Abstract
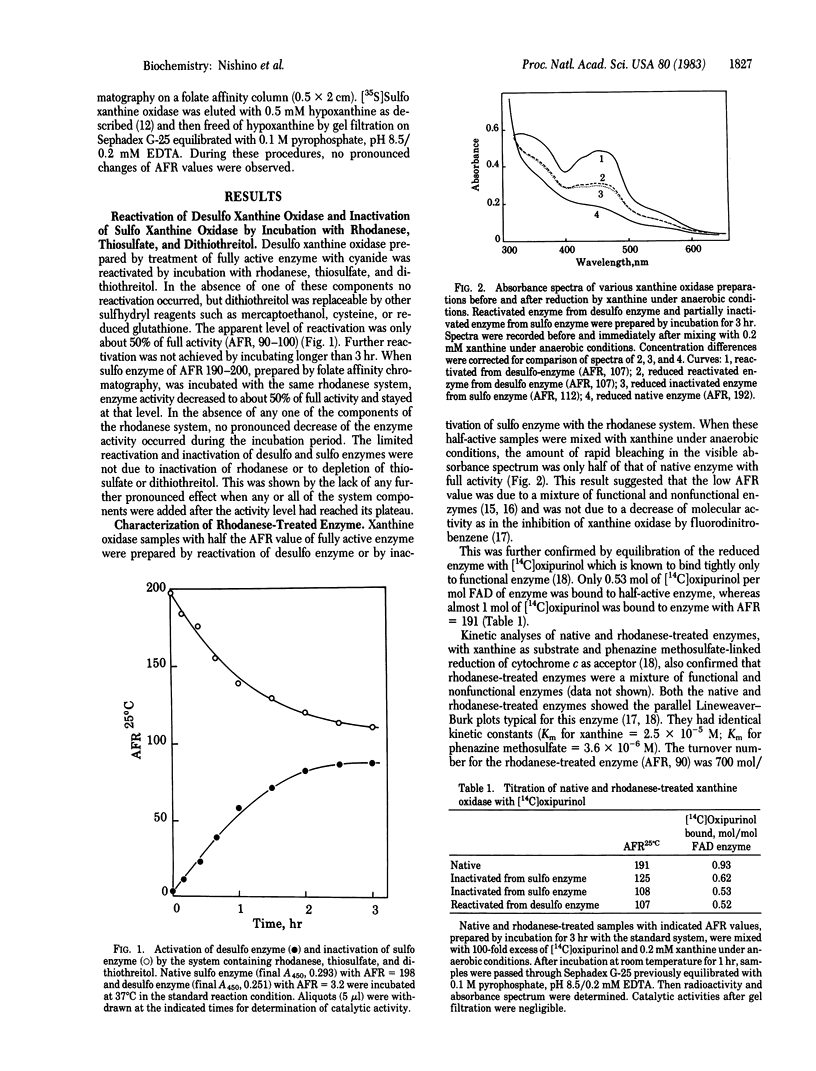
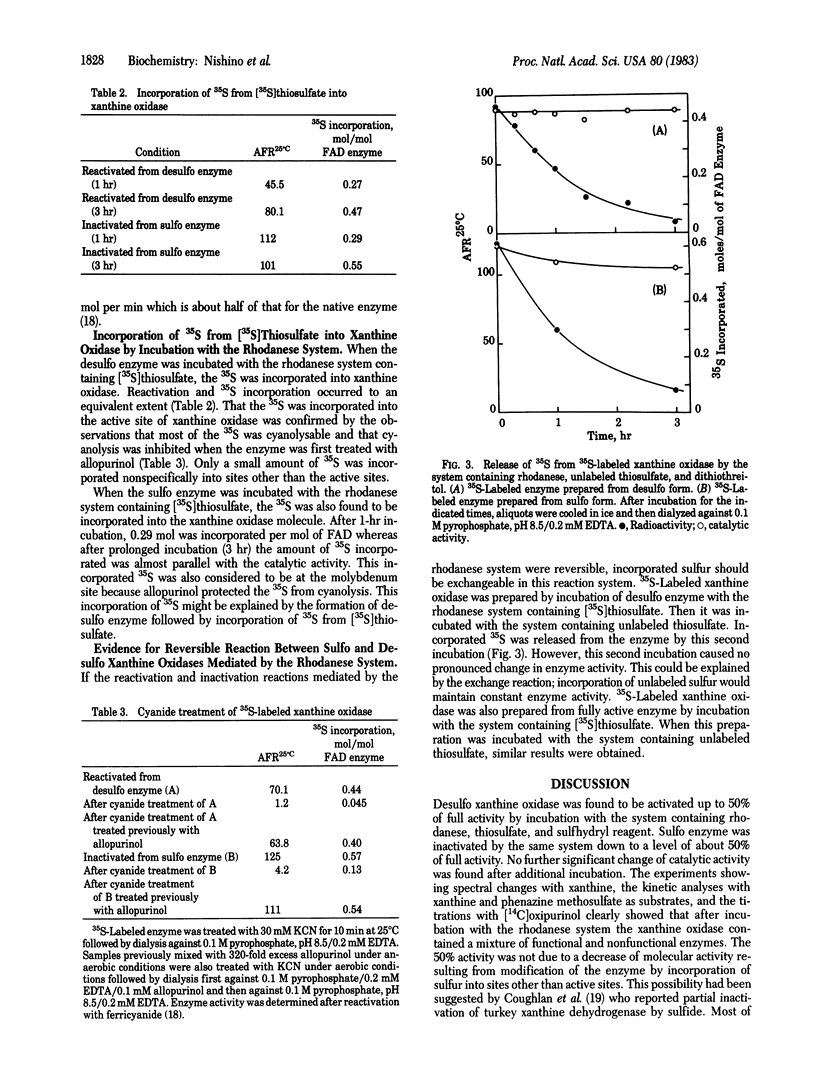
The desulfo form of milk xanthine oxidase (xanthine: oxygen oxidoreductase, EC 1.2.3.2) was reactivated by incubation with rhodanese (thiosulfate: cyanide sulfurtransferase, EC 2.8.1.1), thiosulfate, and sulfhydryl reagent; 50% of full activity was recovered. No further reactivation occurred with additional incubation. It was also found that native enzyme in the sulfo form with full activity was inactivated by incubation with the same system, down to half of full activity and no further inactivation occurred. After these incubations the enzyme was found to be a mixture of functional and nonfunctional enzymes based on spectral changes with xanthine, on [14C]oxipurinol equilibration, and on steady-state kinetics. The 35S of [35S]thiosulfate was incorporated into desulfo xanthine oxidase in parallel with an increase in catalytic activity. Most of the 35S was cyanolysable but was protected from cyanolysis by pretreatment with allopurinol. The 35S was released from 35S-labeled reconstituted xanthine oxidase upon incubation with the rhodanese system containing unlabeled thiosulfate. However, catalytic activity remained unchanged, indicating that the sulfur atom was exchanged during the incubation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bordas J., Bray R. C., Garner C. D., Gutteridge S., Hasnain S. S. X-ray absorption spectroscopy of xanthine oxidase. The molybdenum centres of the functional and the desulpho forms. Biochem J. 1980 Nov 1;191(2):499–508. doi: 10.1042/bj1910499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branzoli U., Massey V. Evidence for an active site persulfide residue in rabbit liver aldehyde oxidase. J Biol Chem. 1974 Jul 25;249(14):4346–4349. [PubMed] [Google Scholar]
- Cleere W. F., Coughlan M. P. Turkey liver xanthine dehydrogenase. Reactivation of the cyanide-inactivated enxyme by sulphide and by selenide. Biochem J. 1974 Nov;143(2):331–340. doi: 10.1042/bj1430331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Kovensky A., Hitchings G. H. Metabolic studies of allopurinol, an inhibitor of xanthine oxidase. Biochem Pharmacol. 1966 Jul;15(7):863–880. doi: 10.1016/0006-2952(66)90163-8. [DOI] [PubMed] [Google Scholar]
- Gutteridge S., Tanner S. J., Bray R. C. Comparison of the molybdenum centres of native and desulpho xanthine oxidase. The nature of the cyanide-labile sulphur atom and the nature of the proton-accepting group. Biochem J. 1978 Dec 1;175(3):887–897. doi: 10.1042/bj1750887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itoh R., Nishino T., Usami C., Tsushima K. An immunochemical study of the changes in chicken liver xanthine dehydrogenase activity during dietary adaptation. J Biochem. 1978 Jul;84(1):19–26. doi: 10.1093/oxfordjournals.jbchem.a132107. [DOI] [PubMed] [Google Scholar]
- MORELL D. B. The nature and catalytic activities of milk xanthine oxidase. Biochem J. 1952 Aug;51(5):657–666. doi: 10.1042/bj0510657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malthouse J. P., Bray R. C. The nature of the sulphur atom liberated from xanthine oxidase by cyanide. Evidence from e.p.r. spectroscopy after 35S substitution. Biochem J. 1980 Oct 1;191(1):265–267. doi: 10.1042/bj1910265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey V., Brumby P. E., Komai H. Studies on milk xanthine oxidase. Some spectral and kinetic properties. J Biol Chem. 1969 Apr 10;244(7):1682–1691. [PubMed] [Google Scholar]
- Massey V., Edmondson D. On the mechanism of inactivation of xanthine oxidase by cyanide. J Biol Chem. 1970 Dec 25;245(24):6595–6598. [PubMed] [Google Scholar]
- Massey V., Komai H., Palmer G., Elion G. B. On the mechanism of inactivation of xanthine oxidase by allopurinol and other pyrazolo[3,4-d]pyrimidines. J Biol Chem. 1970 Jun 10;245(11):2837–2844. [PubMed] [Google Scholar]
- Nishino T., Ito R., Tsushima K. Studies on chicken liver xanthine dehydrogenase with reference to the problem of non-equivalence of FAD moieties. Biochim Biophys Acta. 1975 Sep 22;403(1):17–22. doi: 10.1016/0005-2744(75)90004-2. [DOI] [PubMed] [Google Scholar]
- Nishino T., Nishino T., Tsushima K. Purification of highly active milk xanthine oxidase by affinity chromatography on Sepharose 4B/folate gel. FEBS Lett. 1981 Aug 31;131(2):369–372. doi: 10.1016/0014-5793(81)80406-1. [DOI] [PubMed] [Google Scholar]
- Nishino T. Purification of hepatic xanthine dehydrogenase from chicken fed a high-protein diet. Biochim Biophys Acta. 1974 Mar 21;341(1):93–98. doi: 10.1016/0005-2744(74)90069-2. [DOI] [PubMed] [Google Scholar]
- Nishino T., Tsushima K., Hille R., Massey V. Inhibition of milk xanthine oxidase by fluorodinitrobenzene. J Biol Chem. 1982 Jul 10;257(13):7348–7353. [PubMed] [Google Scholar]
- Wahl R. C., Rajagopalan K. V. Evidence for the inorganic nature of the cyanolyzable sulfur of molybdenum hydroxylases. J Biol Chem. 1982 Feb 10;257(3):1354–1359. [PubMed] [Google Scholar]



