Abstract
Background
Acute kidney injury is a common clinical comorbidity and early diagnosis is crucial for improving prognosis, but there is still no ideal biomarker for early diagnosis.
Material/Methods
miRNA microarray was used for detecting miRNA in kidney subjected to renal ischemia-reperfusion injury 12 h after reperfusion. Real-time PCR was performed to validate the results of microarray. miRNAs in the ischemia group were twice as high as in the sham group. Kidney-enriched miR-10a, miR-192, and miR-194 were detected in rat plasma to screen potential biomarkers for renal ischemia-reperfusion injury. Aberrant expressed miRNA in plasma at 12 h were further detected at 1 h, 2 h, 6 h, 12 h, and 24 h to observe the changing trend of these miRNAs and were compared to blood urea nitrogen and serum creatinine.
Results
Thirty-six miRNAs were aberrantly expressed in kidney of rats with renal ischemia-reperfusion injury, among which 15 miRNAs had a 2-fold greater change. Results of real-time PCR were generally in accordance with microarray results. Levels of the 15 miRNAs differentially expressed in injured kidney were not significantly different from those in sham kidney. However, miR-10a, miR-192, and miR-194 were significantly increased in plasma of rats with renal ischemia-reperfusion injury, among which miR-10a was elevated within 1 h after reperfusion, whereas miR-192 and miR-194 were elevated at 6 h after injury. Blood urea nitrogen was increased at 12 h and serum creatinine was increased at 6 h after injury.
Conclusions
Plasma miR-10a, miR-192, and miR-194 were potential biomarkers for renal ischemia reperfusion injury in rats, and miR-10a might be the most promising plasma biomarker for renal injury because of its elevation within 1 h after renal injury, as well as renal specificity.
Keywords: Reperfusion Injury, Acute Kidney Injury, Biological Markers, MicroRNAs
Background
Acute kidney injury (AKI) is a common co-morbidity among critically ill patients, as well as an important cause of mortality in the intensive care unit [1,2]. Early diagnosis of AKI is a key factor for intervention in disease progression, but the conventional diagnostic method is always delayed by the late elevation of blood urea nitrogen (BUN) and serum creatinine (SCr) [3]. Several biomarkers have been developed for early diagnosis of AKI, such as neutrophil gelatinase-associated lipocalin (NGAL) and kidney injury molecule-1 (KIM-1). NGAL was reported to be elevated within 2 h after acute kidney injury induced by cardiopulmonary bypass in children, with a high diagnostic efficacy [4]. But the area under the curve (AUC) of the receiver operating characteristic (ROC) of NGAL in adult trials was much lower, demonstrating that NGAL level might be affected by confounding factors such as pre-existing disease or infection [5]. Elevation of urinary KIM-1 occurred within 24 h, much later than NGAL [6]. However, recent studies showed that NGAL and KIM-1 might also be biomarkers for heart failure [7,8]. The specificity of NGAL and KIM-1 in diagnosing AKI should be further validated in well-designed clinical trials [9].
microRNAs (miRNAs) are groups of endogenous small non-coding RNAs, regulating translation of mRNA by binding to the 3′ untranslated region and mediating many kinds of physiological and pathophysiological processes [10–12]. They are also detectable in blood or urine in a stable form resistant to RNase A digestion [13,14]. Circulating or urinary miRNAs were believed to be potential biomarkers for many kinds of diseases, among which cancer were first identified to be discriminated by serum miRNAs [13,14]. Organ-enriched miRNAs were thought to be potential biomarkers for organ injury. For example, miR-208a was specifically increased in patients with acute myocardial infarction (AMI) within 4 h after disease onset, but not in patients with non-AMI coronary heart disease [15]. In kidney injury, plasma miR-10a was first reported to be increased 6 h after renal infarction in rats [16]. However, Wang et al. [17] found that serum miR-10a was elevated in urine but not serum at 24 h after renal ischemia-reperfusion injury (IRI) in mice. Other miRNA biomarkers for kidney injury include miR-30d [17], miR-210 [18], and miR-494 [19]. However, the dynamic changes of miRNA after kidney injury were not evaluated in these studies.
Therefore, the present study was performed to detect plasma miRNA levels at different time points after renal IRI in rats. The candidate miRNAs included the aberrantly-expressed miRNAs in IRI kidney, as well as kidney-enriched miR-10a, miR-192, and miR-194 [20].
Material and Methods
Ethics statement
All animal experiments were approved by the Animal Care and Use Committee of Changhai Hospital and carried out in accordance with the National Institutes of Health Guide for Use of Laboratory Animals.
Rat renal ischemia-reperfusion injury model
The rat renal IRI model was established according to Bellini et al. [21]. In brief, rats were anesthetized with 280–350 mg/kg 10% chloral hydrate and a transabdominal midline incision was made after skin disinfection. Bilateral renal pedicles were exposed and occluded by micro vessel clamps. The wound was covered by wet gauzes and 45 min later the clamps were released. The abdominal incision was closed by continuous suture in 2 layers. Rats in the sham group underwent a similar operation without the pedicle clamp. To verify the success of modeling, serum creatinine and urea nitrogen were detected. For miRNA screening with microarray, kidney was harvested 12 h after reperfusion in 3 rats with renal IRI and 3 rats in the sham group. For plasma miRNA detection, 1 ml of blood was collected by the tail-cutting method followed by abdominal infusion with 1 ml of 0.9% saline at 1 h, 2 h, 6 h, 12 h, and 24 h after reperfusion in 6 rats with renal IRI and 6 rats in the sham group.
Total RNA extraction
RNA extraction was performed as we described previously [22]. Briefly, kidney was homogenated in 1 ml of TRIzol solution (Invitrogen, USA), followed by 200 μl of chloroform. After centrifuge at 12000 g, 500 μl of isopropanol was added for RNA precipitation. Then 1 ml 75% ethanol was used for washing the precipitation and DEPC water was used for RNA dissolution. For extraction of plasma RNA, 200 μl of plasma was added into 500 μl of TRIzol solution and 100 fmol f mmu-miR-295 was spiked into the mixture as an internal control. Then 200ìl chloroform was added and the mixture was centrifuged at 12000 g for 15 min. The supernatant was transferred to an absorption column provided by a miRcute miRNA extraction kit (TIANGEN, China). The same volume of Solution C in the kit was added was added for RNA precipitation. RNA was absorbed in the membrane of the column and the waste solution was removed by centrifuge. Finally, RNA in the column was dissolved in 20 μl of eluent.
miRNA microarray assay
miRNA expressions in IRI kidney was screened by microarray assay provided by LC Science (USA). The small RNAs (<300 nt) was isolated from the total RNA by a YM-100 Microcon centrifugal filter (Millipore, USA) and extended with a poly(A) tail using poly(A) polymerase. An oligonucleotide tag was then ligated to the poly(A) tail for later fluorescent dye staining; 2 different tags were used for the 2 RNA samples in dual-sample experiments. Hybridization was performed overnight on a μParaflo™ microfluidic chip using a micro-circulation pump (Atactic Technologies, USA). On the microfluidic chip, each detection probe consisted of a chemically modified nucleotide coding segment complementary to target miRNA (from miRBase, http://microrna.sanger.ac.uk/sequences/) or other RNA (control or customer defined sequences) and a spacer segment of polyethylene glycol to extend the coding segment away from the substrate. The detection probes were made by in situ synthesis using PGR (photo-generated reagent) chemistry. The hybridization melting temperatures were balanced by chemical modifications of the detection probes. Hybridization was performed using 100 μl 6xSSPE buffer (0.90 M NaCl, 60 mM Na2HPO4, 6 mM EDTA, pH 6.8) containing 25% formamide at 34°C. After hybridization, the sample was detected with fluorescence labeling using tag-specific Cy3 and Cy5 dyes. Hybridization images were collected using a laser scanner (GenePix 4000B, Molecular Device, USA) and digitized using Array-Pro image analysis software (Media Cybernetics, USA). Data were analyzed by first subtracting the background and then normalizing the signals using a LOWESS filter (locally-weighted regression). For 2 color experiments, the ratio of the 2 sets of detected signals (log2 transformed, balanced) and p-values of the t-test were calculated; differentially detected signals were those with p values less than 0.01. miRNAs that increased greater than 2-fold were selected for further plasma detection.
Quantitative real-time PCR
Microarray results were validated by quantitative real-time PCR (qRT-PCR). qRT-PCR was also performed to detect plasma miRNA. The candidate miRNA included aberrantly-expressed miRNAs in microarray, as well as miR-10a, miR-192, and miR-194. miRNAs that were altered significantly 12 h after reperfusion were then detected at 1 h, 2 h, 6 h, and 24 h to observe the level at different time points. qRT-PCR was performed using miScript RT II Kit (Qiagen, USA), miScript SYBR Green PCR Kit (Qiagen, USA), and miScript Primer Assay (Qiagen, USA). The procedure for reverse transcription was 37°C for 60 min, followed by 95°C 5 min. PCR procedures included 95°C for 15 min; 94°C for 15 s, 55°C for 30 s, 70°C for 30 s, 40 cycles. ΔΔCt value was calculated for PCR results.
Bioinformatics
Aberrantly expressed miRNAs with a higher than 2-fold change were retrieved in MIRanda (http://www.microrna.com), PicTar (http://pictar.mdc-berlin.de/cgi-bin/new_PicTar_vertebrate.cgi), and TargetScan (http://www.targetscan.org/) to find the potential target genes. Genes potentially related to renal injury and appeared in 2 or more databases and 2 or more species were chosen as possible target genes.
Statistical analysis
All statistical analyses were performed in SPSS 16.0 (USA). Continuous data are presented as mean ±S.E.M. and were compared using Student’s t test. A p value less than 0.05 was considered as statistically significant.
Results
miRNA microarray assay
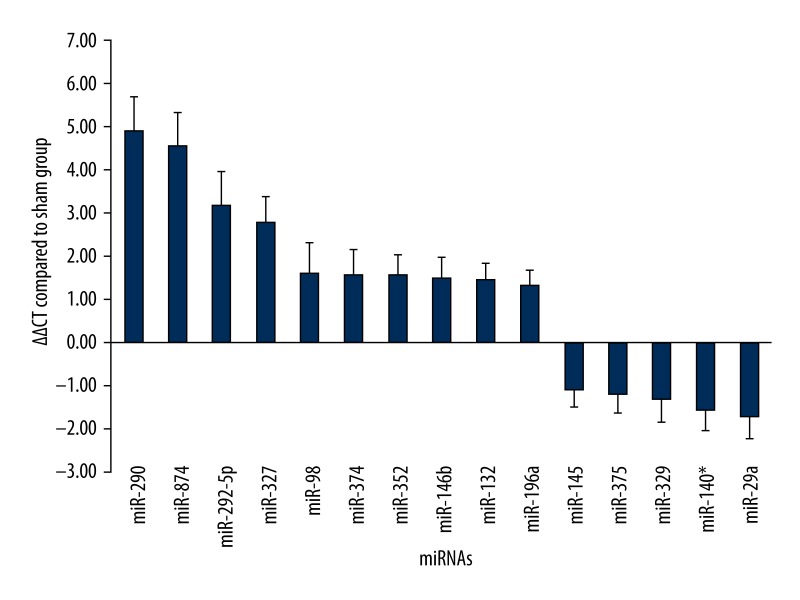
Microarray assay results showed a total of 36 differentially-expressed miRNAs, among which 15 miRNAs were considered as aberrantly expressed with a more than 2-fold change when calculating the ratio of fluorescence intense between the 2 groups. The elevated miRNAs included miR-290, miR-874, miR-292-5p, miR-327, miR-374, miR-98, miR-352, miR-132, miR-146b, and miR-196a. The down-regulated miRNAs included miR-145, miR-329, miR-375, miR-140*, and miR-29a. Validation of microarray assay by qRT-PCR showed similar results and the ΔΔCt value compared to the sham group are shown in Figure 1.
Figure 1.
miRNA levels according to the validation result of quantitative real-time PCR. Y axis referred to the ΔΔCt value of miRNAs with a differential ratio higher than twice.
Potential target genes
Aberrantly expressed miRNAs were classified into inflammation-, autophagy- and apoptosis-, angiogenesis-, and fibrosis-related genes according to their potential target genes (Table 1).
Table 1.
Plasma miRNA levels in rats with renal IRI.
| miRNA | ΔΔCt value |
|---|---|
| miR-29a | 0.71±0.48 |
| miR-98 | −0.41±0.49 |
| miR-132 | 1.44±0.82 |
| miR-140* | −0.26±0.41 |
| miR-145 | −1.01±0.62 |
| miR-146b | −0.08±0.34 |
| miR-196a | 1.35±0.51 |
| miR-290 | 0.47±1.26 |
| miR-292 | 0.80±0.17 |
| miR-374 | 1.93±0.95 |
| miR-874 | 0.70±0.75 |
| miR-10a | 1.35±0.22 |
| miR-192 | 2.38±0.61 |
| miR-194 | 2.22±0.54 |
IRI – ischemia-reperfusion injury.
Plasma miRNA levels in rats with renal IRI
Plasma miRNAs were detected 12 h after reperfusion. miR-327, miR-329, and miR-352 were almost negligible in plasma because of a Ct value of nearly 40. The other 12 miRNAs aberrantly expressed in IRI kidney were comparable to those in the sham group (p>0.05). However, the 3 kidney-enriched miRNAs were all significantly increased in the IRI group compared to the sham group (p<0.05) (Table 2).
Table 2.
Prediction of target genes potentially related to renal ischemia reperfusion injury.
| miRNA | Predicted target gene | Function |
|---|---|---|
| miR-290 | Beclin-1 | Autophage |
| miR-874 | MKK4 | MAPK |
| miR-292-5p | Beclin-1 | Autophage |
| miR-327 | B7-H1 | Apoptosis |
| miR-98 | TGF-β receptor 1 | Fibrosis |
| miR-374 | IL-10 | Inflammation |
| miR-352 | Integrin β8 | Angiogenesis |
| miR-146b | IRAK1 | NF-κB |
| miR-132 | MAPK1 | MAPK |
| miR-196a | IGF-1 | Proliferation |
| miR-145 | Angiopoietin-2 | Angiogenesis |
| miR-375 | CADM1 | Leukocyte adhesion |
| miR-329 | Integrin β3 | Platelet adhesion |
| miR-140* | TGF-β3 | Fibrosis |
| miR-29a | B7-H3 | Inflammation |
miRNA level at different time points in rats with renal IRI
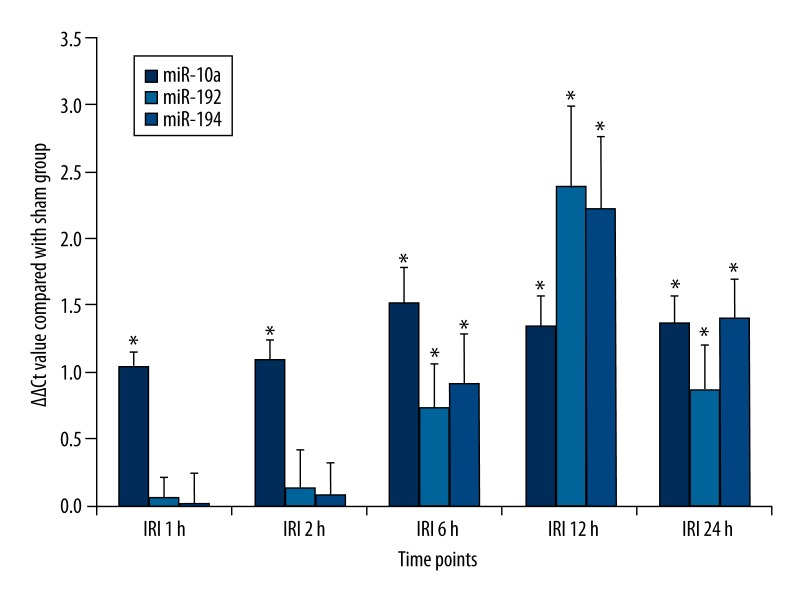
miRNA levels at different time points were determined to investigate how early the miRNAs could be used as biomarkers for renal IRI. As a result, miR-10a was elevated 1 h after reperfusion and peaked at 6 h. Its levels at the 5 time points in the IRI group were all significantly higher than in the sham group (p<0.05). miR-192 and miR-194 shared a similar changing trend, with an initial elevation at 6 h and a peak at 12 h. Their levels at the 3 time points after 6 h in the IRI group were all higher than in the sham group (p<0.05) (Figure 2).
Figure 2.
Plasma levels of miR-10a, miR-192 and miR-194 in rats with renal ischemia-reperfusion injury at different time points. * p<0.05, compared with sham group.
Blood urea nitrogen and serum creatinine
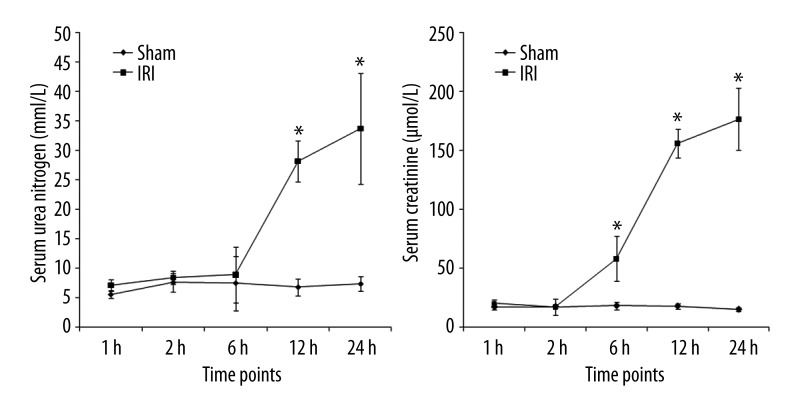
Both BUN and SCr were elevated within 24 h after reperfusion. BUN was elevated after 12 h after reperfusion and SCr was elevated 6 h after reperfusion. Both of them peaked at 24 h (p<0.05) (Figure 3).
Figure 3.
Serum urea nitrogen and creatinine levels in rats with renal ischemia-reperfusion injury at different time points. * p<0.05, compared with sham group.
Discussion
AKI is a serious disease, influencing prognosis of critically ill patients, with a mortality of 45.6% in intensive care units [23]. Renal IRI is the main cause of hospital-acquired AKI. A large-scale epidemiological study involving about 500 hospitals and 29,040 inpatients showed an incidence of 19.2%. Hospital stay of AKI patients was 2 days longer than that of non-AKI patients, with a mortality rate 4.1 times higher [24]. Early diagnosis of AKI is the key process for AKI treatment, but the gold standard of AKI diagnosis is based on BUN and SCr [25,26], which are both elevated at a relatively late stage and are affected by age, muscular content, and protein diet [3]. NGAL and KIM-1 were among the promising biomarkers for early diagnosis of AKI, but both require further validation by well-designed trials because of potentially low specificity [9].
miRNAs were first reported to exist in the circulation in 2008, in a stable form without degradation, despite a 3-h RNase digestion [11,12]. Circulating and urinary miRNAs have been demonstrated to be potential biomarkers for various diseases. miR-21 is regarded as a biomarker for cancer, with both diagnostic and prognostic value [27]. In humans or rats with myocardial injury, miR-1, miR-126, and miR-208 were elevated in the circulation [13,28,29]. In a previous study of ours, serum miR-223 and miR-146a were identified to be potential biomarker for sepsis [22]. Several miRNAs were also revealed to be potential biomarkers for kidney injury, including urinary and plasma miR-10a [14,15], plasma miR-210 [16], urinary miR-30d [15], and miR-494 [17]. However, a disparity in miR-10a findings exists between rat and murine studies. Ji et al. [14] reported that plasma miR-10a was elevated at 6 h after renal infarction in rats, but Wang et al. [15] found no significant difference between renal IRI mice and sham-operated mice regarding plasma miR-10a. In the latter study, miR-10a was reported to be increased in urine of IRI mice. The results of the rat experiments of our present study are in accordance to the previous study. The disparity among species might explain the difference of our findings from the results of Wang et al. [15].
It was not clear why circulating miRNA levels changed during disease progression, and both aberrantly-expressed and organ-enriched miRNAs might be the derivation. However, in the present study, miRNAs highly expressed in IRI kidney did not differ from those in sham kidney, and the 3 miRNAs elevated in plasma were all kidney-enriched miRNAs. This phenomenon might suggest that circulating miRNAs as biomarkers for organ injury comes from the impaired cells. miR-10a was highly expressed in kidney during nephrogenesis [30]. miR-192 and miR-194, although reported to be enriched in kidney and absent in heart, spleen, lung, muscle, and prostate [31], shown to be enriched in liver and to be potential biomarkers for liver injury [31,32]. On the other hand, peak time of miR-192 and miR-194 was at 6 h after reperfusion, similarly to SCr. Therefore, plasma miR-10a might be the optimal biomarker for rat renal IRI.
The microarray results identified a total of 15 miRNAs with at least a 2-fold increase. There were also some other studies that did similar microarray analysis. Liu et al. [33] found that 76 miRNAs were aberrantly expressed in IRI kidney, among which miR-290 had a similar trend to that reported in the present study. In the study of Muratsu-Ikeda et al. [34], miR-29a was significantly up-regulated at 3 h and 10 h in human renal tubular cells after hypoxia and re-oxygenation, b it was down-regulated in our study. The different derivation and species might explain this difference. However, according to the conservation rule, miR-29a and miR-290 might be important miRNAs in the pathogenesis of renal ischemia-reperfusion injury.
The bioinformatics showed that miR-29a and miR-290 were both involved in inflammatory responses. Beclin-1 and B7-H3 might be their target genes, respectively. Chung et al. [35] demonstrated that Beclin-1 was significantly up-regulated in tubular cells from rats with urinary obstruction, accompanied by increased apoptosis and pyroptosis. Blockade of beclin-1 with siRNA might aggregate aristolochic acid-induced tubular apoptosis [36]. Up-regulation of miR-290 might target beclin-1 and inhibit beclin-1 expression, thereby resulting in tubular apoptosis. B7-H3 is a co-stimulatory molecule that inhibits T cell activation. Blockade of B7-H3 might accelerate rejection response after transplantation [37]. Down-regulation of miR-29a in the kidney might induce up-regulation of B7-H3, which might be a negative feedback reaction during renal ischemia-reperfusion injury. The exact pathophysiological effect of these changes remains to be further investigated. Other potential target genes include those involved in inflammation (m mTOR, MAPK, and NF-κB), cell death, and hypoxia-induced proliferation (transforming growth factor and insulin-like growth factor).
There are several limitations to our study. First, disparity among species might hamper the application of results from rats to humans, and we did not replicate our study in clinical trials. Clinical trials is a much larger project, which will be performed by us in a future study. Second, we found that changes in levels of miRNAs in plasma were not as high as changes in blood urea nitrogen and serum creatinine, which might make detection in clinical settings challenging because of the possibility of false-negative or false-positive errors.
Conclusions
In conclusion, the present study screened the aberrantly expressed miRNAs in IRI kidney, but their level in plasma of IRI rats was not significantly different from sham rats. Plasma miR-10a, miR-192, and miR-194 were all elevated in plasma of IRI rats after 12 h, among which miR-10a might be the most promising biomarker for AKI because of the early change (within 1 h after onset of IRI) as well as the renal specificity.
Footnotes
Competing interests
The authors declare that they have no competing interests.
Source of support: This study was supported by the National Natural Scientific Foundation of China (81372038), Scientific Research Grant from Shanghai Health Bureau (2009115), and the 1255 Scientific Grant from Changhai Hospital (CH125541600)
References
- 1.Safian RD, Textor SC. Renal-artery stenosis. N Engl J Med. 2001;344:431–42. doi: 10.1056/NEJM200102083440607. [DOI] [PubMed] [Google Scholar]
- 2.Schrier RW, Wang W. Acute renal failure and sepsis. N Engl J Med. 2004;351:159–69. doi: 10.1056/NEJMra032401. [DOI] [PubMed] [Google Scholar]
- 3.Waikar SS, Bonventre JV. Biomarkers for the diagnosis of acute kidney injury. Curr Opin Nephrol Hypertens. 2007;16:557–64. doi: 10.1097/MNH.0b013e3282f08745. [DOI] [PubMed] [Google Scholar]
- 4.Mishra J, Dent C, Tarabishi R, et al. Neutrophil gelatinase-associated lipocalin (NGAL) as a biomarker for acute renal injury after cardiac surgery. Lancet. 2005;365:1231–38. doi: 10.1016/S0140-6736(05)74811-X. [DOI] [PubMed] [Google Scholar]
- 5.Wagener G, Jan M, Kim M, et al. Association between increases in urinary neutrophil gelatinase-associated lipocalin and acute renal dysfunction after adult cardiac surgery. Anesthesiology. 2006;105:485–91. doi: 10.1097/00000542-200609000-00011. [DOI] [PubMed] [Google Scholar]
- 6.Han WK, Waikar SS, Johnson A, et al. Urinary biomarkers in the early diagnosis of acute kidney injury. Kidney Int. 2008;73:863–69. doi: 10.1038/sj.ki.5002715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cruz DN, Gaiao S, Maisel A, et al. Neutrophil gelatinase-associated lipocalin as a biomarker of cardiovascular disease: a systematic review. Clin Chem Lab Med. 2012;50(9):1533–45. doi: 10.1515/cclm-2012-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlsson AC, Larsson A, Helmersson-Karlqvist J, et al. Urinary kidney injury molecule 1 and incidence of heart failure in elderly men. Eur J Heart Fail. 2013;15:441–46. doi: 10.1093/eurjhf/hfs187. [DOI] [PubMed] [Google Scholar]
- 9.Obermüller N, Geiger H, Weipert C, Urbschat A. Current developments in early diagnosis of acute kidney injury. Int Urol Nephrol. 2013 doi: 10.1007/s11255-013-0448-5. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 10.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116:281–97. doi: 10.1016/s0092-8674(04)00045-5. [DOI] [PubMed] [Google Scholar]
- 11.Xu S, Jin C, Shen X, et al. MicroRNAs as potential novel therapeutic targets and tools for regulating paracrine function of endothelial progenitor cells. Med Sci Monit. 2012;18(7):HY27–31. doi: 10.12659/MSM.883193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zheng Z, Zeng Y, Huang H, et al. MicroRNA-132 may play a role in coexistence of depression and cardiovascular disease: A hypothesis. Med Sci Monit. 2014;20:438–43. doi: 10.12659/MSM.883935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mitchell PS, Parkin RK, Kroh EM, et al. Circulating microRNAs as stable blood-based markers for cancer detection. Proc Natl Acad Sci. 2008;105:10513–18. doi: 10.1073/pnas.0804549105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen X, Ba Y, Ma L, et al. Characterization of microRNAs in serum: a novel class of biomarkers for diagnosis of cancer and other diseases. Cell Res. 2008;18:997–1006. doi: 10.1038/cr.2008.282. [DOI] [PubMed] [Google Scholar]
- 15.Wang GK, Zhu JQ, Zhang JT, et al. Circulating microRNA: a novel potential biomarker for early diagnosis of acute myocardial infarction in humans. Eur Heart J. 2010;31:659–66. doi: 10.1093/eurheartj/ehq013. [DOI] [PubMed] [Google Scholar]
- 16.Ji X, Takahashi R, Hiura Y, et al. Plasma miR-208 as a biomarker of myocardial injury. Clin Chem. 2009;55:1944–49. doi: 10.1373/clinchem.2009.125310. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Zhou Y, Jiang L, et al. Urinary microRNA-10a and microRNA-30d serve as novel, sensitive and specific biomarkers for kidney injury. PLoS One. 2012;7:e51140. doi: 10.1371/journal.pone.0051140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lorenzen JM, Kielstein JT, Hafer C, et al. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin J Am Soc Nephrol. 2011;6:1540–46. doi: 10.2215/CJN.00430111. [DOI] [PubMed] [Google Scholar]
- 19.Lan YF, Chen HH, Lai PF, et al. MicroRNA-494 reduces ATF3 expression and promotes AKI. J Am Soc Nephrol. 2012;23:2012–23. doi: 10.1681/ASN.2012050438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sun Y, Koo S, White N, et al. Development of a microarray to detect human and mouse microRNAs and characterization of expression in human organs. Nucleic Acids Res. 2004;32:e188. doi: 10.1093/nar/gnh186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bellini MH, Coutinho EL, Filgueiras TC, et al. Endostatin expression in the murine model of ischaemia/reperfusion-induced acute renal failure. Nephrology. 2007;12:459–65. doi: 10.1111/j.1440-1797.2007.00850.x. [DOI] [PubMed] [Google Scholar]
- 22.Wang JF, Yu ML, Yu G, et al. Serum miR-146a and miR-223 as potential new biomarkers for sepsis. Biochem Biophys Res Commun. 2010;394:184–88. doi: 10.1016/j.bbrc.2010.02.145. [DOI] [PubMed] [Google Scholar]
- 23.Ostermann M, Chang RW. Acute kidney injury in the intensive care unit according to RIFLE. Crit Care Med. 2007;35:1837–43. doi: 10.1097/01.CCM.0000277041.13090.0A. [DOI] [PubMed] [Google Scholar]
- 24.Liangos O, Wald R, O’Bell JW, et al. Epidemiology and outcome of acute renal failure in hospitalized patients: a national survey. Clin J Am Soc Nephrol. 2006;1:43–51. doi: 10.2215/CJN.00220605. [DOI] [PubMed] [Google Scholar]
- 25.McCullough PA, Shaw AD, Haase M, et al. Diagnosis of acute kidney injury using functional and injury biomarkers: workgroup statements from the tenth acute dialysis quality initiative consensus conference. Contrib Nephrol. 2013;182:13–29. doi: 10.1159/000349963. [DOI] [PubMed] [Google Scholar]
- 26.Ricci Z, Cruz DN, Ronco C. Classification and staging of acute kidney injury: beyond the RIFLE and AKIN criteria. Nat Rev Nephrol. 2011;7:201–8. doi: 10.1038/nrneph.2011.14. [DOI] [PubMed] [Google Scholar]
- 27.Tanaka Y, Kamohara H, Kinoshita K, et al. Clinical impact of serum exosomal microRNA-21 as a clinical biomarker in human esophageal squamous cell carcinoma. Cancer. 2013;119:1159–67. doi: 10.1002/cncr.27895. [DOI] [PubMed] [Google Scholar]
- 28.Zhou X, Mao A, Wang X, et al. Urine and Serum MicroRNA-1 as Novel Biomarkers for Myocardial Injury in Open-Heart Surgeries with Cardiopulmonary Bypass. PLoS One. 2013;8:e62245. doi: 10.1371/journal.pone.0062245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Long G, Wang F, Duan Q, et al. Human circulating microRNA-1 and microRNA-126 as potential novel indicators for acute myocardial infarction. Int J Biol Sci. 2012;8:811–18. doi: 10.7150/ijbs.4439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ho J, Pandey P, Schatton T, et al. The pro-apoptotic protein Bim is a microRNA target in kidney progenitors. J Am Soc Nephrol. 2011;22(6):1053–63. doi: 10.1681/ASN.2010080841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Starkey Lewis PJ, Dear J, et al. Circulating microRNAs as potential markers of human drug-induced liver injury. Hepatology. 2011;54(5):1767–76. doi: 10.1002/hep.24538. [DOI] [PubMed] [Google Scholar]
- 32.Farid WR, Pan Q, van der Meer AJ, et al. Hepatocyte-derived microRNAs as serum biomarkers of hepatic injury and rejection after liver transplantation. Liver Transpl. 2012;18:290–97. doi: 10.1002/lt.22438. [DOI] [PubMed] [Google Scholar]
- 33.Liu F, Lou YL, Wu J, et al. Upregulation of microRNA-210 regulates renal angiogenesis mediated by activation of VEGF signaling pathway under ischemia/perfusion injury in vivo and in vitro. Kidney Blood Press Res. 2012;35:182–91. doi: 10.1159/000331054. [DOI] [PubMed] [Google Scholar]
- 34.Muratsu-Ikeda S, Nangaku M, Ikeda Y, et al. Downregulation of miR-205 modulates cell susceptibility to oxidative and endoplasmic reticulum stresses in renal tubular cells. PLoS One. 2012;7:e41462. doi: 10.1371/journal.pone.0041462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Chung SD, Lai TY, Chien CT, Yu HJ. Activating Nrf-2 signaling depresses unilateral ureteral obstruction-evoked mitochondrial stress-related autophagy, apoptosis and pyroptosis in kidney. PLoS One. 2012;7:e47299. doi: 10.1371/journal.pone.0047299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zeng Y, Yang X, Wang J, et al. Aristolochic acid I induced autophagy extenuates cell apoptosis via ERK 1/2 pathway in renal tubular epithelial cells. PLoS One. 2012;7:e30312. doi: 10.1371/journal.pone.0030312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ueno T, Yeung MY, McGrath M, et al. Intact B7-H3 signaling promotes allograft prolongation through preferential suppression of Th1 effector responses. Eur J Immunol. 2012;42:2343–53. doi: 10.1002/eji.201242501. [DOI] [PMC free article] [PubMed] [Google Scholar]