Abstract
Background
Cysteinyl leukotrienes (cys-LTs) are very important factors in the pathophysiology of bronchial asthma. Cys-LT receptor antagonists (LTRAs) decrease allergic airway inflammation. The aim of the present study was to determine the differential effects of LTRAs and corticosteroids on allergic airway inflammation and allergen-specific cytokine production from lymphoid tissues using a murine model of asthma.
Material/Methods
Four groups of female BALB/c mice [control (Cont); Dermatophagoides farinae allergen-sensitized (AS); pranlukast (Prl), an LTRA-treated AS; and dexamethasone (Dex)-treated AS] were examined. Lung pathology and cytokine production by prepared mononuclear cells isolated from mediastinal lymph nodes (MLNs) and spleen were compared among these groups.
Results
AS mice exhibited allergic airway inflammation and significant increases in allergen-specific Th1 and Th2 cytokines in MLNs and spleen. Prl-treated mice showed significant attenuation of allergic airway inflammation concomitant with reduction of Th2 cytokines and IFN-γ in MLNs but not in spleen. In contrast, Dex significantly decreased Th1 and Th2 cytokines in MLNs and also decreased them (except IL-13 and IL-2) in spleen.
Conclusions
The inflammatory effects of cys-LTs could differ in lymphoid organs. LTRAs potentially regulate allergic airway inflammation in an organ- and cytokine-specific manner, while systemic corticosteroid shows nonspecific effects.
Keywords: Leukotriene Antagonists, Lymphoid Tissue, Pranlukast, Asthma
Background
Bronchial asthma is a chronic airway inflammatory disease [1–4]. Many factors affect a patient’s asthma status. For example, mental condition and environmental factors are known to be important factors [5,6]. The main strategy in the treatment of asthma focuses on suppression of airway inflammation. Inhaled corticosteroid (ICS) is the main controller of asthma therapy due to its effectiveness and safety [2–4,7]. Cysteinyl leukotrienes (cys-LTs) cause airway smooth muscle contraction, airway inflammation, and airway edema [4,8]. Leukotriene receptor antagonists (LTRAs) suppress cys-LTs actions to decrease airway inflammation [4,8–10]. Many reports have shown the effects of LTRAs, and LTRAs are now recognized as being among the main controllers of asthma [11–13]. Our previous reports also showed the efficacy and safety of pranlukast, an LTRA [9–11].
Many kinds of inflammatory cells are involved in the mechanism of asthma [1,2,14–16]. Th1 and Th2 cell balance is an important aspect of the pathophysiology of asthma [1,2,15,16]. Th2 cells are the dominant cells in the allergic airway inflammation of asthma [15,16]. Many reports showed that cys-LTs also affect Th1 and Th2 balance [2,4,8]. Our previous report showed that cys-LTs activate nuclear factor kappa-B (NF-κB) and, upon activation, regulated normal T cell-expressed and secreted (RANTES) production in the same murine model used in this report [17]. Nonetheless, the exact mechanism involved in the interaction between cys-LTs and allergen-specific cytokine production has yet to be determined.
Thus, the present study was designed to determine the effects of cys-LTs on allergen-specific cytokine production from lymphoid tissues, using an LTRA in a murine model of asthma. The effects of LTRA treatment were also compared with those of corticosteroids.
Material and Methods
Animal immunization protocol
Female, 4-week-old, BALB/c mice (Charles River Japan, Inc., Yokohama, Japan) were housed at the Laboratory Animal Center for Biochemical Research, Nagasaki University School of Medicine. The mice were divided into 4 groups according to our previous reports [17,18]. Each group contained 4 mice. All mice were immunized twice intraperitoneally on days 1 and 14 with 0.5 mg/mouse of Dermatophagoides farinae (Df) (LG-5339, Cosmo Bio, Tokyo, Japan) precipitated in aluminum hydroxide. These mice were then challenged intranasally with 50 μL of phosphate-buffered saline (PBS, control group; Cont) or 50 μg/50 μL of Df allergen (allergen-sensitized group; AS) on days 14, 16, and 18. Following this, 0.5 mg/mouse of pranlukast (Ono Pharmaceutical Co., Osaka, Japan), an LTRA, or 0.02 mg/mouse of dexamethasone (Sigma, St. Louis, MO) were injected subcutaneously in Df allergen-sensitized and challenged AS mice from days 13 to 19 [Pranlukast (Prl) and Dexamethasone (Dex) groups, respectively]. On day 21, all mice were sacrificed by dislocation of the cervical vertebrae, and tissues were obtained from each group. The procedures were reviewed and approved by Nagasaki University School of Medicine Committee on Animal Research (No. 917). All experiments were repeated 3 times.
Pathological estimation
Lung specimens were obtained from each mouse and embedded in paraffin, as described previously [17–19]. Inflammation of the lung was assessed after staining the sections with hematoxylin and eosin. Lung specimens were evaluated at least twice in a blinded fashion by 3 different observers as previously described (magnification ×400) [17–19].
Cell counts of bronchoalveolar lavage fluid
Samples of bronchoalveolar lavage fluid (BALF) were evaluated using a hemocytometer and light microscopy. Each BALF sample was centrifuged for 10 minutes at 400 × g at 4°C. The cell pellets were resuspended in 1 mL of PBS. The total number of cells in the BALF was counted using a hemocytometer, and cells on Cytospin slides were fixed and visualized by May-Giemsa staining. Three observers performed differential counts of 200 cells. Absolute cell numbers were calculated as the product of the total and differential cell counts in the BALF.
Cytokine analysis
Mononuclear cells (MNCs) were prepared from 4 groups of mice to determine cytokine production according to our previous reports [17–19]. Mediastinal lymph nodes (MLNs) tissues and spleen were removed from the mice. The removed tissues were chopped by sterile scissors, and digested in a 37°C water bath for 2 hours in digestion buffer containing 1.5 mg/mL collagenase A (type IA, Boehringer Mannheim, Mannheim, Germany), 0.02 mg/mL DNase I (type I, Boehringer Mannheim), and 0.75 mg/mL hyaluronidase (type I, Sigma). After the digestion procedure, the cell pellets were filtered using a metal mesh. Then, filtered digestives were washed 3 times by RPMI 1640 (Gibco BRL, Rockville, MD) containing 10% fetal bovine serum (FBS, Gibco BRL) and 1% penicillin/streptomycin (PC/SM, Gibco BRL), followed by the density gradient method to purify the MNCs. These MNCs were cultured at a density of 1×106/200 μL/well in 96-well plate in an incubator under a 95%O2–5%CO2 gas mixture, at 37°C for 48 hours. Two forms of stimulation were used to culture: no stimulation (none) and 100 μg/mL of Df allergen (Df). The concentrations of IFN-γ, IL-2, IL-4, IL-5, and IL-13 in the culture supernatants were determined by ELISA (Quantikine, R&D Systems Inc., Minneapolis, MN), using the procedures described in the respective instruction manuals.
Statistical analysis
Results are expressed as means ± standard error of mean (SEM). Data were evaluated using repeated-measures ANOVA with a Bonferroni multiple comparison test. A p value <0.05 was considered significant.
Results
LTRA attenuates allergen-induced airway inflammation
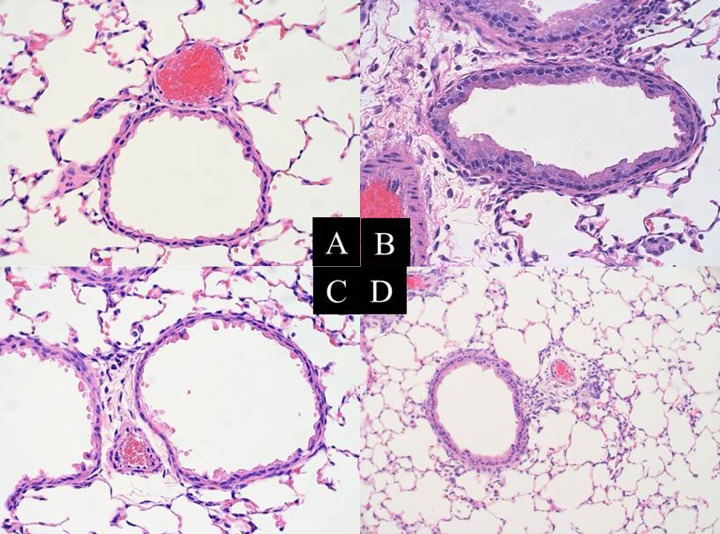
Airway inflammation was examined histopathologically (Figure 1). Cont mice showed normal histology. AS mice showed allergic airway inflammation characterized by inflammatory cellular infiltration and goblet cell hyperplasia. These inflammatory changes were inhibited in Prl and Dex mice. Airway inflammation was also quantified by analysis of the cellular components of BALF (Table 1). In comparison with Cont mice, AS mice showed significant increases in macrophages, lymphocytes, and eosinophils. Prl and Dex significantly reduced allergic airway inflammation, with Dex showing stronger effects than Prl.
Figure 1.
Anti-inflammatory effects of an LTRA and dexamethasone in the allergen-induced lung inflammation of this model. Lung tissue was harvested from 4 groups of mice and stained with hematoxylin and eosin. Representative photomicrographs (×400) from each group (n=12 for each); Cont (A), AS (B), Prl (C), and Dex (D) groups are shown. Inflammatory changes are significantly inhibited in Prl mice. Dex mice show almost complete inhibition of these Df-allergen-induced inflammatory changes.
Table 1.
Cell counts of BALF.
| Macrophages | Neutrophils | Lymphocytes | Eosinophils | |
|---|---|---|---|---|
| Cont | 7.3±2.1 | 0.8±0.2 | 0.2±0.1 | 0 |
| AS | 11.8±4.1 | 2.1±1.9 | 4.2±1.8** | 8.3±2.9**,† |
| Prl | 6.9±2.3 | 1.1±1.0 | 2.3±1.5* | 4.1±3.1** |
| Dex | 7.6±2.5 | 1.2±0.8 | 0.9±1.1 | 1.2±1.1 |
Cell counts of BALF are expressed as mean ± SEM (×105 cells).
p<0.05 compared to Cont;
p<0.01 compared to Cont;
p<0.05 compared to Prl.
Effects of cys-LTs on allergen-specific cytokine production from lymphoid tissues
MNCs prepared from the MLNs or from the spleen were cultured with medium stimulation (none) or specific allergen stimulation (Df). The concentrations of IFN-γ, IL-2, IL-4, IL-5, and IL-13 in the cultured supernatant were determined by ELISA (Figures 2 and 3).
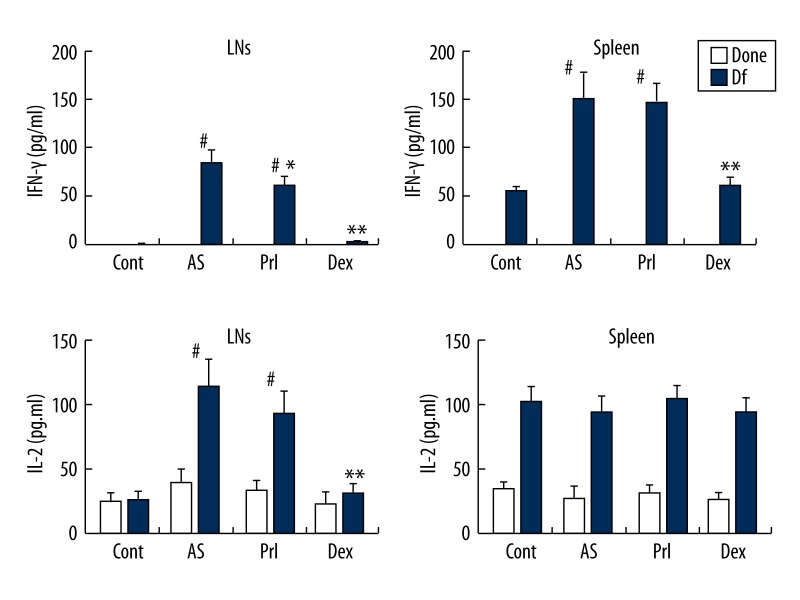
Figure 2.
Th1 cytokine production. Cytokine production from MLN MNCs and splenic MNCs. These MNCs were cultured with medium (negative control, none) or specific allergen (Df). AS mice show significant increases in IFN-γ both in MLNs and splenic MNCs. LTRA inhibits this increase only for IFN-γ production of MLNs. LTRA fails to inhibit the overproduction of IFN-γ from splenic MNCs and IL-2. Systemic steroid inhibits IFN-γ and IL-2 production from MLNs. Bars represents mean (n=12 for each) ±SEM. # p<0.01 vs. Cont, * p<0.05 and ** p<0.01 vs. AS.
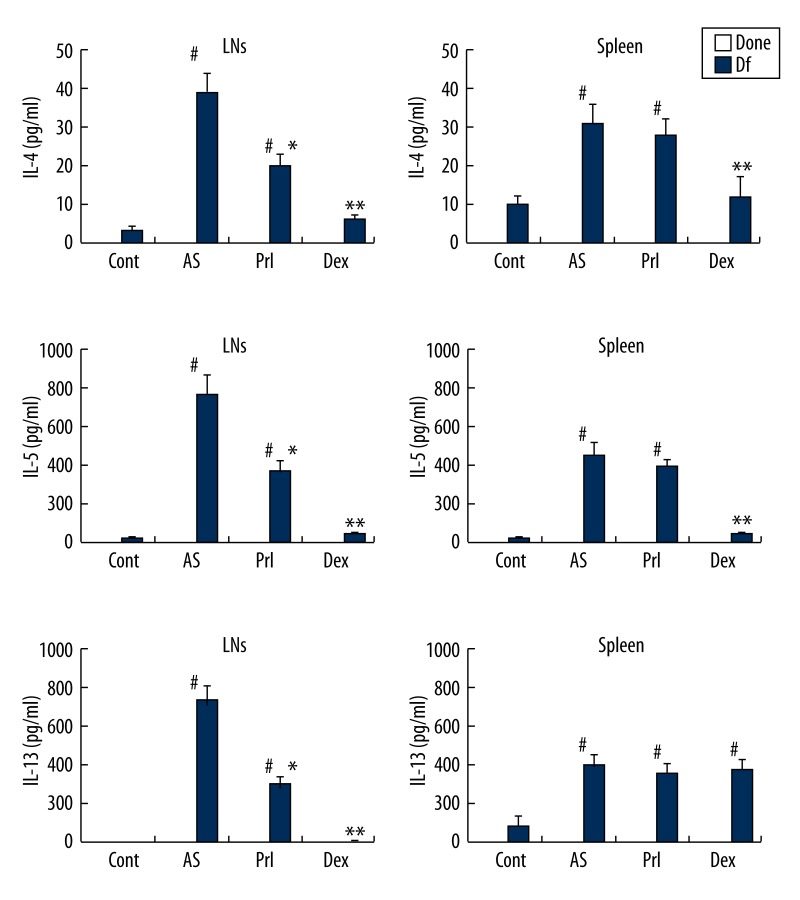
Figure 3.
Th2 cytokine production. Cytokine production from MLN MNCs and splenic MNCs. These MNCs were cultured with medium (negative control, none) or specific allergen (Df). MNCs of MLNs show the same results as for Th2 cytokines (IL-4, IL-5, IL-13). The AS group shows a significant increase in Th2 cytokines with Df stimulation. LTRA and steroid significantly inhibit this increase. Steroid inhibits Th2 cytokines more strongly than LTRA. Bars represents mean (n=12 for each) ±SEM. # p<0.01 vs. Cont, * p<0.05 and ** p<0.01 vs. AS.
Figure 2 shows Th1 cytokine production. AS mice showed significant increases in allergen-specific IFN-γ in MLNs and spleen MNCs. Prl significantly decreased allergen-specific IFN-γ production only in MLNs, while Dex caused significant decreases in MLNs and spleen. Allergen-specific IL-2 production was significantly enhanced in MLNs in AS mice. Prl failed to inhibit allergen-specific IL-2 production in MLNs, while Dex significantly decreased it. No significant differences occurred in allergen-specific IL-2 production in spleen of either group.
AS mice showed significantly increased productions of Th2 cytokines in MLNs and spleen (Figure 3). Prl and Dex significantly attenuated allergen-specific Th2 cytokine production in MLNs of AS mice, with Dex showing significantly stronger effects. In splenic MNCs, LTRA failed to significantly inhibit allergen-specific Th2 cytokine production, while Dex significantly decreased allergen-specific IL-4 and IL-5 productions, but not IL-13 production.
Discussion
The major findings of the present study were: (1) sensitization to Df increased organ-specific cytokine production; (2) LTRA regulated allergic airway inflammation in an organ- and cytokine-specific manner; (3) in contrast, corticosteroids showed non-specific inhibition of cytokine production; and (4) the inhibitory effects of corticosteroids were significantly stronger than those of LTRA.
In the present study, in vivo antagonism of cys-LTs by an LTRA in a murine model of allergic asthma resulted in significant attenuation of allergic airway inflammation concomitant with inhibition of Th2 cytokines (IL-4, IL-5, IL-13) and IFN-γ. Previous reports suggested distinct immunological effects of cys-LTs in bronchial asthma. Underwood et al reported that LTD4-induced airway eosinophilia in the guinea pig was inhibited by IL-5 monoclonal antibody [20]. Montelukast, an LTRA, decreased the number of IL-5-expressing T cells in the airways of sensitized rats [21]. LTD4 induced NF-κB activation and RANTES production in a murine model of asthma [17]. Pranlukast, an LTRA, inhibited the production of mite allergen-specific cytokines from peripheral blood mononuclear cells of patients with asthma [22]. Consistent with these reports, in vivo antagonism of cys-LTs by LTRA in a murine model of allergic asthma resulted in significant attenuation of allergic airway inflammation concomitant with inhibition of Th2 cytokines (IL-4, IL-5, IL-13). In addition to Th2 cytokines, IFN-γ was also inhibited by an LTRA. IFN-γ represents a Th1-like cytokine, but it also shows pro-inflammatory effects, including prolongation of eosinophil survival and increased expression of cell adhesion molecules of the airway epithelium [2,12,15,16]. Thus, in vivo antagonism of cys-LTs by an LTRA resulted in an anti-inflammatory effect in the lung.
The anti-inflammatory effects of the LTRA were localized in MLNs, but not in splenic MNCs, in the present study. Thus, the LTRA showed a different effect in each lymphoid tissue and acted only in inflamed lung-draining LNs. Corticosteroids are the most effective controller medicine for asthma. They show stronger anti-inflammatory effects than LTRAs, but they potentially cause severe systemic adverse effects, including systemic infection due to their immunosuppressive effects. In fact, Dex non-selectively inhibited cytokines in MLNs and spleen. LTRAs have been considered safe for the control of asthma [3,9–11,13]. We previously reported the efficacy and long-term safety of pranlukast [9,11]. The LTRA failed to inhibit allergen-specific cytokine production in splenic MNCs in the present study. The present result that the effects of the LTRA were limited in inflamed tissue could also support the safety of LTRA. In agreement with the present study, an LTRA showed protective effects against viral infection by increasing anti-viral cytokines (IL-10, IL-12) from the dendritic cells in a murine viral infection model, while systemic corticosteroid non-selectively inhibited cytokine production [19].
The critical limitation of this study is that the LTRA mechanism of the action related to organ-specific cytokine production is not clear. The cys-LT1 receptor has been identified in airway smooth muscle cells, eosinophils, macrophages, monocytes, and mast cells [23]. Because the spleen is composed of many MNCs, it is expected that LTRAs may also act on splenic MNCs. This result suggests that cys-LTs may have immunological actions only in inflammatory organs. Cys-LTs may act differently in each lymphoid tissue in the pathophysiology of bronchial asthma. Previous reports showed that corticosteroids could not inhibit cys-LT production in humans [24,25]. Using an LTRA with a corticosteroid may lead to better control of inflammation compared to using a corticosteroid alone.
Conclusions
The inflammatory effects of cys-LTs could differ in different lymphoid organs. LTRAs potentially regulate allergic airway inflammation in an organ- and cytokine-specific manner, while systemic corticosteroids show nonspecific effects. Since corticosteroids cannot inhibit cys-LT production and action, the present study supports the therapeutic approach of using an LTRA with a corticosteroid, which may lead to organ-specific and safe control of the inflammation of bronchial asthma.
Footnotes
Source of support: Departmental sources
References
- 1.Busse WW, Lemanske RF. Asthma (Review) N Engl J Med. 2001;344:350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 2.Boyce JA, Bochner B, Finkelman FD, Rothenberg ME. Advances in mechanisms of asthma, allergy, and immunology in 2011. J Allergy Clin Immunol. 2011;129(2):335–41. doi: 10.1016/j.jaci.2011.12.968. [DOI] [PubMed] [Google Scholar]
- 3.O’Byrne PM. Therapeutic strategies to reduce asthma exacerbations. J Allergy Clin Immunol. 2011;128(2):257–63. doi: 10.1016/j.jaci.2011.03.035. [DOI] [PubMed] [Google Scholar]
- 4.Tantisira KG, Drazen JM. Genetics and pharmacogenetics of the leukotriene pathway. J Allergy Clin Immunol. 2009;124(3):422–27. doi: 10.1016/j.jaci.2009.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Trzcinska H, Przybylski G, Kozlowski B, Derdowski S. Analysis of the relation between level of asthma control and depression and anxiety. Med Sci Monit. 2012;18(3):CR190–94. doi: 10.12659/MSM.882524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yalcin AD, Basaran S, Bisgin A, et al. Pollen aero allergens and the climate in Mediterranean region and allergen sensitivity in allergic rhinoconjunctivitis and allergic asthma patients. Med Sci Monit. 2014;20:102–10. doi: 10.12659/MSM.883762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erceg D, Nanadic N, Placec D, et al. Inhaled corticosteroids used for the control of asthma in a “real-life” setting do not affect linear growth velocity in prepubertal children. Med Sci Monit. 2012;18(9):CR564–68. doi: 10.12659/MSM.883352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ogawa Y, Calhoun WJ. The role of leukotrienes in airway inflammation. J Allergy Clin Immunol. 2006;118(4):788–98. doi: 10.1016/j.jaci.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 9.Obase Y, Shimoda T, Matsuse H, et al. The position of pranlukast, a cysteinyl leukotriene receptor antagonist, in the long-term treatment of asthma. 5-year follow-up study. Respiration. 2004;71(3):225–32. doi: 10.1159/000077419. [DOI] [PubMed] [Google Scholar]
- 10.Tomari S, Shimoda T, Kawano T, et al. Effects of pranlukast, a cysteinyl leukotriene receptor 1 antagonist, combined with inhaled beclomethasone in patients with moderate or severe asthma. Ann Allergy Asthma Immunol. 2001;87:156–61. doi: 10.1016/S1081-1206(10)62212-0. [DOI] [PubMed] [Google Scholar]
- 11.Obase Y, Shimoda T, Tomari S, et al. Efficacy and safety of long-term treatment of asthmatic patients with pranlukast, a cysteinyl-leukotriene-receptor antagonist: four-year follow-up study. Ann Allergy Asthma Immunol. 2001;87:43–47. doi: 10.1016/s1081-1206(10)62321-6. [DOI] [PubMed] [Google Scholar]
- 12.Holgate ST. Pathophysiology of asthma: what has our current understanding taught us about new therapeutic approaches? J Allergy Clin Immunol. 2011;128(3):495–505. doi: 10.1016/j.jaci.2011.06.052. [DOI] [PubMed] [Google Scholar]
- 13.Montuschi P, Peters-Golden ML. Leukotriene modifiers for asthma treatment. Clin Exp Allergy. 2010;40(12):1732–41. doi: 10.1111/j.1365-2222.2010.03630.x. [DOI] [PubMed] [Google Scholar]
- 14.Lan F, Liu K, Zhang J, et al. Th17 response is augmented in OVA-induced asthmatic mice exposed to HDM. Med Sci Monit. 2011;17(5):BR132–38. doi: 10.12659/MSM.881759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nguyen TH, Casale TB. Immune modulation for treatment of allergic disease. Immuno Rev. 2011;242(1):258–71. doi: 10.1111/j.1600-065X.2011.01034.x. [DOI] [PubMed] [Google Scholar]
- 16.Robinson DS. The role of the T cell in asthma. J Allergy Clin Immunol. 2010;126(6):1081–91. doi: 10.1016/j.jaci.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 17.Kawano T, Matsuse H, Kondo Y, et al. Cysteinyl leukotrienes induce nuclea factor kappa b activation and RANTES production in a murine model of asthma. J Allergy Clin Immunol. 2003;112(2):369–74. doi: 10.1067/mai.2003.1636. [DOI] [PubMed] [Google Scholar]
- 18.Matsuse H, Behera AK, Kumar M, et al. Recurrent respiratory syncytial virus infections in allergen-sensitized mice lead to persistent airway inflammation and hyperresponsiveness. J Immunol. 2000;164:6583–92. doi: 10.4049/jimmunol.164.12.6583. [DOI] [PubMed] [Google Scholar]
- 19.Matsuse H, Hirose H, Fukahori S, et al. Regulation of dendritic cell functions against harmful respiratory pathogens by a cysteinyl leukotrienes receptor antagonists. Allergy Rhinol. 2012;3(1):e30–34. doi: 10.2500/ar.2012.3.0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uunderwood DC, Osborn RR, Newsholme SJ, et al. Persistent airway eosinophilia after leukotriene (LT). D4 administration in the guinea pig: modulation by the LTD4 receptor antagonist, pranlukast, or an interleukin-5 monoclonal antibody. Am J Respir Crit Care Med. 1996;154:850–57. doi: 10.1164/ajrccm.154.4.8887574. [DOI] [PubMed] [Google Scholar]
- 21.Ihaku D, Cameron L, Suzuki M, et al. Montelukast, a leukotriene receptor antagonist, inhibits the late airway response to antigen, airway eosinophilia, and IL-5-expressing cells in Brown Norway rats. J Allergy Clin Immunol. 1999;104:1147–54. doi: 10.1016/s0091-6749(99)70006-0. [DOI] [PubMed] [Google Scholar]
- 22.Tohda Y, Nakahara H, Kubo H, et al. Effects of ONO-1078 (pranlukast). on cytokine production in peripheral blood mononuclear cells of patients with bronchial asthma. Clin Exp Allergy. 1999;29:1532–36. doi: 10.1046/j.1365-2222.1999.00710.x. [DOI] [PubMed] [Google Scholar]
- 23.Singh RK, Gupta S, Dastidar S, Ray A. Cysteinyl leukotrienes and their receptors: Molecular and functional characteristics. Pharmacol. 2010;85:336–49. doi: 10.1159/000312669. [DOI] [PubMed] [Google Scholar]
- 24.Schleimer RP, Schulman ES, MacGlashan DW, Jr, et al. Effects of dexamethasone on mediator release from human lung fragments and purified human lung mast cells. J clin Invest. 1983;71(6):1830–35. doi: 10.1172/JCI110938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Shaughnessy KM, Wellings R, Gillies B, Fuller RW. Differential effects of fluticasone propionate on allergen-evoked bronchocomstriction and increased erinary leukotriene E4 excretion. Am Rev Respir Dis. 1993;147(6 Pt 1):1472–76. doi: 10.1164/ajrccm/147.6_Pt_1.1472. [DOI] [PubMed] [Google Scholar]