Abstract
Background
High recombinant protein productivity in mammalian cell lines is often associated with phenotypic changes in protein content, energy metabolism, and cell growth, but the key determinants that regulate productivity are still not clearly understood. The mammalian target of rapamycin (mTOR) signalling pathway has emerged as a central regulator for many cellular processes including cell growth, apoptosis, metabolism, and protein synthesis. This role of this pathway changes in response to diverse environmental cues and allows the upstream proteins that respond directly to extracellular signals (such as nutrient availability, energy status, and physical stresses) to communicate with downstream effectors which, in turn, regulate various essential cellular processes.
Results
In this study, we have performed a transcriptomic analysis using a pathway-focused polymerase chain reaction (PCR) array to compare the expression of 84 target genes related to the mTOR signalling in two recombinant CHO cell lines with a 17.4-fold difference in specific monoclonal antibody productivity (q p ). Eight differentially expressed genes that exhibited more than a 1.5-fold change were identified. Pik3cd (encoding the Class 1A catalytic subunit of phosphatidylinositol 3-kinase [PI3K]) was the most differentially expressed gene having a 71.3-fold higher level of expression in the high producer cell line than in the low producer. The difference in the gene’s transcription levels was confirmed at the protein level by examining expression of p110δ.
Conclusion
Expression of p110δ correlated with specific productivity (q p ) across six different CHO cell lines, with a range of expression levels from 3 to 51 pg/cell/day, suggesting that p110δ may be a key factor in regulating productivity in recombinant cell lines.
Keywords: CHO, mTOR, Monoclonal antibody, Phosphotidylinositol 3-kinase, Cell engineering
Background
The production of biopharmaceutical products is one of the fastest growing areas in the pharmaceutical industry with sales of $99 billion in 2010 [1,2]. Many of these biopharmaceutical products are used to treat critical diseases, like hepatitis, cancer, heart disease, hemophilia, rheumatoid arthritis, and diabetes. Monoclonal antibodies (mAbs) currently constitute more than 30% of total biopharmaceuticals and the portfolio of products is expected to grow between 7-15% per annum in the coming years. Whilst the future does looks bright for this sector, the emergence of biosimilar competition is ensuring that manufacturers of these products are continuously seeking methods of increasing productivity of cell culture processes to maximise yields.
Numerous attempts have been made to generate cell lines that can produce large amounts of recombinant products. Among the cell lines available for mAb production are myeloma, hybridoma, and Chinese hamster ovary (CHO) cell lines [3,4]. The use of CHO cells in large-scale production is common [3,5-7] because of their ability to express high recombinant protein levels [8,9], grow to high cell densities [10-13], and to grow in serum-free suspension culture [6,14-16]. CHO cells are also suitable for use with expression systems, such as dihydrofolate reductase (DHFR) and glutamine synthetase (GS) [17-19].
High productivity is usually accompanied by slower growth rates [20-25], and generating high-producing cell lines with high growth rates should be advantageous to further improve mAb production. However, maintaining cell-specific productivity at high growth rates is challenging because both processes require high energy. Glutamine and glucose are often consumed in the synthesis of precursors for recombinant proteins rather than in cell proliferation [26], which eventually leads to growth repression.
The correlation between cell volume (size) and productivity has been clearly demonstrated [27] and is reflected in the phenotypic changes in protein content and cell biomass [13,28,29]. This increase in protein content may lead to an increase in cell size [25,29,30]. The integration of both protein synthesis and cell proliferation in response to diverse environmental cues has been reported to be regulated by a signalling pathway known as the mammalian target of rapamycin (mTOR) signalling pathway [31,32].
As shown in Figure 1, upon receiving signals from growth factors and hormones, mTOR is activated by Akt/P13K signalling [33,34]. P13K catalyses the formation of phosphoinositides-3-phosphate (PIP3) from phosphoinositides-2-phosphate (PIP2), which then activates a protein kinase known as Akt [35]. The activated mTOR initiates protein synthesis via two principal pathways; firstly through the inhibition of eukaryotic initiation factor 4E binding protein (4EBP1) and secondly through the activation of ribosomal protein S6 kinase (S6K) [36,37]. These downstream effectors activate protein synthesis by activating translation initiation and elonga tion factors, including eIF4E, eIF4B, S6, and eEF2 [32,38].
Figure 1.
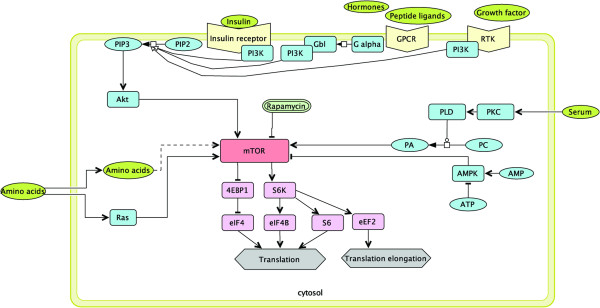
The mTOR pathway signaling pathway. This pathway involves communication between the upstream proteins that respond directly to such extracellular signals as nutrient availability, energy status, and physical stresses.
In addition to growth factors and hormones, mTOR also receives signals from phosphatidic acid (PA) [39] and amino acids [38,40,41]. In the presence of serum, protein kinase C (PKC) is activated, and it sequentially activates phospholipase D (PLD) which is responsible for converting PA to phosphatidylcholine (PC) [39]. In contrast, the activation of mTOR by amino acids is unclear [32]. It was reported to be regulated by the Ras protein [41] and/or intracellular amino acids [38,42]. Moreover, adenosine monophosphate kinase (AMPK) can also precisely modulate mTOR activity based on the adenosine monophosphate (AMP) to adenosine triphosphate (ATP) ratio [43]. In the presence of high intracellular ATP levels, AMPK can be deactivated, leading to an increase in protein synthesis [44,45].
A recent study highlighted the role of mTOR in improving viability, cell growth, antibody production, and robustness of the CHO cell line [46]. However, the mechanism underlying these improvements has not yet been elucidated. We believe that using a transcriptomic approach, such as the pathway-focused polymerase chain reaction (PCR) array, which has proven to be useful in a similar study [47], we can elucidate the connections between mTOR, cell growth, and productivity. Thus, this study initially employed two recombinant CHO cell lines with a 17.4-fold difference in specific productivity to study the differential expressions of genes related to the mTOR signaling pathway. High productivity has been associated partly with enhanced translation mechanisms [48]. We therefore expected to see upregulation of genes encoding mTOR upstream regulators such as, Akt [49,50], PI3K [51,52], PLD [39,53], and Ras-related GTP binding proteins (RagC) [41,54,55], as well as downstream effectors involved in translation mechanisms such as S6 and S6K [56-59]. Since high gene expression does not necessarily mean that the corresponding protein is also highly upregulated in the cell, we decided to perform Western blot analysis to confirm that the selected potential marker p110δ was expressed in increasing amount in a panel of 6 CHO cell lines with varying levels of productivity.
Methods
Cell lines and maintenance
This study employed a panel of six GS-CHO cell lines (referred to as CL38, CL47, CL76, CL150, CL160 and CL164) producing cB72.3 IgG4 monoclonal antibody with varying levels of productivity. The cell lines were kindly provided by Lonza Biologics (Slough, UK). The cell lines were generated by transfection of the suspension-variant derivatives CHO-K1 (CHOK1SV) with the glutamine synthetase (GS) expression vector pcB72.3 containing light chain (LC) and heavy chain (HC) cDNA, each driven by the hCMV-MIE promoter [60,61]. Cells were maintained in CD-CHO medium supplemented with 25 μM MSX. Using three biological replicates, our experiments were conducted in 125 ml shake flasks (SCHOTT Inc., Elmsford, USA) at 37°C and 140 rpm agitation.
The six cell lines were numbered in descending order according to their specific productivity, with [1] referring to the most productive cell line and [6] referring to the least productive cell line. These cell lines were thus referred to as CL47[1], CL76[2], CL150[3], CL164[4], CL38[5] and CL160[6] throughout this study . Based on specific productivity, CL47[1] was categorised as the high producer; CL76[2], CL150[3], CL164 [4], and CL38[5], as medium producers; and CL160[6], as the low producer.
Flow cytometric analysis of cell number and viability
Cell suspensions (490 μl) were removed from flasks and placed in a flow cytometry tube and 10 μl propidium iodide (PI) solution added from the stock solution (0.5 μg/ml). Samples were mixed by gentle shaking and analysed immediately for cell numbers and viability, using Cell Lab Quanta SC flow cytometry (Beckman Coulter Inc., CA, USA) equipped with an argon laser (488 nm). Red fluorescence (PI) was collected using a 635 nm band pass filter. Analysis was undertaken by loading an appropriate protocol for the acquired parameters: electronic volume (EV), log side scatter (SS), and PI integral; 10 000 observations were collected for analysis. The evaluation of cell number was achieved by gating areas in the EV versus log SS dot plot in which living cells and dead cells appear.
We used the following equation to calculate the specific growth rate, μ:
Where and symbolise the viable cell density at time points t 1 and t 0 , respectively.
Determination of antibody concentration
Enzyme-linked immunosorbent assay (ELISA) was used to determine the concentration of mAb secreted by the cell lines, as described previously [30]. The antibody was sandwiched between monoclonal anti-human IgG Fc (Sigma-Aldrich, St. Louis, USA) and anti-human kappa light chain horseradish peroxidase (HRP) conjugates (Sigma-Aldrich). The concentration of antibody in the samples was determined using o-phenylenediamine (OPD; Sigma-Aldrich) as a substrate, and the specific productivity, q p , was calculated using the following equation:
where [MAb] represents the antibody concentration at a particular time, while cumulative viable cell time (CVCT) is the sum of the individual areas given by
Where x 0 represents the cell number at the particular time t 0 and x 1 represents the cell number at the elapsed time t 1 .
Quantitative real-time PCR (qRT-PCR) analysis
3 × 106 cells were removed from the flask at the mid-exponential phase (day three of the batch culture), centrifuged, and washed twice by PBS. The sample was treated with RNAlater and stored at −80°C until analysis, at which point it was centrifuged to remove the RNAlater stabilization reagent. RNA isolation was carried out using the RNeasy Mini Kit (QIAGEN, Valencia, CA, USA) according to the manufacturer’s instructions. The concentration of RNA was determined using a NanoDrop ND-1000 UV–vis Spectrophotometer (Nanodrop Technologies, Wilmington, DE, USA), and the integrity of RNA was checked using an Agilent Bioanalyzer (Santa Clara, CA, USA).
The expression levels of mTOR-related genes were quantified using a mouse-mTOR-pathway-focused qRT-PCR array from SA Biosciences (Frederick, Maryland, USA). The DNA elimination treatment was carried out, and complementary DNA (cDNA) was synthesized from the RNA samples, using the RT2 First Strand Kit (SA Biosciences) according to the manufacturer’s instructions. The cDNA samples were mixed with RT2 SYBR Green/ROX qRT-PCR Master Mix reagents (SA Biosciences) according to the manufacturer’s instructions, and the qRT-PCR was performed on these samples using ABI Prism 7500 FAST sequence detection system (Applied Biosystems, Carlsbad, CA, USA). The Ct values obtained from the qRT-PCR analysis were normalised to five housekeeping genes (beta glucuronidase [Gusb], hypoxanthine quinine phosphoribosyl transferase 1 [Hprt1], heat shock protein 90 alpha [Hsp90ab1], glyceraldehyde-3-phosphate dehydrogenase [Gapdh], and beta actin [Actb]). The normalization was calculated by using the 2 -∆∆CT method, as previously described [62].
Total protein content
The total protein content was determined using the QuantiPro BCA Assay Kit (Sigma-Aldrich). 3 × 106 cells were removed from the flask, centrifuged and then washed once with sterile-filtered PBS. The cells were then centrifuged at 500 g for five minutes and supernatant removed. A mixture of CelLytic M Cell Lysis Reagent (Sigma-Aldrich) and a protease inhibitor cocktail (Sigma-Aldrich) was added to disrupt the cells. The samples were mixed by vortexing for one minute, centrifuged at 15 000 g for 15 minutes at 4°C. The supernatant was transferred to a new microcentrifuge tube and stored at −20°C until analysis.
Western blot analysis
For western blot analysis, 20 μg of total protein was mixed in 2× Laemmli buffer (Sigma-Aldrich) and denatured at 95°C. The samples were then subjected to electrophoresis on an 8% polyacrylamide gel (Thermo Scientific, Waltham, MA, USA) using a Mini Protean 3 electrophoresis system (Bio-Rad Laboratories, Waltham, MA, USA) for 90 minutes. The proteins were transferred electrophoretically to a PVDF membrane (Millipore Corporation, Cheshire, NH, USA) overnight. The membrane was blocked in non-fat milk powder in PBST followed by an overnight incubation with an anti-mouse p110δ PI3K antibody (Santa Cruz Biotechnology, Santa Crus, CA, USA). The membrane was probed with a secondary antibody solution containing anti-goat IgG peroxidase conjugates prior to detection using a SuperSignal West Pico kit (Thermo Scientific) according to the manufacturer’s instructions. Upon reaction, the membrane was exposed, developed, and fixed on a film. The band intensity was measured by the AlphaDigiDoc RT2 gel documentation system software (Alpha Innotech Corporation, CA, USA).
Results
Comparing the growth and productivity of the high and low producers
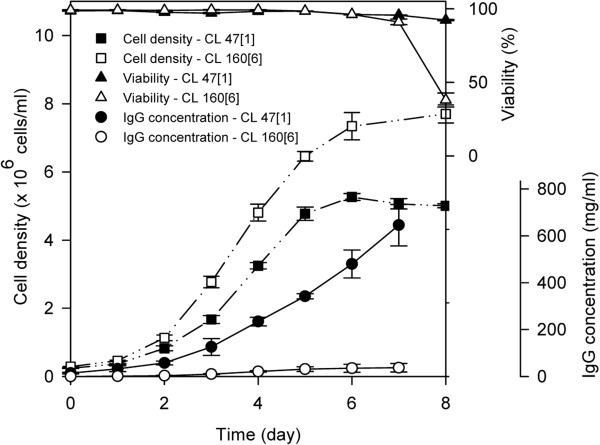
The C47 [1] and CL160 [6] lines, which expressed the recombinant chimeric IgG4 mAb, were grown in batch cultures in three biological replicates. The cell numbers and antibody concentrations, determined by flow cytometric analysis and ELISA, respectively, were determined routinely throughout the culture duration until the cells reached the death phase. The cell growth rates and cumulative viable cell time (CVCT) were determined throughout the exponential phase, from day one to day five of batch culture as shown in Table 1. The viable cell density, viability, and antibody concentration profiles are shown in Figure 2. Following a normal growth curve, CL160[6] outperformed CL47[1] in terms of growth, as demonstrated by the calculated CVCT and growth rate (μ) of 329.66 ± 17.99 (109 cell.h/L) and 0.67 ± 0.01 (day-1) in CL160[6], compared to 244.48 ± 4.58 (109 cell.h/L) and 0.63 ± 0.01 (day-1) in CL47[1], respectively. However, CL47 [1] sustained high viability towards the decline phase (day 8), while the viability of CL160[6], on the contrary, started to decrease sharply on day seven. In CL 47[1], the specific productivity and total antibody concentration, as indicated in Table 1, were 17.4- and 11-fold higher than CL160[6], respectively.
Table 1.
The culture characteristics and cell productivity of high producer (CL47[1]) and low producer (160[6]) cell lines during batch culture
| 47[1] | 160[6] | |
|---|---|---|
| CVCT (109 cell.hr/L) |
244.48 ± 4.58 |
329.66 ± 17.99 |
| Antibody concentration (μg/ml) |
575.24 ± 137.55 |
52.08 ± 0.77 |
| Growth rate (day-1) |
0.63 ± 0.01 |
0.67 ± 0.01 |
| q p (pg/cell/day;pcd) | 50.54 ± 3.18 | 2.91 ± 1.45 |
The symbol “±” represents the standard deviation calculated between the three biological replicates.
Figure 2.
Viable cell density, viability, and antibody concentration profiles of high producer (CL47[1]) and low producer (CL160[6]) cells lines during batch culture. Error bars symbolize the standard deviation as calculated between three biological replicates.
Differential mTOR-related gene expression between high and low producers
We hypothesized that the differences in CL47 [1] and CL160[6] productivity levels are a consequence of the diverse mechanisms of cellular functions that are controlled by mTOR. Using a commercially available pathway-focused PCR array, the expression of 84 genes related to the mTOR signalling pathway were screened in the template cDNA which was prepared from the samples collected at the mid-exponential phase (day three of the batch culture). Figure 3 demonstrates the relative expression of the mTOR-related genes exhibited by the high producer (CL47[1], treated) in comparison to the low producer (CL160[6], control). Student’s t-tests were conducted to determine the significance of the gene expression changes. Out of the 84 mTOR-related genes in this array, eight genes expressed at least a 1.5-fold difference (Pik3cd, Pik3cg, Pld1, Rragc, Ins2, Telo2, Rps6 and Prkab1), with a corresponding statistical significance (P-value < 0.05) between the high and low producers (Figure 4). These genes were all upregulated.
Figure 3.
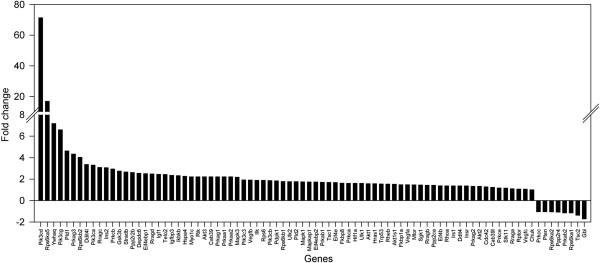
The fold changes of mTOR-related gene expressions of GS-CHO cell lines at the mid-exponential phase of batch culture. Values were calculated based on a relative quantification made between high producer (CL47[1]) and low producer (160[6];control) lines by using the 2-∆∆CT method.
Figure 4.
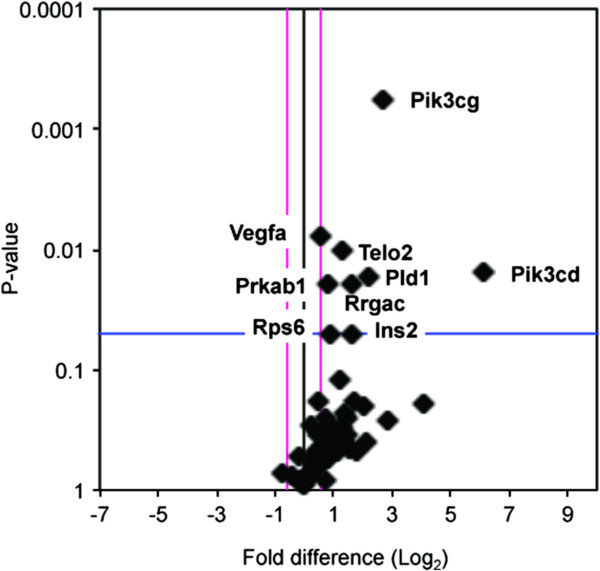
The log2 fold changes in gene expression between high producer (CL47[1]) and low producer (160[6]) lines against t-test P-values. Vertical line represents the threshold of fold changes (i.e., 1.5); horizontal line represents the significant difference of t-test P-values (i.e., 0.05). Significantly expressed genes (P-values < 0.05) are tabulated in Table 2.
Expression of genes in mTOR signalling pathway
The list of mTOR-related genes with significantly altered expression in CL47[1] relative to CL160[6] is presented in Table 2. Three differentially expressed genes involved in insulin signalling were upregulated. The most upregulated gene, pik3cdat 71.3-fold (P-value < 0.05), encodes the catalytic subunit of the delta polypeptide p110δ. The gene encoding a p110δ sibling, the gamma polypeptide (p110γ) subunit, was also significantly expressed but to a lesser extent (6.6-fold; P-value < 0.05). The p110δ and p110γ subunits are classified as Class 1A and 1B, respectively and both receive signals from insulin and various types of growth factors [51,63,64]. Another gene related to insulin signalling that was also significantly expressed was insulin 2 (Ins2), with a 3.06-fold change (P-value < 0.05).
Table 2.
The mTOR-related genes with at least a 1.5-fold change in expression level (P-values < 0.05)
| Gene symbol | Description | Fold change | P-value |
|---|---|---|---|
|
Pik3cd |
Phosphatidylinositol 3-kinase catalytic delta polypeptide |
(+) 71.33 |
0.02 |
|
Pik3cg |
Phosphoinositide-3-kinase, catalytic, gamma polypeptide |
(+) 6.59 |
<0.001 |
|
Pld1 |
Phospholipase D1 |
(+) 4.63 |
0.02 |
|
Rragc |
Ras-related GTP binding C |
(+) 3.08 |
0.05 |
|
Ins2 |
Insulin II |
(+) 3.06 |
0.02 |
|
Telo2 |
TEL2, telomere maintenance 2, homolog (S. cerevisiae) |
(+) 2.44 |
0.01 |
|
Rps6 |
Ribosomal protein S6 |
(+) 1.89 |
0.05 |
| Prkab1 | Protein kinase, AMP-activated, beta 1 non-catalytic subunit | (+) 1.71 | 0.02 |
Ras-related GTP-binding protein C (Rragc) and phospholipase D1 (Pld1) were also expressed significantly, with fold changes of 3.08 and 4.63, respectively (P-value < 0.05). Unlike RagC, which has a response specific to amino acid availability [41,54,55], PLD1 is activated by mitogens such as serum [39]. Moreover, the expression of the regulatory gamma subunit of AMPK gene (Prkag3) was also observed to be significantly differentially expressed (1.71-fold, P-value < 0.05) though the expression was observed to be minimal compared to other significant differential mTOR-related genes. The activity of AMPK, precisely controlled by the ratio of AMP to adenosine triphosphate (ATP), reflects the cell energy status [43].
Among the genes that encode for downstream effectors of mTOR, only ribosomal protein S6 (Rps6) was significantly differentially expressed (1.89-fold, P-value < 0.05) in CL47[1] relative to the CL160[6]. The interactions among the upstream regulators, mTOR, and the downstream effectors are indicated in Figure 1. Although mTOR was not significantly expressed in this study, a gene that could be linked to its presence, known as Telo2, was upregulated by 2.44-fold (P-value < 0.05). This gene encodes telomere maintenance 2 (TEL2), which is responsible for mTOR stabilisation and maturation [65,66]. However, the mechanism by which TEL2 stabilizes mTOR remains poorly understood [65,66].
The relationship among p110δ expression and specific productivity, cell growth, and cell size
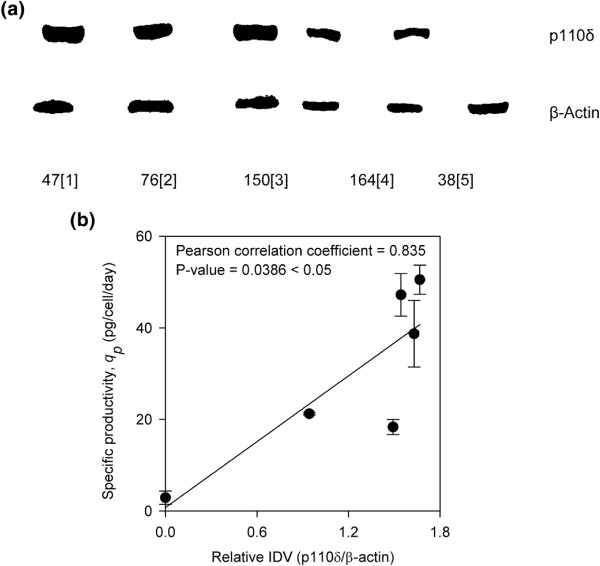
To investigate whether Pik3cd mRNA levels in the high and low producers are predictive of p110δ expression, western blot analysis was performed on samples harvested from the mid-exponential phase (day three) of the batch cultures. Figure 5a shows the expression of the p110δ subunit in GS-CHO cell lines with different q p levels ranging from 18 to 40 pg/cell/day. (CL47[1] = 50.54 pcd; CL76[2] = 47.23 pcd; CL150[3] = 38.73 pcd; CL164[4] = 21.23 pcd; CL38[5] = 18.34 pcd and CL160[6] = 2.91 pcd). The molecular characteristics of these cell lines have been extensively studied previously [67]. The integrated density values (IDV) corresponding to the band intensities were measured. Pearson correlation coefficient analysis was performed to determine the relationship between p110δ subunit expression and the q p levels. The analysis revealed that the expression of the p110δ subunit correlated with the overall q p (ρ = 0.83, P-value < 0.05; Figure 5b). This demonstrates the possibility that the p110δ subunit could be a potential marker describing q p . Previous studies have demonstrated that PI3K overexpression could influence cell growth and proliferation [68-70]. Thus, we attempted to correlate expression of the p110δ subunit with cell size and CVCT. A Pearson correlation coefficient analysis indicated that neither cell size nor CVCT (data not shown) correlated with the expression of the p110δ subunit (P-value > 0.05).
Figure 5.
Comparison of p110σ subunit expressions between (a) six GS-CHO cell lines with different productivity levels at the mid-exponential phase of batch culture and (b) the correlation between p110σ expression and qp. Error bars symbolize the standard deviation as calculated between three biological replicates.
Discussion
Since Dreesen and Fussenegger [46] recently showed that overexpression of mTOR in a CHO cell line significantly improved recombinant antibody production, we decided to investigate expression of members of the mTOR signalling in a panel of CHO cell lines with different levels of productivity. In order to address this question we used the mTOR signalling PCR array for mouse due to the unavailability of the hamster array at that time. Given that the mTOR signalling pathway is conserved from yeast to mammals [71], we used mouse sequences instead of hamster to quantify the levels of mTOR-related gene expressions in this study. Previous transcriptomic studies which relied on cross-hybridization to mouse DNA microarrays have showed considerable success [72] and in general, results from alignment to both mouse and rat reference genomes suggest that CHO genomic sequences are generally more similar to mouse genomic sequences [73]. Of the 84 mTOR-related genes present on the array only eight genes including, Pik3cd, Pik3cg, Ins2, Pld1, Rragc, Prkag3, Rps6 and Telo2 were identified as significantly (p < 0.05) upregulated in CL47[1] in comparison to CL160[6]. This panel of candidate genes could potentially determine high specific productivity in CHO cell lines. Two of the genes that were highly expressed, Pik3cd and Pik3cg, encode for p110δ and p110γ polypeptides, respectively and are also present on the SA Biosciences hamster mTOR signalling PCR array (PAJJ-098Z). These polypeptides differ at the regulatory subunit structure that is responsible for mediating p110δ and p110γ recruitment to the receptors of interest. The presence of p110δ as a regulatory subunit facilitates the binding of p110γ to the G protein beta subunit-like (Gβl) in response to a stimulated G-protein couple receptor (GPCR). The recruitment of the p110δ subunit to the activated receptor tyrosine kinase (RTK) is, however, mediated by a different regulatory subunit, p85, in response to various extracellular growth factors and insulin signals [35,74,75]. Hence, the different receptors as targets imply that upregulation of pik3cd gene could be independent of pik3cg (and vice versa), even though these polypeptides share a common role in catalysing phosphorylation of the inositol ring at the D3 position of their downstream effectors, the phosphoinositides.
Although the effects of p110δ in recombinant protein production have yet to be clarified, its associations with growth are better understood. The effects of p110δ overexpression have been correlated to cell growth and cell size in Drosophila, where expansion of the wing blade was due to an increase in cell number and cell size. A reverse effect on cell growth was observed in p110δ-deficient cells [68]. The real cause, which affects the lack of correlation between the CVCT and cell size with p110δ expression, is not clear. The correlation could be weakened by the presence of the medium producer cell line in which other signalling pathways could have compensated for the lack of effects of p110δ expression on cell growth. However, this expression effect is more profound when only two extremes (high and low producers) were considered. CL160 [6] has a smaller cell size (data not shown) than CL47[1], and this could partly result from the defective p110δ expression in CL160[6] [51,68]. There has been some studies published which examined the use of genetic control of the cell cycle to increase q p . However, these studies cannot confirm that the production of recombinant protein is related to any specific phase of the cell cycle. However, the increase in q p can be related to cell size rather than the cell cycle phase [17]. This has been shown previously by Bi et al. [30] in a cytostatic system utilising inducible expression of p21CIP1 to arrest CHO cells where a fourfold increase of q p was obtained concomitant to a fourfold increase in cell volume. Regardless of cell cycle phase, the cell size could also be controlled by p110δ expression through protein synthesis, a mechanism that is important for cell growth and proliferation. As cells have to retain a constant cell size during proliferation, a tight coordination between cell growth and cell cycle progression is essential for maintaining cell size and these processes are controlled by p110δ expression as the upstream regulator of mTOR signalling pathway [33,76,77].
While the regulation of p110δ activity is related to cell growth, the activity of p110γ involves the regulation of cell survival [78-80]. An increased resistance to cell death was also observed as a result of the simultaneous inhibition of p53 and activation of nuclear factor κB (NF-κB) signalling pathways by Akt phosphorylation following the overexpression of the p110γ subunit in human cell lines [80].
In addition, Prkag3, Pld1, and Rragc genes were also significantly expressed in CL47 [1], and these could be related to high specific productivity. These genes encode AMPK, PLD, and Ras-related GTP-binding protein C, which represent upstream regulators of mTOR. The altered expression of these genes may implicate the expression of the Rsp6 gene, which encodes the S6 protein. The S6 protein regulates the translation of ribosomal protein, elongation factor, and polyA-binding protein, that could lead to ribosome biogenesis [81-83]. This suggests that the improved specific productivity in CL47 [1] could be due to the altered expression of the Rsp6 gene. Our results were supported by a study conducted by Bi et al. [30]. A significant increase in mAb titre was shown to correlate with higher S6 protein expression in an isopropyl-β-d-thiogalactoside (IPTG)-induced p21cip-arrested CHO cell line [30].
We also found altered expression of the Ins2 gene in the CL47[1] cell line; this gene is not commonly expressed in non-pancreatic β cells. It is known that all cells contain an insulin gene, but its expression can vary in different cell types. The expression of Ins2 could be controlled at the transcriptional level. It was shown previously by Kuroda et al. [84] that the Ins2 gene was fully methylated and becomes demethylated as the cells differentiate into insulin-expressing cells in vitro[84]. If CHO cells could produce insulin, this would make the cells less dependent on exogenous insulin, which is certainly an advantageous trait in recombinant protein production.
Conclusion
This study provides a better understanding of the role of the mTOR signalling pathway in GS-CHO cell lines and identifies the importance of this pathway in recombinant protein production. We also highlight the presence of the p110δ subunit of PI3K as a potential key regulator in productivity that may represent a future target for cell-engineering strategies for enhancing productivity. Cell-engineering approaches such as overexpression and/or gene silencing could be used to verify the roles of p110δ and p110γ in cellular functions that may be involved in recombinant protein production.
Competing interests
The authors declare no competing interests.
Authors’ contribution
RE carried out the molecular genetic and cell culture studies, and participated in the sequence alignment. All authors wrote, edited and approved the manuscript.
Contributor Information
Raihana Edros, Email: Raihana.Edros@ucdconnect.ie.
Susan McDonnell, Email: susan.mcdonnell@ucd.ie.
Mohamed Al-Rubeai, Email: m.al-rubeai@ucd.ie.
Acknowledgements
The work was funded by grant from HEA, Ireland: PRTLI cycle 4- (Bio)Pharmaceutical and Pharmacological Sciences project and from Majlis Amanah Rakyat, MARA, Malaysia.
References
- Walsh G. Biopharmaceutical benchmarks. Nat Biotechnol. 2000;18(8):831–3. doi: 10.1038/78720. [DOI] [PubMed] [Google Scholar]
- Walsh G. Biopharmaceutical benchmarks. Nat Biotechnol. 2010;28(9):917–24. doi: 10.1038/nbt0910-917. [DOI] [PubMed] [Google Scholar]
- Chu L, Robinson DK. Industrial choices for protein production by large-scale cell culture. Curr Opin Biotechnol. 2001;12(2):180–187. doi: 10.1016/S0958-1669(00)00197-X. [DOI] [PubMed] [Google Scholar]
- Seth G, Charaniya S, Wlaschin KF, Hu W-S. In pursuit of a super producer—alternative paths to high producing recombinant mammalian cells. Curr Opin Biotechnol. 2007;18:8. doi: 10.1016/j.copbio.2007.10.012. [DOI] [PubMed] [Google Scholar]
- Derouazi M, Girard P, Van Tilborgh F, Iglesias K, Muller N, Bertschinger M, Wurm FM. Serum-free large-scale transient transfection of CHO cells. Biotechnol Bioeng. 2004;87(4):537–545. doi: 10.1002/bit.20161. [DOI] [PubMed] [Google Scholar]
- Haldankar R, Li D, Saremi Z, Baikalov C, Deshpande R. Serum-free suspensin large-scale transient transfection of CHO cells in WAVE bioreactors. Mol Biotechnol. 2006;34(2):191–199. doi: 10.1385/MB:34:2:191. [DOI] [PubMed] [Google Scholar]
- Xing Z, Kenty BM, Li ZJ, Lee SS. Scale-up analysis for a CHO cell culture process in large-scale bioreactors. Biotechnol Bioeng. 2009;103(4):733–746. doi: 10.1002/bit.22287. [DOI] [PubMed] [Google Scholar]
- Lucas BK, Giere LM, DeMarco RA, Shen A, Chisholm V, Crowley CW. High-level production of recombinant proteins in CHO cells using a dicistronic DHFR intron expression vector. Nucleic Acids Res. 1996;24(9):1774–1779. doi: 10.1093/nar/24.9.1774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pu H, Cashion LM, Kretschmer PJ, Liu Z. Rapid establishment of high-producing cell lines using dicistronic vectors with glutamine synthetase as the selection marker. Mol Biotechnol. 1998;10(1):17–25. doi: 10.1007/BF02745860. [DOI] [PubMed] [Google Scholar]
- Hilal-Alnaqbi A, Hu A, Zhang Z, Al-Rubeai M. Growth, metabolic activity, and productivity of immobilized and freely suspended CHO cells in perfusion culture. Biotechnol Appl Biochem. 2013;60:436–445. doi: 10.1002/bab.1103. [DOI] [PubMed] [Google Scholar]
- Kaneko Y, Sato R, Aoyagi H. Evaluation of Chinese hamster ovary cell stability during repeated batch culture for large-scale antibody production. J Biosci Bioeng. 2010;109(3):274–280. doi: 10.1016/j.jbiosc.2009.09.044. [DOI] [PubMed] [Google Scholar]
- Konstantinov KB, Tsai Y, Moles D, Matanguihan R. Control of long-term perfusion chinese hamster ovary cell culture by glucose auxostat. Biotechnol Prog. 2008;12(1):100–109. doi: 10.1021/bp950044p. [DOI] [PubMed] [Google Scholar]
- Kou TC, Fan L, Ye ZY, Zhou Y, Liu XP, Zhao L, Tan WS. Process analysis of reduced specific productivity of TNFR-Fc in Chinese hamster ovary cells at high cell density. Process Biochem. 2011;46(7):1492–1499. doi: 10.1016/j.procbio.2011.04.001. [DOI] [Google Scholar]
- Gasser F, Mulsant P, Gillois M. Long-term multiplication of the Chinese hamster ovary (CHO) cell line in a serum-free medium. In Vitro Cell Dev Biol Plant. 1985;21(10):588–592. doi: 10.1007/BF02620890. [DOI] [PubMed] [Google Scholar]
- Haldankar R, Kopchick JJ, Ridgway D. Stable production of a human growth hormone antagonist from CHO cells adapted to serum,ÄêFree suspension culture. Biotechnol Prog. 1999;15(3):336–346. doi: 10.1021/bp9900460. [DOI] [PubMed] [Google Scholar]
- Sinacore M, Charlebois T, Harrison S, Brennan S, Richards T, Hamilton M, Scott S, Brodeur S, Oakes P, Leonard M. CHO DUKX cell lineages preadapted to growth in serum-free suspension culture enable rapid development of cell culture processes for the manufacture of recombinant proteins. Biotechnol Bioeng. 2000;52(4):518–528. doi: 10.1002/(SICI)1097-0290(19961120)52:4<518::AID-BIT7>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- Dinnis DM, James DC. Engineering mammalian cell factories for improved recombinant monoclonal antibody production: lessons from nature? Biotechnol Bioeng. 2005;91(2):180–189. doi: 10.1002/bit.20499. [DOI] [PubMed] [Google Scholar]
- Page MJ, Sydenham MA. High level expression of the humanized monoclonal antibody CAMPATH-1H in chinese hamster ovary cells. Nat Biotech. 1991;9(1):64–68. doi: 10.1038/nbt0191-64. [DOI] [PubMed] [Google Scholar]
- Wurm FM. Production of recombinant protein therapeutics in cultivated mammalian cells. Nat Biotechnol. 2004;22(11):1393–1398. doi: 10.1038/nbt1026. [DOI] [PubMed] [Google Scholar]
- Chusainow J, Yang YS, Yeo JH, Toh PC, Asvadi P, Wong NS, Yap MG. A study of monoclonal antibody-producing CHO cell lines: what makes a stable high producer? Biotechnol Bioeng. 2009;102(4):1182–1196. doi: 10.1002/bit.22158. [DOI] [PubMed] [Google Scholar]
- Fann CH, Guarna MM, Kilburn DG, Piret JM. Relationship between recombinant activated protein C secretion rates and mRNA levels in baby hamster kidney cells. Biotechnol Bioeng. 1999;63(4):464–472. doi: 10.1002/(SICI)1097-0290(19990520)63:4<464::AID-BIT10>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- Gu MB, Todd P, Kompala DS. Metabolic burden in recombinant CHO cells: effect ofdhfr gene amplification andlacZ expression. Cytotechnology. 1995;18(3):159–166. doi: 10.1007/BF00767763. [DOI] [PubMed] [Google Scholar]
- Jiang Z, Huang Y, Sharfstein ST. Regulation of recombinant monoclonal antibody production in chinese hamster ovary cells: a comparative study of gene copy number, mRNA level, and protein expression. Biotechnol Prog. 2008;22(1):313–318. doi: 10.1021/bp0501524. [DOI] [PubMed] [Google Scholar]
- Kim SJ, Kim NS, Ryu CJ, Hong HJ, Lee GM. Characterization of chimeric antibody producing CHO cells in the course of dihydrofolate reductase-mediated gene amplification and their stability in the absence of selective pressure. Biotechnol Bioeng. 1998;58(1):73–84. doi: 10.1002/(SICI)1097-0290(19980405)58:1<73::AID-BIT8>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Pendse GJ, Karkare S, Bailey JE. Effect of cloned gene dosage on cell growth and hepatitis B surface antigen synthesis and secretion in recombinant CHO cells. Biotechnol Bioeng. 1992;40(1):119–129. doi: 10.1002/bit.260400117. [DOI] [PubMed] [Google Scholar]
- Kidane A, Guan Y, Evans P, Kaderbhai M, Kemp R. Comparison of heat flux in wild-type and genetically-engineered chinese hamster ovary cells. J Therm Anal Calorim. 1997;49(2):771–783. doi: 10.1007/BF01996760. [DOI] [Google Scholar]
- Lloyd DR, Holmes P, Jackson LP, Emery AN, Al-Rubeai M. Relationship between cell size, cell cycle and specific recombinant protein productivity. Cytotechnology. 2000;34(1):59–70. doi: 10.1023/A:1008103730027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhal AV, Marcelino I, Carrondo MJT. Metabolic changes during cell growth inhibition by p27 overexpression. Appl Microbiol Biotechnol. 2003;63(2):164–173. doi: 10.1007/s00253-003-1385-5. [DOI] [PubMed] [Google Scholar]
- Khoo SH, Al-Rubeai M. Detailed understanding of enhanced specific antibody productivity in NS0 myeloma cells. Biotechnol Bioeng. 2009;102(1):188–199. doi: 10.1002/bit.22041. [DOI] [PubMed] [Google Scholar]
- Bi JX, Shuttleworth J, Al-Rubeai M. Uncoupling of cell growth and proliferation results in enhancement of productivity in p21CIP1-arrested CHO cells. Biotechnol Bioeng. 2004;85(7):741–749. doi: 10.1002/bit.20025. [DOI] [PubMed] [Google Scholar]
- Arsham AM, Neufeld TP. Thinking globally and acting locally with TOR. Curr Opin Cell Biol. 2006;18(6):589–97. doi: 10.1016/j.ceb.2006.09.005. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Richardson CJ, Tee AR, Cheatham L, Tsou C, Blenis J. mTOR controls cell cycle progression through its cell growth effectors S6K1 and 4E-BP1/eukaryotic translation initiation factor 4E. Mol Cell Biol. 2004;24(1):200–216. doi: 10.1128/MCB.24.1.200-216.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XM, Blenis J. Molecular mechanisms of mTOR-mediated translational control. Nat Rev Mol Cell Biol. 2009;10(5):307–18. doi: 10.1038/nrm2672. [DOI] [PubMed] [Google Scholar]
- Polak P, Hall MN. mTOR and the control of whole body metabolism. Curr Opin Cell Biol. 2009;21(2):209–18. doi: 10.1016/j.ceb.2009.01.024. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Luo J, Cantley LC. The evolution of phosphatidylinositol 3-kinases as regulators of growth and metabolism. Nat Rev Genet. 2006;7(8):606–19. doi: 10.1038/nrg1879. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17(6):596–603. doi: 10.1016/j.ceb.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441(7092):424–30. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- Proud CG. mTOR-mediated regulation of translation factors by amino acids. Biochem Biophys Res Commun. 2004;313(2):429–36. doi: 10.1016/j.bbrc.2003.07.015. [DOI] [PubMed] [Google Scholar]
- Fang Y, Vilella-Bach M, Bachmann R, Flanigan A, Chen J. Phosphatidic acid-mediated mitogenic activation of mTOR signaling. Sci Signal. 2001;294(5548):1942. doi: 10.1126/science.1066015. [DOI] [PubMed] [Google Scholar]
- Peng T, Golub TR, Sabatini DM. The immunosuppressant rapamycin mimics a starvation-like signal distinct from amino acid and glucose deprivation. Mol Cell Biol. 2002;22(15):5575–84. doi: 10.1128/MCB.22.15.5575-5584.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Peterson TR, Shaul YD, Lindquist RA, Thoreen CC, Bar-Peled L, Sabatini DM. The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Sci Signal. 2008;320(5882):1496. doi: 10.1126/science.1157535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie GR, Hajduch E, Hundal HS, Proud CG, Taylor PM. Intracellular sensing of amino acids in xenopus laevis oocytes stimulates p70 S6 kinase in a target of rapamycin-dependent manner. J Biol Chem. 2002;277(12):9952–7. doi: 10.1074/jbc.M107694200. [DOI] [PubMed] [Google Scholar]
- Bolster DR, Crozier SJ, Kimball SR, Jefferson LS. AMP-activated protein kinase suppresses protein synthesis in rat skeletal muscle through down-regulated mammalian target of rapamycin (mTOR) signaling. J Biol Chem. 2002;277(27):23977–23980. doi: 10.1074/jbc.C200171200. [DOI] [PubMed] [Google Scholar]
- Chong WPK, Sim LC, Wong KTK, Yap MGS. Enhanced IFNγ production in adenosine-treated CHOCells: a mechanistic study. Biotechnol Prog. 2009;25(3):866–873. doi: 10.1002/btpr.118. [DOI] [PubMed] [Google Scholar]
- Dennis PB, Jaeschke A, Saitoh M, Fowler B, Kozma SC, Thomas G. Mammalian TOR: a homeostatic ATP sensor. Science. 2001;294(5544):1102–5. doi: 10.1126/science.1063518. [DOI] [PubMed] [Google Scholar]
- Dreesen IA, Fussenegger M. Ectopic expression of human mTOR increases viability, robustness, cell size, proliferation, and antibody production of chinese hamster ovary cells. Biotechnol Bioeng. 2011;108(4):853–66. doi: 10.1002/bit.22990. [DOI] [PubMed] [Google Scholar]
- Browne SM, Al-Rubeai M. Analysis of an artificially selected GS-NS0 variant with increased resistance to apoptosis. Biotechnol Bioeng. 2011;108(4):880–92. doi: 10.1002/bit.22994. [DOI] [PubMed] [Google Scholar]
- O’Callaghan PM, McLeod J, Pybus LP, Lovelady CS, Wilkinson SJ, Racher AJ, Porter A, James DC. Cell line-specific control of recombinant monoclonal antibody production by CHO cells. Biotechnol Bioeng. 2010;106(6):938–951. doi: 10.1002/bit.22769. [DOI] [PubMed] [Google Scholar]
- Calvisi DF, Wang C, Ho C, Ladu S, Lee SA, Mattu S, Destefanis G, Delogu S, Zimmermann A, Ericsson J. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterol. 2011;140(3):1071–1083. doi: 10.1053/j.gastro.2010.12.006. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Missiaglia E, Dalai I, Barbi S, Beghelli S, Falconi M, della Peruta M, Piemonti L, Capurso G, Di Florio A, delle Fave G. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28(2):245–255. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boller D, Schramm A, Doepfner KT, Shalaby T, von Bueren AO, Eggert A, Grotzer MA, Arcaro A. Targeting the phosphoinositide 3-kinase isoform p110δ impairs growth and survival in neuroblastoma cells. Clin Cancer Res. 2008;14(4):1172–81. doi: 10.1158/1078-0432.CCR-07-0737. [DOI] [PubMed] [Google Scholar]
- Kang S, Bader AG, Vogt PK. Phosphatidylinositol 3-kinase mutations identified in human cancer are oncogenic. Proc Natl Acad Sci USA. 2005;102(3):802–807. doi: 10.1073/pnas.0408864102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toschi A, Lee E, Xu L, Garcia A, Gadir N, Foster DA. Regulation of mTORC1 and mTORC2 complex assembly by phosphatidic acid: competition with rapamycin. Mol Cell Biol. 2009;29(6):1411–1420. doi: 10.1128/MCB.00782-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E, Goraksha-Hicks P, Li L, Neufeld TP, Guan KL. Regulation of TORC1 by Rag GTPases in nutrient response. Nat Cell Biol. 2008;10(8):935–945. doi: 10.1038/ncb1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sancak Y, Bar-Peled L, Zoncu R, Markhard AL, Nada S, Sabatini DM. Ragulator-Rag complex targets mTORC1 to the lysosomal surface and is necessary for its activation by amino acids. Cell. 2010;141(2):290–303. doi: 10.1016/j.cell.2010.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holz MK, Ballif BA, Gygi SP, Blenis J. mTOR and S6K1 mediate assembly of the translation preinitiation complex through dynamic protein interchange and ordered phosphorylation events. Cell. 2005;123(4):569–80. doi: 10.1016/j.cell.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Kimball SR, Shantz LM, Horetsky RL, Jefferson LS. Leucine regulates translation of specific mRNAs in L6 myoblasts through mTOR-mediated changes in availability of eIF4E and phosphorylation of ribosomal protein S6. J Biol Chem. 1999;274(17):11647–11652. doi: 10.1074/jbc.274.17.11647. [DOI] [PubMed] [Google Scholar]
- Neshat MS, Mellinghoff IK, Tran C, Stiles B, Thomas G, Petersen R, Frost P, Gibbons JJ, Wu H, Sawyers CL. Enhanced sensitivity of PTEN-deficient tumors to inhibition of FRAP/mTOR. Proc Natl Acad Sci. 2001;98(18):10314–10319. doi: 10.1073/pnas.171076798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahin F, Kannangai R, Adegbola O, Wang J, Su G, Torbenson M. mTOR and P70 S6 kinase expression in primary liver neoplasms. Clin Cancer Res. 2004;10(24):8421–8425. doi: 10.1158/1078-0432.CCR-04-0941. [DOI] [PubMed] [Google Scholar]
- Hayward BE, Hussain A, Wilson RH, Lyons A, Woodcock V, McIntosh B, Harris TJR. The cloning and nucleotide sequence of cDNA for an amplified glutamine synthetase gene from the Chinese hamster. Nucl Acids Res. 1986;14(2):999–1008. doi: 10.1093/nar/14.2.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalwy S, Rance J, Young R. Toward more efficient protein expression. Mol Biotechnol. 2006;34(2):151–156. doi: 10.1385/MB:34:2:151. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25(4):402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Latres E, Amini AR, Amini AA, Griffiths J, Martin FJ, Wei Y, Lin HC, Yancopoulos GD, Glass DJ. Insulin-like growth factor-1 (IGF-1) inversely regulates atrophy-induced genes via the phosphatidylinositol 3-kinase/Akt/mammalian target of rapamycin (PI3K/Akt/mTOR) pathway. J Biol Chem. 2005;280(4):2737–2744. doi: 10.1074/jbc.M407517200. [DOI] [PubMed] [Google Scholar]
- Sanchezmargalet V, Goldfine ID, Vlahos CJ, Sung CK. Role of phosphatidylinositol-3-kinase in insulin receptor signaling: studies with inhibitor, LY294002. Biochem Biophys Res Commun. 1994;204(2):446–452. doi: 10.1006/bbrc.1994.2480. [DOI] [PubMed] [Google Scholar]
- Takai H, Wang RC, Takai KK, Yang H, de Lange T. Tel2 regulates the stability of PI3K-related protein kinases. Cell. 2007;131(7):1248–1259. doi: 10.1016/j.cell.2007.10.052. [DOI] [PubMed] [Google Scholar]
- Takai H, Xie Y, de Lange T, Pavletich NP. Tel2 structure and function in the Hsp90-dependent maturation of mTOR and ATR complexes. Genes Dev. 2010;24(18):2019–2030. doi: 10.1101/gad.1956410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edros RZ, McDonnell S, Al-Rubeai M. Using molecular markers to characterize productivity in chinese hamster ovary cell lines. PLoS One. 2013;8(10):e75935. doi: 10.1371/journal.pone.0075935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leevers SJ, Weinkove D, MacDougall LK, Hafen E, Waterfield MD. The Drosophila phosphoinositide 3-kinase Dp110 promotes cell growth. EMBO J. 1996;15(23):6584–94. [PMC free article] [PubMed] [Google Scholar]
- Luo J, McMullen JR, Sobkiw CL, Zhang L, Dorfman AL, Sherwood MC, Logsdon MN, Horner JW, DePinho RA, Izumo S. Class IA phosphoinositide 3-kinase regulates heart size and physiological cardiac hypertrophy. Mol Cell Biol. 2005;25(21):9491–9502. doi: 10.1128/MCB.25.21.9491-9502.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shioi T, Kang PM, Douglas PS, Hampe J, Yballe CM, Lawitts J, Cantley LC, Izumo S. The conserved phosphoinositide 3-kinase pathway determines heart size in mice. EMBO J. 2000;19(11):2537–2548. doi: 10.1093/emboj/19.11.2537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Houghton PJ. Targeting mTOR signaling for cancer therapy. Curr Opin Pharmacol. 2003;3(4):371–377. doi: 10.1016/S1471-4892(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Yee JC, Wlaschin KF, Chuah SH, Nissom PM, Hu WS. Quality assessment of cross-species hybridization of CHO transcriptome on a mouse DNA oligo microarray. Biotechnol Bioeng. 2008;101:1359–1365. doi: 10.1002/bit.21984. [DOI] [PubMed] [Google Scholar]
- Wlaschin KF, Nissom PM, Gatti Mde L, Ong PF, Arleen S, Tan KS, Rink A, Cham B, Wong K, Yap M, Hu WS. EST sequencing for gene discovery in Chinese hamster ovary cells. Biotechnol Bioeng. 2005;91:592–606. doi: 10.1002/bit.20511. [DOI] [PubMed] [Google Scholar]
- Fry MJ. Structure, regulation and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1994;1226(3):237–68. doi: 10.1016/0925-4439(94)90036-1. [DOI] [PubMed] [Google Scholar]
- Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436(1–2):127–50. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Blenis J. Target of rapamycin (TOR): an integrator of nutrient and growth factor signals and coordinator of cell growth and cell cycle progression. Oncogene. 2004;23(18):3151–71. doi: 10.1038/sj.onc.1207542. [DOI] [PubMed] [Google Scholar]
- Fingar DC, Salama S, Tsou C, Harlow E, Blenis J. Mammalian cell size is controlled by mTOR and its downstream targets S6K1 and 4EBP1/eIF4E. Genes Dev. 2002;16(12):1472–1487. doi: 10.1101/gad.995802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazzatti JA, Klingler-Hoffmann M, Haylock-Jacobs S, Harata-Lee Y, Niu M, Higgins MD, Kochetkova M, Hoffmann P, McColl SR. Differential roles for the p101 and p84 regulatory subunits of PI3Kγ in tumor growth and metastasis. Oncogene. 2012;31(18):2350–61. doi: 10.1038/onc.2011.414. [DOI] [PubMed] [Google Scholar]
- Dogra C, Changotra H, Wergedal JE, Kumar A. Regulation of phosphatidylinositol 3-kinase (PI3K)/Akt and nuclear factor-kappa B signaling pathways in dystrophin-deficient skeletal muscle in response to mechanical stretch. J Cell Physiol. 2006;208(3):575–585. doi: 10.1002/jcp.20696. [DOI] [PubMed] [Google Scholar]
- Guo C, Gasparian AV, Zhuang Z, Bosykh DA, Komar AA, Gudkov AV, Gurova KV. 9-Aminoacridine-based anticancer drugs target the PI3K/AKT/mTOR, NF-kappaB and p53 pathways. Oncogene. 2009;28(8):1151–61. doi: 10.1038/onc.2008.460. [DOI] [PubMed] [Google Scholar]
- Avni D, Biberman Y, Meyuhas O. The 5′ terminal oligopyrimidine tract confers translational control on TOP mRNAs in a cell type-and sequence context-dependent manner. Nucleic Acids Res. 1997;25(5):995–1001. doi: 10.1093/nar/25.5.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jefferies H, Reinhard C, Kozma S, Thomas G. Rapamycin selectively represses translation of the “polypyrimidine tract” mRNA family. Proc Natl Acad Sci. 1994;91(10):4441–4445. doi: 10.1073/pnas.91.10.4441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terada N, Patel HR, Takase K, Kohno K, Nairn AC, Gelfand EW. Rapamycin selectively inhibits translation of mRNAs encoding elongation factors and ribosomal proteins. Proc Natl Acad Sci. 1994;91(24):11477–11481. doi: 10.1073/pnas.91.24.11477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroda A, Rauch TA, Todorov I, Ku HT, Al-Abdullah IH, Kandeel F, Mullen Y, Pfeifer GP, Ferreri K. Insulin gene expression is regulated by DNA methylation. PLoS One. 2009;4(9):e6953. doi: 10.1371/journal.pone.0006953. [DOI] [PMC free article] [PubMed] [Google Scholar]