonds has been associated with physical and emotional distress. Studies in monogamous mammalian species underscore the central role of oxytocin (OT) in pair-bonding and human imaging studies implicate OT-rich brain areas in early romantic love. To assess the role of OT in romantic attachment, we examined plasma OT in 163 young adults: 120 new lovers (60 couples) three months after the initiation of their romantic relationship and 43 non-attached singles. Twenty-five of the 36 couples who stayed together were seen again six months later. Couples were observed in dyadic interactions and were each interviewed regarding relationship-related thoughts and behaviors. OT was significantly higher in new lovers compared to singles, F(1, 152) = 109.33, p < .001, which may suggest increased activity of the oxytocinergic system during the early stages of romantic attachment. These high levels of OT among new lovers did not decrease six months later and showed high individual stability. OT correlated with the couples’ interactive reciprocity, including social focus, positive affect, affectionate touch, and synchronized dyadic states, and with anxieties and worries regarding the partner and the relationship, findings which parallel those described for parent–infant bonding. OT levels at the first assessment differentiated couples who stayed together six months later from those who separated during this period. Regression analysis showed that OT predicted interactive reciprocity independent of sex, relationship duration, and the partner’s OT. Findings suggest that OT may play an important role at the first stages of romantic attachment and lend support to evolutionary models suggesting that parental and romantic attachment share underlying bio-behavioral mechanisms.
Keywords: Oxytocin, Romantic attachment, Pair bonding, Reciprocity
Introduction
Romantic relationships have a profound effect on adult life. Happy and stable intimate bonds have been associated with physical and psychological health (Burman and Margolin, 1992), whereas the inability to establish or maintain close relationships is linked with physical and emotional distress (Bloom et al., 1978; Simon and Marcussen, 1999). Yet, neuroedocrine processes supporting the formation of human romantic attachment and their relations with the couple’s behavioral repertoire has received little empirical attention.
The formation of romantic attachment in humans is a developmental process involving changes in the relationship over time, for instance, a gradual increase in the degree of closeness between partners as the relationship progresses. As such, it is likely that this process would be accompanied by alterations in brain activity and neurohormonal processes. Indeed, evidence suggests that romantic love represents a dynamic process and each stage is marked by distinct neural and endocrine features. In particular, the initial stage of a romantic relationship has been associated with greater neural activity in the left posterior cingulate cortex and caudate regions (Aron et al., 2005; Bartels and Zeki, 2000; Kim et al., 2009) as compared to later stages of the relationship. On the other hand, activations of cortical regions including the anterior cingulate, insular cortex, and ventral pallidum were increased in long-term pair-bonds (Aron et al., 2005; Bartels and Zeki, 2000; Kim et al., 2009). Interestingly, brain regions implicated in parental–infant attachment were also found to be activated in prolonged romantic relationships as assessed by both fMRI (Acevedo et al., 2011) and ERP (Weisman et al., 2011) measures, suggesting that the formation of parental and romantic attachment share underlying mechanisms (Feldman, in press). In addition to differences, studies have also described substantial overlap in the neural activations implicated in the different stages of a romantic relationship. Both the initial period of romantic love and long-term relationships are associated with neural activity in dopamine-rich reward and basal ganglia systems, such as the ventral tegmental area (VTA) and dorsal striatum (Acevedo et al., 2011; Aron et al., 2005). These studies point to the centrality of reward-related systems for the development of romantic attachment and may suggest that elements within the relationship that are associated with reward, such as warm touch or affective matching, may be related to individual variability in physiological systems that undergo reorganization during the initial stages of romantic love.
Brain areas supporting the formation romantic attachment are those rich in oxytocin (OT) receptors (Acevedo et al., 2011), underscoring the potential role of OT in romantic bonding. OT is a nonapeptide hormone associated with affiliative bonding in mammals (Insel et al., 1997) that is known to mediate social behavior, pair-bonding, and parental attachment across a variety of species (Carter, 1998). Specifically, OT has been shown to play a critical role in the regulation of pair-bond formation in monogamous mammals (Ross and Young, 2009). In humans, intranasal administration of OT was found to increase bonding-related behavior, including gaze to eye region (Guastella et al., 2008), interpersonal trust and empathy (Hurlemann et al., 2010; Kosfeld et al., 2005), and social cognition (Kirsch et al., 2005). OT has also been shown to play a role in human parenting and peripheral levels of OT have been associated with reciprocal parent–infant interactions in both mothers and fathers (Feldman et al., 2010). In addition, research has pointed to the central role of transmembrane glycoprotein CD38 in OT neuropathways and in the release of OT from axon terminalis (Jin et al., 2007), and recent findings showed associations between plasma OT, genetic variability in the CD38 rs3796863 SNP, and the degree of reciprocity and touch between parents and their infants (Feldman et al., in press), pointing to the potential involvement of OT in the development of reciprocal interactions between attachment partners.
Human studies lend support to the involvement of OT in romantic attachment. Acevedo et al. (2011) found higher activations among romantically attached individuals in regions implicated in pair-bonding in monogamous rodents, and OT administration has shown to increase couples’ positive communication (Ditzen et al., 2009). However, findings regarding peripheral OT are mixed. Whereas some found links between plasma OT and positive communication, affiliation, and emotional support (Gonzaga et al., 2006; Grewen et al., 2005; Holt-Lunstad et al., 2008), others showed associations with negative emotions, anxiety, and distress in romantic couples (Marazziti et al., 2006; Tabak et al., 2010; Taylor et al., 2010). In addition, research has shown that the early stages of romantic love may be linked to alterations in plasma levels of cortisol, sex steroids, nerve growth factor (NGF), and brain derived neurotrophic factor (BDNF) (Marazziti et al., 2009), as well as decreased density of the serotonin transporter in platelets (Emanuele et al., 2006; Marazziti et al., 1999; Marazziti and Canale, 2004), suggesting that the initial period of romantic attachment is linked with neuroendocrine changes. Similarly, warm contact between romantic partners has been associated with elevations in a subset of serum proteins (Matsunaga et al., 2009). However, we are aware of no study that examined peripheral levels of OT across the initial period of romantic love.
In light of the above, the present study sought to extend research on the role of OT in human romantic attachment, particularly the expression of OT across the dynamic period of pair-bonding. We focused on the initial stages of romantic attachment and assessed new romantic partners during the period of falling in love and six months later in comparison to romantically unattached singles. Plasma OT, the couple’s interactive behavior, and the partners’ mental representations of the relationship were assessed using hormonal, observational, and interview methodologies. Specifically, we examined whether (a) plasma OT levels in new lovers would be higher than those observed in singles and would be stable across the period of pair-bonding in humans, and (b) OT levels would be associated with the couple’s reciprocal behavior, similar to the findings reported for parent–infant interactions (Gordon et al., 2010a). Consistent with animal research, our overall hypothesis was that OT is essential for bond formation and we expected higher levels of the hormone among new lovers as compared to singles. We also expected to find a correlation between peripheral OT and the degree of reciprocity between partners, consistent with the perspective that periods of parental and romantic bond formation share underlying bio-behavioral mechanisms (Feldman, in press) and with research linking OT and reciprocal interactions between parents and infants (Feldman et al., 2011; Gordon et al., 2010b).
Methods and materials
Participants
One hundred and sixty-three individuals participated in the study in two groups. The “new lovers” group included 120 young heterosexual adults (60 couples) who began their romantic relationship on average 2.4 months prior to their entry into the study (relationship duration range = 2 weeks to 4 months). Men were on average 25.03 years (SD = 8.78) and women’s age averaged 22.84 (SD = 4.50). Of the 54 couples we were able to contact six month after the first assessment, thirty-six were still together (66.6%) and 18 couples had split-up (six couples could not be reached). Exclusion criteria included individuals who did not complete high school education (12 years of schooling), were above 35 years, were taking medication for a physical or psychiatric condition, or reported not being generally healthy.
The “singles” group included 43 young adults who were not involved in any kind of a romantic relationship and did not separate from a former romantic partner within the past three months (mean age = 24.63 years, SD = 3.16 years). Of the couples who stayed together, 25 were seen again six months after the first assessment. Second assessment of OT for new lovers who were no longer in a romantic relationship was not conducted because of substantial variability between separated couples that would have likely impacted the findings. Some of the separated couples had split immediately after the first experiment, whereas others separated a few months later, just prior to our call for the next visit. Moreover, a few of the separated lovers were already in a new romantic relationship at the time of second OT assessment. No differences in demographic conditions were found between new lovers and singles, between new lovers who did or did not stay together, or between those who did or did not return for the follow-up assessment.
Procedure
Participants were recruited by advertisements posted in a university campus and surrounding area. Experiments were conducted in a comfortable laboratory during the mid-afternoon hours, in light of previous research suggesting stability in the diurnal cycle of plasma OT in the afternoon and evening hours (Forsling et al., 1998). After receiving a brief explanation of the experimental procedure and signing an informed consent, participants completed self-report measures that assessed a range of demographic and health variables (e.g., weight, height, smoking, medication, time since last meal prior the experiment, and contraceptives). Next, blood was drawn by a nurse from the antecubital vein of participants into a 9 mL chilled vacutainer tubes containing lithium heparin that were supplemented with 400 KIU of Trasylol (Bayer, Germany) per 1 mL blood. Blood samples were kept ice-chilled for up to 2 h before being centrifuged at 4 °C at 1000 × g for 15 min. Supernatants were collected and stored at −70 °C until assayed. For new lovers, but not for singles, visits included an interview and videotaped interactions following the blood draw. Each partner was interviewed independently (counterbalanced for sex) using the Romantic Couples Interview (Feldman and Schneiderman, 2006) an interview lasting approximately 30 min that taps typical thoughts, worries, behaviors, hopes, and plans of new lovers. Interviews were audiotaped, transcribed verbatim, and were coded offline by blind coders. Following the individual interviews, couples were re-united for a videotaped interaction that included the couple discussing a shared positive experience for approximately 5 min. Interactions were coded offline using the Coding Interactive Behavior Manual (CIB) (Feldman, 1998), a well-validated system for coding dyadic interactions, by individuals blind to other information. Each single participant received a gift of about 13 USD and each new lover received 35 USD for participation. The research was approved by the University Institutional Review Board and all participants signed an informed consent.
Oxytocin assay
Determination of OT concentrations in plasma was performed using a commercial OT enzyme-linked immunosorbent assay (ELISA) kit (Assay Design, Ann Arbor, MI) consistent with previous research (Carter et al., 2007; Feldman et al., 2007; Gordon et al., 2008). Samples were diluted 1–5 in assay buffer, measurements were performed in duplicate, and the concentrations of samples were calculated by using MATLAB software (The MathWorks, Inc., Natick, Massachusetts) according to relevant standard curves (ranges from 15.6 pM to 1000 pM). The limit of detection is 11.5 pM. The intra-assay coefficients are 5.4% for the medium-high range and 12.4% for low range. The inter-assay coefficients were 8.7% for the medium–high range and 14.5%, for low range. The biochemist conducting the analysis was blind to any information related to relational status (single, in relationship), sex, or behavioral and interview data.
Behavioral measures
Interactive reciprocity
Interactions were coded with the Coding Interactive Behavioral Manual (CIB) (Feldman, 1998), adult version. The CIB is a global rating system of dyadic interactions with versions for newborns, infants, children, adolescents, and adults. The CIB has good psychometric properties and has shown sensitivity to differences related to child age, interactive partner, cultural background, and risk conditions, and has been validated in numerous studies (Feldman, 2010; Feldman and Eidelman, 2009; Feldman and Klein, 2003; Feldman et al., 2001).
The adult–adult version of the CIB included 33 codes for partners: 28 identical codes for each partner and 5 for the dyad as a whole rated on a Likert scale of 1 = low to 5 = high. Consistent with the constructs used by the CIB system, the “Interactive Reciprocity” construct for each partner was computed by averaging following codes: positive affect, interpersonal focus, affectionate touch, dyadic reciprocity, and matching of emotional state (α = .60). The Interactive Reciprocity construct of the CIB has been extensively used in research spanning infancy to adolescence and has shown to be stable over the first 13 years of life, to predict adjustment in adolescence, and to be sensitive to cultural background and high-risk parenting (Feldman, 2010; Feldman and Masalha, 2010; Feldman et al., 2003). In infancy, early childhood, and adolescence, the Interactive Reciprocity construct includes the following codes: give-and-receive reciprocity, adaptation-affective matching, gaze synchrony, and positive-fluent affect. In the current study, we also included affectionate touch in the construct of reciprocity, based on the assumption that touch is a central and dyadic component of romantic relationships, whereas in the parent–child relationship it is a central but parental component of the exchange. Similarly, in adult–adult interaction “interpersonal focus” was considered the age-matched parallel to the code of “gaze synchrony” in infancy. Coding was conducted by coders trained to reliability and inter-rated reliability, conducted on 10% of the sample, exceeded 85% on all codes. Coders were blind to all other information.
Interview measures
Worries
Couples were interviewed on their positive and negative thoughts with regards to the partner and the relationship, worries, and typical behaviors that accompanied the formation of romantic attachment using the Romantic Couple Interview (Feldman and Schneiderman, 2006). The instrument was developed for the current study on the basis of past research on parental bonding (Feldman et al., 1999; Leckman et al., 1999). The Romantic Couple Interview includes 17 topics that are elicited during the interview and are coded offline on a scale of 1–5. Interviews were audiotaped and were then coded by two trained coders, with inter-rated reliability on 10% of the sample exceeding 90% on all topics. Coders did not participate in conducting the interviews and were blind to all other hormonal, behavioral, self-report and demographic information. The Worries score, used in this study, was the average of two topics – amount of thoughts regarding the partner and the romantic relationships and the level of anxiety in the relationships. Consistent with perspectives suggesting that periods of parental and romantic bonding share underlying mechanisms, this factor is similar to the one used in parenting research, describes a unique mental state in parents during the postpartum period (Feldman et al., 1999; Leckman et al., 1999), and has shown to correlate with parental OT (Feldman et al., 2007).
Attachment style
In order to evaluate whether the individual’s attachment style was related to OT levels, subjects completed the Experience in Close Relationships scale (ECR), a well-known measure of attachment in adults (Brennan et al., 1998).
Statistical analysis
Differences in OT levels between singles and new lovers were examined with Analysis of Variance (ANOVA). Stability and change in mean levels of OT between the first and second assessments among new lovers was examined with Repeated Measures ANOVA. Pearson’s correlations examined individual stability in couples’ OT and relations between OT, behavior, and interview measures. Hierarchical multiple regression was used to predict OT levels from behavior and interview measures. All p-values were two-tailed. Data were analyzed using SPSS software for Windows, version 18 (SPSS Inc., Chicago, IL, USA).
Results
Plasma OT levels of singles and new lovers in the first and second assessments are presented in Table 1. These levels are similar to those reported in previous research involving adults using ELISA methodology (Feldman et al., 2007; Gordon et al., 2010b; Levine et al., 2007). Plasma OT values at the first assessment, D(52) = .26, p < .05, as well as at the follow up, D(52) = .17, p < .05, were non-normal. Therefore, prior to statistical analysis OT values were log-transformed to correct for their non-Gaussian distribution.
Table 1.
Plasma OT Concentrations in Singles and New Lovers at the Initial Stage of Romantic Attachment and Six Months Later.
| OT Levels (pg/mL) | Means (SD) (pg/mL) | Minimum (pg/mL) | Maximum (pg/mL) | N |
|---|---|---|---|---|
| Singles Women | 263.76 (240.68) | 114.10 | 1196.20 | 23 |
| Single Men | 250.98 (245.10) | 124.30 | 1255.03 | 20 |
| New Lovers-Women (time 1) | 509.83 (228.67) | 180.36 | 1620.00 | 53 |
| New Lovers-Men (time 1 | 480.76 (211.58) | 219.28 | 1331.74 | 60 |
| New Lovers-Women (time 2) | 469.69 (210.94) | 81.46 | 1046.09 | 21 |
| New Lovers-Men (time 2) | 505.30 (296.29) | 218.12 | 1413.48 | 25 |
Pearson correlations indicated that OT was not associated with sex, body weight, height, smoking, contraceptive pills (58% of women were taking contraceptive pills), time since last meal, or sexual activity of the subject. Period of the menstrual cycle in those women who did not take contraceptive pills was not controlled in the study, but no correlation was found between number of days since the last menstruation of these women and their OT levels.
To examine whether new lovers differ from singles in plasma OT levels, we computed a two-way Univariate ANOVA with group (singles/new lovers) and sex as the between-subject factors and OT values at the first assessment as the dependent variable. Results indicated a significant group effect, F(1, 152) = 109.33, p < .001, partial η2 = .42, with substantially higher levels of plasma OT among new lovers compared to non-attached singles (Fig. 1).
Fig. 1.
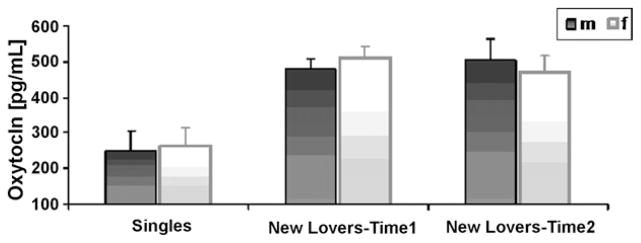
Plasma OT Levels in Singles and New Lovers at the First and the Second Assessments.
To examine individual stability in OT levels, paired-samples t-test was conducted to compare OT levels in the first and second assessments. No change over time in OT levels was found, t(43) = .91, p = .37 (Fig. 1). In addition, Pearson’s correlations were computed between the first and the second assessments. OT levels were highly stable over time, r = .85, p < .000. Individual stability was similar for women (r = .84, p < .000) and men (r = .84, p < .000).
In order to evaluate whether new lovers who were still together six months after the first assessment had different levels of plasma OT levels already at the first assessment as compared to new lovers who had separated during this period, a two-way ANOVA was computed with two independent variables, sex, and relationship duration (couples who stayed together vs. couples who were separated six months later), and OT values at first assessment as the dependent variable. A significant effect of relationship duration was found, F(1, 97) = 4.67, p < .05, partial η2 = .046. In addition, a logistic regression was conducted to assess whether OT levels at the initial period of falling in love can predict whether the couple will remain together six months later. Results revealed that OT levels at the first assessment were modestly predictive of the couple’s relationship status six months later, χ2(1) = 4.64, p < .05, Nagelkerke R2 = 0.06 (Table 2, Fig. 2).
Table 2.
Predicting New Lovers’ Relationship Status at the Second Assessment by their OT Levels in the Initial Stage of Romantic Attachment.
| 95% Cl for exp b
|
||||
|---|---|---|---|---|
| B (SE) | Lower | exp b | Upper Included | |
| Constant | −7.74 (4.20) | |||
| Log OT | 3.21* (1.59) | 1.10 | 24.80 | 561.94 |
R2 = .07 (Hosmer & Lemeshow), .05 (Cox & Snell), .06 (Nagelkerke). Model X2 (1) = 4.64.
p < .05.
p < .01.
p < .001.
Fig. 2.
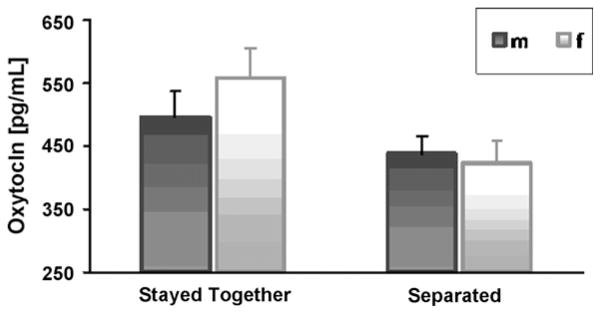
Plasma OT Levels at the First Assessment among Couples who Stayed Together or Separated Six Months Later.
To examine the relations between hormones, behavior, interview, and self-report measures, Pearson’s correlations were conducted. Couples who exhibited higher level of Interactive Reciprocity had higher plasma OT levels at the first (r = .29, p < .01) assessment (Fig. 3). Similarly, couples whose narrative was marked by greater Worry, indicating more time spent in thoughts of the partner and the relationship, had higher OT at the initial assessment (r = .19, p < .05). No associations between OT and self-reported attachment anxiety or attachment avoidance was found (ranxiety = .10, p = .35; ravoidance = −.07, p = .51).
Fig. 3.
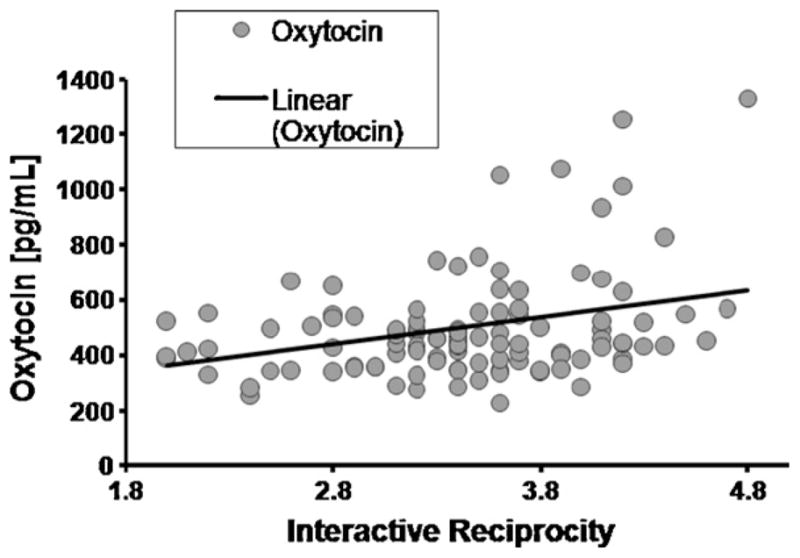
Correlation between New Lovers’ Plasma OT Levels at the First Assessment and their Reciprocity Score.
A multiple regression model predicting OT levels from the partners’ behavior and representations was computed. Predictors were entered in three steps. In the first step, sex and age were entered to control for demographic factors. In the second step, the individual’s Worry score was entered and in the third, the couple’s Interactive Reciprocity score. Results indicate that both the positive dyadic behavioral measure of the relationship and the anxious mental aspect of pair-bonding were each independently predictive of new lovers’ OT levels at the initial period of a romantic attachment (Table 3).
Table 3.
Predicting OT at the Initial Stage of Romantic Attachment.
| Beta | R2 change | F change | |
|---|---|---|---|
| Sex | −.07 | ||
| Age | .00 | .01 | .27 |
| Worries | .20* | .04 | 4.03* |
| Interactive Reciprocity | .26** | .07 | 7.67** |
R2 Total = .11, F(4, 107) = 3.14, p < .05.
p < .05.
p < .01.
In order to examine potential independence in the partners’ OT values, Pearson’s correlation between OT levels of romantic partners was conducted. No association between partners’ hormonal levels were found in the first (r = .06, p = .70) or second assessments (r = .01, p = .98). Finally, a multiple regression model was computed to predict Interactive Reciprocity at the early stages of romantic attachment from the individual’s OT levels while controlling for the potential confounding effects of the partner’s OT. Predictors were entered in four steps. In the first two steps sex and relationship duration (whether the couple did or did not stay together) were entered to control for variability associated with these factors. In the third step, the partner’s OT was entered, and in the final step, the individual’s OT was entered to examine its contribution to the prediction of the person’s reciprocal style independent of other factors in the model. Results show that the partner’s OT was marginally predictive of the reciprocal style of the partner; however, the individual’s OT level had an independent contribution to the prediction of his or her Interactive Reciprocity above and beyond the partner’s OT (Table 4).
Table 4.
Predicting New Lovers Interactive Reciprocity at the Initial Stage of Romantic Attachment.
| Criterion: Interactive Reciprocity
| |||
|---|---|---|---|
| Beta | R2 Change | F Change | |
| Sex | −.00 | .00 | .02 |
| Relationship durationa | −.01 | .00 | .24 |
| Partner’s OT | .20* | .03 | 2.38 |
| Individual’s OT | .33** | .10 | 10.31 |
R2 Total = .13, F(4, 91) = 3.31, p < .05.
Stayed together or not.
p < .05.
p < .01.
Discussion
Although much research has focused on the neurobiological basis of bond formation, studies have mainly addressed the maternal–infant bond (Gordon et al., 2011). Significantly less is known about one of the most important forms of social affiliation in adult life – romantic attachment – and little research examined circulating OT levels during the initial period of falling in love. Yet, romantic relationships have a profound effect on adult life. Dysfunctional romantic relationships and unreciprocated love have been associated with substantial psychological distress and suicidal behavior (Canetto and Lester, 2002; Emanuele, 2009). Indeed, love themes were significantly more common than other problems mentioned in suicide notes among both men and women (Canetto and Lester, 2002), underscoring the need to further explore the neurobiology of romantic attachment. In this study, we examined the role of OT during the initial stages of pair-bonding in humans. To our knowledge, this is the first longitudinal study to investigate romantic attachment from a developmental biochemical perspective.
As predicted, new lovers had substantially higher plasma levels of OT as compared to non-attached singles. These high hormonal levels were individually stable and showed no decrease during the first nine months of successful pair-bonding. However, since we did not examine the same individuals prior to falling in love and again at the initial stages of romantic attachment, it is not possible to fully determine whether OT levels increased in response to the onset of a romantic relationship or whether OT represents a trait-like dimension of the individual and individuals with higher OT are more likely to fall in love. Yet, high levels of OT at the beginning of a romantic relationship suggest the involvement of this hormone in processes of partner attachment in humans. These findings are consistent with those reported for other mammals, particularly monogamous rodent species in which OT has shown to play a critical role in the formation of pair-bonds (Cho et al., 1999; Insel and Hulihan, 1995; Williams et al., 1994).
Couples who stayed together showed higher OT levels at the initial period of romantic attachment. These findings suggest that OT in the first months of romantic love may serve as an index of relationship duration. OT is known to function as a bio-behavioral feedback loop and research in mammals showed that more touch and contact increased OT receptor density (Champagne et al., 2001). It is possible that the link between OT and the couple’s Interactive Reciprocity, a behavioral construct which includes affectionate touch, points to a similar bio-behavioral feedback loop whereby higher levels of reciprocity and touch increased the couple’s involvement in the relationship at the physiological, behavioral, and representational levels.
The current findings lend some support to evolutionary models suggesting that parental and romantic attachment share some underlying mechanisms and are mediated by the oxytocinergic system (Carter, 1998; Feldman, in press). In a parallel study conducted at our lab on parents and their firstborn infant during the first month of parenting and six month later (Gordon et al., 2010a), we similarly found that at the transition to parenthood, mothers’ and fathers’ OT levels were higher as compared to those observed in non-attached singles. However, in comparing the current sample of new lovers with the sample of new parents, OT levels at the initial stages of romantic attachment were significantly higher in new lovers than those observed in new parents, indicating that the initial period of romantic love may induce the most intense activity of the oxytocinergic system, but such hypothesis requires further research and a longitudinal experimental design for conclusive findings. It is possible that the very high OT levels observed during the initiation of romantic love may somewhat attenuate by the time couples become parents but do not drop to the level of non-attached singles and following individuals from the stage of romantic love to the stage of early parenting may answer this question. Overall, three key features were similar in new parents and new lovers. First, OT levels did not differ between men and women and showed high individual stability over the course of a six-month period, suggesting that baseline OT may represent a trait-like dimension of the individual. Second, OT levels did not decrease during the first half-year of bonding in both parental and romantic attachment. Finally, OT was related to specific bonding-related behaviors and to preoccupations and worries in both parents and romantic partners.
Consistent with the parental bonding literature, associations were found between OT levels and the couple’s reciprocal interactive behavior and mental preoccupations and worries. Both the early stages of romantic love and early parental love are characterized by typical behaviors, mental states, and activations of specific brain structures (Atzil et al., 2011; Bartels and Zeki, 2004; Leckman et al., 1999; van Anders et al., 2011). As seen, interactive behaviors such as affectionate touch, interpersonal focus, and matched dyadic states, which are central for the formation of parent–infant synchrony in both mothers and fathers (Feldman and Eidelman, 2007) and are associated with parental OT (Feldman et al., 2011), were central components of the reciprocity formed between romantic partners and similarly correlated with OT. In addition, the early stages of parenting has shown to be accompanied by heightened sensitivity to infant cues and mental preoccupations and worries, which similarly correlate with OT (Feldman et al., 2007) and are essential for the formation of the parent’s bond to the infant (Feldman et al., 1999; Leckman et al., 1999). The current findings may suggest that preoccupations with the romantic partner and the relationship are just as essential for the formation of the selective and enduring romantic bond.
Limitations of the study are important to consider in the interpretation of the findings. First, due to obvious constraints in human research, we did not measure OT at the neurochemical brain level. Because the links between central and peripheral OT are not fully understood, it is not clear whether plasma levels reflect central activity of the system, although studies in both humans and animals point to coordination between central and peripheral levels (Burri et al., 2008; Wotjak et al., 1998). Overall, our findings provide evidence for the central role of OT in romantic attachment, but much further research is required to assess the involvement of OT in human pair bonding at the brain and genetic levels in healthy and pathological forms of love. Second, it is important to note that the results do not imply a causal relationship between the beginning of a romantic relationship and higher OT values and it is possible that individuals with high OT also form romantic attachments more readily. Third, although increasing literature suggests that arginine vasopressin is involved in pair bonding along with OT (Donaldson et al., 2010), the current study did not measure vasopressin. Such assessment would be highly informative and we hope to address this hormone in future research. Another limitation of the study is the absence of information about sexual activity and sexual orientation of the participants. Although we know that lovers were heterosexual, we did not collect this information from singles. Previous research reported an increase in plasma OT levels during sexual arousal and orgasm in both men and women (Carmichael et al., 1987, 1994). In the present study we did not find differences in plasma OT levels between new lovers who were involved in sexual activity and those who were not. It is possible that the increase in plasma OT during sexual activity is time-limited, as is the case for nursing (White-Traut et al., 2009), and does not affect baseline OT as it was measured in the present study. In addition, the ELISA OT assay that had been conducted in the study had relatively high intra- and interassay CVs. Finally, we did not measure genetic variability in the OT receptor gene, the CD38 gene, or allelic variations that may impact physiological and behavioral processes at the initiation of romantic love.
Understanding romantic attachment in humans from a biological perspective may help shed further light on the normative as well as pathological processes of romantic attachment, provide further directions for research on the psychological distress that follows romantic failure, and offer more concrete interventions. Future studies are needed to integrate brain, hormonal, and behavioral analysis to further assess the formation of romantic bonds and specify the neurological substrates implicated in human pair bonding. Future research should also address the involvement of other biological agents important to processes of romantic attachment, such as arginine-vasopressin, CD38, and sex hormones, in typical and pathological love, and it is important to conduct research to assess the clinical utility of OT in cases of high romantic stress (Emanuele et al., 2010). Similarly, since OT administration has shown to improve interactions between couples (Ditzen et al., 2009), it is possible that OT-based treatments may improve specific relational components among couples in distress. Finally, specifying the biobehavioral processes that underpin the formation of romantic bonds may illuminate the contribution of such relationships to the individual’s well-being, health, longevity, and personal growth.
Acknowledgments
Role of the funding sources
The US-Israeli Bi-National Science Foundation supported the specific study.
The NARSAD foundation, ISF, and Katz family foundation supported research at Prof. Feldman laboratory during the study period.
The study was supported by the US-Israel Bi-National Foundation (2005-273), by the NARSAD independent investigator award to R.F., by the Israel Science Foundation (#1318), and by the Katz Family Fund.
Footnotes
Conflict of interest
The authors have no conflict of interest to declare.
References
- Acevedo BP, Aron A, Fisher HE, Brown LL. Neural correlates of long-term intense romantic love. Soc Cogn Affect Neurosci. 2011 doi: 10.1093/scan/nsq092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aron A, Fisher H, Mashek DJ, Strong G, Li H, Brown LL. Reward, motivation, and emotion systems associated with early-stage intense romantic love. J Neurophysiol. 2005;94:327–337. doi: 10.1152/jn.00838.2004. [DOI] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R. Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology. 2011;36:2603–2615. doi: 10.1038/npp.2011.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural basis of romantic love. Neuroreport. 2000;11:3829–3834. doi: 10.1097/00001756-200011270-00046. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. Neuroimage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Bloom BL, Asher SJ, White SW. Marital disruption as a stressor: a review and analysis. Psychol Bull. 1978;85:867–894. [PubMed] [Google Scholar]
- Brennan KA, Clark CL, Shaver PR. Self-report measurement of adult attachment: An integrative overview. In: Simpson JA, Rholes WS, editors. Attachment theory and close relationships. Guilford Press; New York: 1998. pp. 46–76. [Google Scholar]
- Burman B, Margolin G. Analysis of the association between marital relationships and health problems: an interactional perspective. Psychol Bull. 1992;112:39–63. doi: 10.1037/0033-2909.112.1.39. [DOI] [PubMed] [Google Scholar]
- Burri A, Heinrichs M, Schedlowski M, Kruger TH. The acute effects of intranasal oxytocin administration on endocrine and sexual function in males. Psychoneuroendocrinology. 2008;33:591–600. doi: 10.1016/j.psyneuen.2008.01.014. [DOI] [PubMed] [Google Scholar]
- Canetto SS, Lester D. Love and achievement motives in women’s and men’s suicide notes. J Psychol. 2002;136:573–576. doi: 10.1080/00223980209605552. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Humbert R, Dixen J, Palmisano G, Greenleaf W, Davidson JM. Plasma oxytocin increases in the human sexual response. J Clin Endocrinol Metab. 1987;64:27–31. doi: 10.1210/jcem-64-1-27. [DOI] [PubMed] [Google Scholar]
- Carmichael MS, Warburton VL, Dixen J, Davidson JM. Relationships among cardiovascular, muscular, and oxytocin responses during human sexual activity. Arch Sex Behav. 1994;23:59–79. doi: 10.1007/BF01541618. [DOI] [PubMed] [Google Scholar]
- Carter CS. Neuroendocrine perspectives on social attachment and love. Psychoneuroendocrinology. 1998;23:779–818. doi: 10.1016/s0306-4530(98)00055-9. [DOI] [PubMed] [Google Scholar]
- Carter CS, Pournajafi-Nazarloo H, Kramer KM, Ziegler TE, White-Traut R, Bello D, Schwertz D. Oxytocin: behavioral associations and potential as a salivary biomarker. Ann NY Acad Sci. 2007;1098:312–322. doi: 10.1196/annals.1384.006. [DOI] [PubMed] [Google Scholar]
- Champagne F, Diorio J, Sharma S, Meaney MJ. Naturally occurring variations in maternal behavior in the rat are associated with differences in estrogen-inducible central oxytocin receptors. Proc Natl Acad Sci USA. 2001;98:12736–12741. doi: 10.1073/pnas.221224598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho MM, DeVries AC, Williams JR, Carter CS. The effects of oxytocin and vasopressin on partner preferences in male and female prairie voles (Microtus ochrogaster) Behav Neurosci. 1999;113:1071–1079. doi: 10.1037//0735-7044.113.5.1071. [DOI] [PubMed] [Google Scholar]
- Ditzen B, Schaer M, Gabriel B, Bodenmann G, Ehlert U, Heinrichs M. Intranasal oxytocin increases positive communication and reduces cortisol levels during couple conflict. Biol Psychiatry. 2009;65:728–731. doi: 10.1016/j.biopsych.2008.10.011. [DOI] [PubMed] [Google Scholar]
- Donaldson ZR, Spiegel L, Young LJ. Central vasopressin V1a receptor activation is independently necessary for both partner preference formation and expression in socially monogamous male prairie voles. Behav Neurosci. 2010;124:159–163. doi: 10.1037/a0018094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuele E. Of love and death: the emerging role of romantic disruption in suicidal behavior. Suicide Life Threat Behav. 2009;39:240. doi: 10.1521/suli.2009.39.2.240. [DOI] [PubMed] [Google Scholar]
- Emanuele E, Bertona M, Minoretti P, Geroldi D. An open-label trial of L-5-hydroxytryptophan in subjects with romantic stress. Neuro Endocrinol Lett. 2010;31:663–666. [PubMed] [Google Scholar]
- Emanuele E, Politi P, Bianchi M, Minoretti P, Bertona M, Geroldi D. Raised plasma nerve growth factor levels associated with early-stage romantic love. Psychoneuroendocrinology. 2006;31:288–294. doi: 10.1016/j.psyneuen.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Feldman R. Coding interactive behavior manual. 1998 Unpublished manual. [Google Scholar]
- Feldman R. The relational basis of adolescent adjustment: trajectories of mother–child interactive behaviors from infancy to adolescence shape adolescents’ adaptation. Attach Hum Dev. 2010;12:173–192. doi: 10.1080/14616730903282472. [DOI] [PubMed] [Google Scholar]
- Feldman R. Parent–infant synchrony: a bio-behavioral model of mutual influences in the formation of affiliative bonds. Monogr Soc Res Child Dev. in press. [Google Scholar]
- Feldman R, Eidelman AI. Maternal postpartum behavior and the emergence of infant–mother and infant–father synchrony in preterm and full-term infants: the role of neonatal vagal tone. Dev Psychobiol. 2007;49:290–302. doi: 10.1002/dev.20220. [DOI] [PubMed] [Google Scholar]
- Feldman R, Eidelman AI. Triplets across the first 5 years: the discordant infant at birth remains at developmental risk. Pediatrics. 2009;124:316–323. doi: 10.1542/peds.2008-1510. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- Feldman R, Gordon I, Zagoory-Sharon O. Maternal and paternal plasma, salivary, and urinary oxytocin and parent–infant synchrony: considering stress and affiliation components of human bonding. Dev Sci. 2011;14:752–761. doi: 10.1111/j.1467-7687.2010.01021.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Klein PS. Toddlers’ self-regulated compliance to mothers, caregivers, and fathers: implications for theories of socialization. Dev Psychol. 2003;39:680–692. doi: 10.1037/0012-1649.39.4.680. [DOI] [PubMed] [Google Scholar]
- Feldman R, Masalha S. Parent–child and triadic antecedents of children’s social competence: cultural specificity, shared process. Dev Psychol. 2010;46:455–467. doi: 10.1037/a0017415. [DOI] [PubMed] [Google Scholar]
- Feldman R, Masalha S, Nadam R. Cultural perspective on work and family: dual-earner Israeli-Jewish and Arab families at the transition to parenthood. J Fam Psychol. 2001;15:492–509. doi: 10.1037//0893-3200.15.3.492. [DOI] [PubMed] [Google Scholar]
- Feldman R, Schneiderman . Romantic Couples Interview. Bar-Ilan University; Ramat-Gan, Israe: 2006. Unpublished Manual. [Google Scholar]
- Feldman R, Weller A, Leckman JF, Kuint J, Eidelman AI. The nature of the mother’s tie to her infant: maternal bonding under conditions of proximity, separation, and potential loss. J Child Psychol Psychiatry. 1999;40:929–939. [PubMed] [Google Scholar]
- Feldman R, Weller A, Sirota L, Eidelman AI. Testing a family intervention hypothesis: the contribution of mother–infant skin-to-skin contact (kangaroo care) to family interaction, proximity, and touch. J Fam Psychol. 2003;17:94–107. [PubMed] [Google Scholar]
- Feldman R, Weller A, Zagoory-Sharon O, Levine A. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother–infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Maoz R, Weisman O, Gordon I, Schneiderman I, Shalev I, Ebstein RP. Sensitive parenting is associated with plasma oxytocin and polymorphisms in the oxytocin receptor (OXTR) and CD38 genes. Biol Psychiatry. doi: 10.1016/j.biopsych.2011.12.025. in press. [DOI] [PubMed] [Google Scholar]
- Forsling ML, Montgomery H, Halpin D, Windle RJ, Treacher DF. Daily patterns of secretion of neurohypophysial hormones in man: effect of age. Exp Physiol. 1998;83:409–418. doi: 10.1113/expphysiol.1998.sp004124. [DOI] [PubMed] [Google Scholar]
- Gonzaga GC, Turner RA, Keltner D, Campos B, Altemus M. Romantic love and sexual desire in close relationships. Emotion. 2006;6:163–179. doi: 10.1037/1528-3542.6.2.163. [DOI] [PubMed] [Google Scholar]
- Gordon I, Martin C, Feldman R, Leckman JF. Oxytocin and social motivation. Dev Cogn Neurosci. 2011;1:471–493. doi: 10.1016/j.dcn.2011.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin and the development of parenting in humans. Biol Psychiatry. 2010a;68:377–382. doi: 10.1016/j.biopsych.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin, cortisol, and triadic family interactions. Physiol Behav. 2010b;101:679–684. doi: 10.1016/j.physbeh.2010.08.008. [DOI] [PubMed] [Google Scholar]
- Gordon I, Zagoory-Sharon O, Schneiderman I, Leckman JF, Weller A, Feldman R. Oxytocin and cortisol in romantically unattached young adults: associations with bonding and psychological distress. Psychophysiology. 2008;45:349–352. doi: 10.1111/j.1469-8986.2008.00649.x. [DOI] [PubMed] [Google Scholar]
- Grewen KM, Girdler SS, Amico J, Light KC. Effects of partner support on resting oxytocin, cortisol, norepinephrine, and blood pressure before and after warm partner contact. Psychosom Med. 2005;67:531–538. doi: 10.1097/01.psy.0000170341.88395.47. [DOI] [PubMed] [Google Scholar]
- Guastella AJ, Mitchell PB, Dadds MR. Oxytocin increases gaze to the eye region of human faces. Biol Psychiatry. 2008;63:3–5. doi: 10.1016/j.biopsych.2007.06.026. [DOI] [PubMed] [Google Scholar]
- Holt-Lunstad J, Birmingham WA, Light KC. Influence of a “warm touch” support enhancement intervention among married couples on ambulatory blood pressure, oxytocin, alpha amylase, and cortisol. Psychosom Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- Hurlemann R, Patin A, Onur OA, Cohen MX, Baumgartner T, Metzler S, Dziobek I, Gallinat J, Wagner M, Maier W, Kendrick KM. Oxytocin enhances amygdala-dependent, socially reinforced learning and emotional empathy in humans. J Neurosci. 2010;30:4999–5007. doi: 10.1523/JNEUROSCI.5538-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insel TR, Hulihan TJ. A gender-specific mechanism for pair bonding: oxytocin and partner preference formation in monogamous voles. Behav Neurosci. 1995;109:782–789. doi: 10.1037//0735-7044.109.4.782. [DOI] [PubMed] [Google Scholar]
- Insel TR, Young L, Wang Z. Molecular aspects of monogamy. Ann NY Acad Sci. 1997;807:302–316. doi: 10.1111/j.1749-6632.1997.tb51928.x. [DOI] [PubMed] [Google Scholar]
- Jin D, Liu HX, Hirai H, Torashima T, Nagai T, Lopatina O, Shnayder NA, Yamada K, Noda M, Seike T, Fujita K, Takasawa S, Yokoyama S, Koizumi K, Shiraishi Y, Tanaka S, Hashii M, Yoshihara T, Higashida K, Islam MS, Yamada N, Hayashi K, Noguchi N, Kato I, Okamoto H, Matsushima A, Salmina A, Munesue T, Shimizu N, Mochida S, Asano M, Higashida H. CD38 is critical for social behaviour by regulating oxytocin secretion. Nature. 2007;446:41–45. doi: 10.1038/nature05526. [DOI] [PubMed] [Google Scholar]
- Kim W, Kim S, Jeong J, Lee KU, Ahn KJ, Chung YA, Hong KY, Chae JH. Temporal changes in functional magnetic resonance imaging activation of heterosexual couples for visual stimuli of loved partners. Psychiatry Invest. 2009;6:19–25. doi: 10.4306/pi.2009.6.1.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch P, Esslinger C, Chen Q, Mier D, Lis S, Siddhanti S, Gruppe H, Mattay VS, Gallhofer B, Meyer-Lindenberg A. Oxytocin modulates neural circuitry for social cognition and fear in humans. J Neurosci. 2005;25:11489–11493. doi: 10.1523/JNEUROSCI.3984-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosfeld M, Heinrichs M, Zak PJ, Fischbacher U, Fehr E. Oxytocin increases trust in humans. Nature. 2005;435:673–676. doi: 10.1038/nature03701. [DOI] [PubMed] [Google Scholar]
- Leckman JF, Mayes LC, Feldman R, Evans DW, King RA, Cohen DJ. Early parental preoccupations and behaviors and their possible relationship to the symptoms of obsessive–compulsive disorder. Acta Psychiatr Scand Suppl. 1999;396:1–26. doi: 10.1111/j.1600-0447.1999.tb10951.x. [DOI] [PubMed] [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A. Oxytocin during pregnancy and early postpartum: individual patterns and maternal-fetal attachment. Peptides. 2007;28:1162–1169. doi: 10.1016/j.peptides.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Akiskal HS, Rossi A, Cassano GB. Alteration of the platelet serotonin transporter in romantic love. Psychol Med. 1999;29:741–745. doi: 10.1017/s0033291798007946. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Canale D. Hormonal changes when falling in love. Psychoneuroendocrinology. 2004;29:931–936. doi: 10.1016/j.psyneuen.2003.08.006. [DOI] [PubMed] [Google Scholar]
- Marazziti D, Dell’Osso B, Baroni S, Mungai F, Catena M, Rucci P, Albanese F, Giannaccini G, Betti L, Fabbrini L, Italiani P, Del Debbio A, Lucacchini A, Dell’Osso L. A relationship between oxytocin and anxiety of romantic attachment. Clin Pract Epidemiol Ment Health. 2006;2:28. doi: 10.1186/1745-0179-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marazziti D, Roncaglia I, Del Debbio A, Bianchi C, Massimetti G, Origlia N, Domenici L, Piccinni A, Dell’Osso L. Brain-derived neurotrophic factor in romantic attachment. Psychol Med. 2009;39:1927–1930. doi: 10.1017/S0033291709990742. [DOI] [PubMed] [Google Scholar]
- Matsunaga M, Sato S, Isowa T, Tsuboi H, Konagaya T, Kaneko H, Ohira H. Profiling of serum proteins influenced by warm partner contact in healthy couples. Neuro Endocrinol Lett. 2009;30:227–236. [PubMed] [Google Scholar]
- Ross HE, Young LJ. Oxytocin and the neural mechanisms regulating social cognition and affiliative behavior. Front Neuroendocrinol. 2009;30:534–547. doi: 10.1016/j.yfrne.2009.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon RW, Marcussen K. Marital transitions, marital beliefs, and mental health. J Health Soc Behav. 1999;40:111–125. [PubMed] [Google Scholar]
- Tabak BA, McCullough ME, Szeto A, Mendez AJ, McCabe PM. Oxytocin indexes relational distress following interpersonal harms in women. Psychoneuroendocrinology. 2010;36:115–122. doi: 10.1016/j.psyneuen.2010.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor SE, Saphire-Bernstein S, Seeman TE. Are plasma oxytocin in women and plasma vasopressin in men biomarkers of distressed pair-bond relationships? Psychol Sci. 2010;21:3–7. doi: 10.1177/0956797609356507. [DOI] [PubMed] [Google Scholar]
- van Anders SM, Goldey KL, Kuo PX. The steroid/peptide theory of social bonds: integrating testosterone and peptide responses for classifying social behavioral contexts. Psychoneuroendocrinology. 2011;36:1265–1275. doi: 10.1016/j.psyneuen.2011.06.001. [DOI] [PubMed] [Google Scholar]
- Weisman O, Feldman R, Goldstein A. Parental and romantic attachment shape brain processing of infant cues. Biol Psychol. 2011 doi: 10.1016/j.biopsycho.2011.11.008. [DOI] [PubMed] [Google Scholar]
- White-Traut R, Watanabe K, Pournajafi-Nazarloo H, Schwertz D, Bell A, Carter CS. Detection of salivary oxytocin levels in lactating women. Dev Psychobiol. 2009;51:367–373. doi: 10.1002/dev.20376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JR, Insel TR, Harbaugh CR, Carter CS. Oxytocin administered centrally facilitates formation of a partner preference in female prairie voles (Microtus ochrogaster) J Neuroendocrinol. 1994;6:247–250. doi: 10.1111/j.1365-2826.1994.tb00579.x. [DOI] [PubMed] [Google Scholar]
- Wotjak CT, Ganster J, Kohl G, Holsboer F, Landgraf R, Engelmann M. Dissociated central and peripheral release of vasopressin, but not oxytocin, in response to repeated swim stress: new insights into the secretory capacities of peptidergic neurons. Neuroscience. 1998;85:1209–1222. doi: 10.1016/s0306-4522(97)00683-0. [DOI] [PubMed] [Google Scholar]


