Abstract
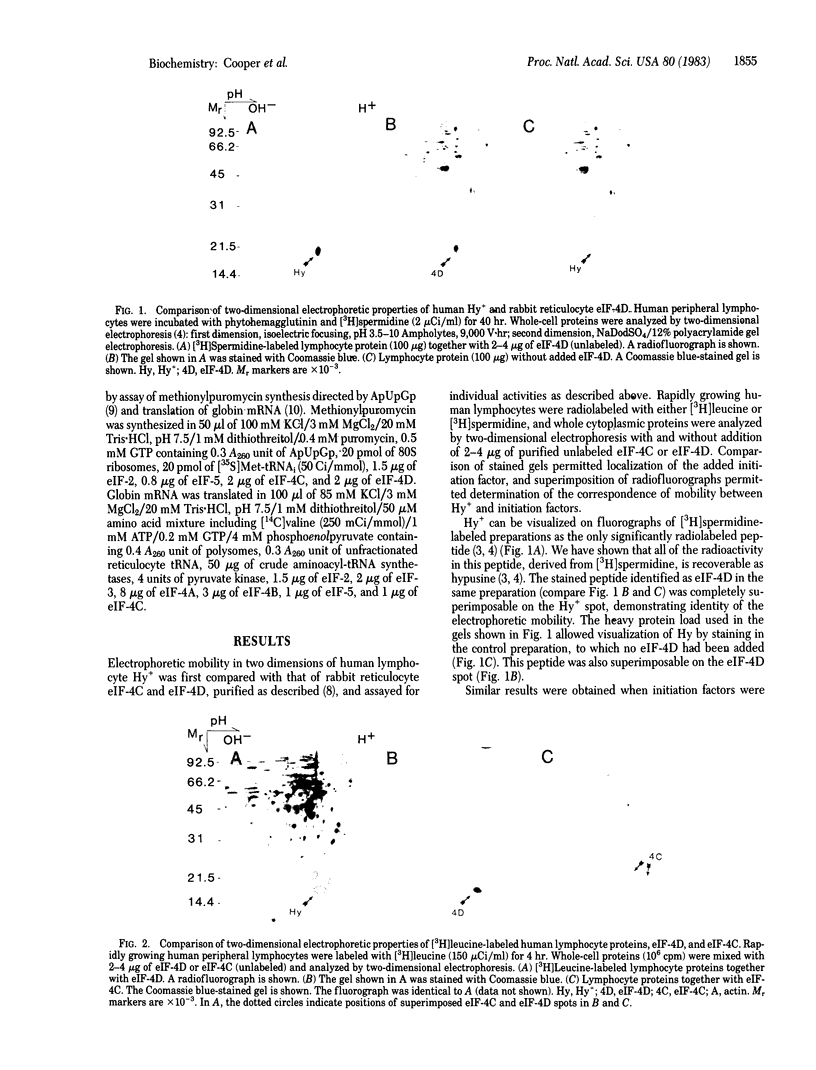
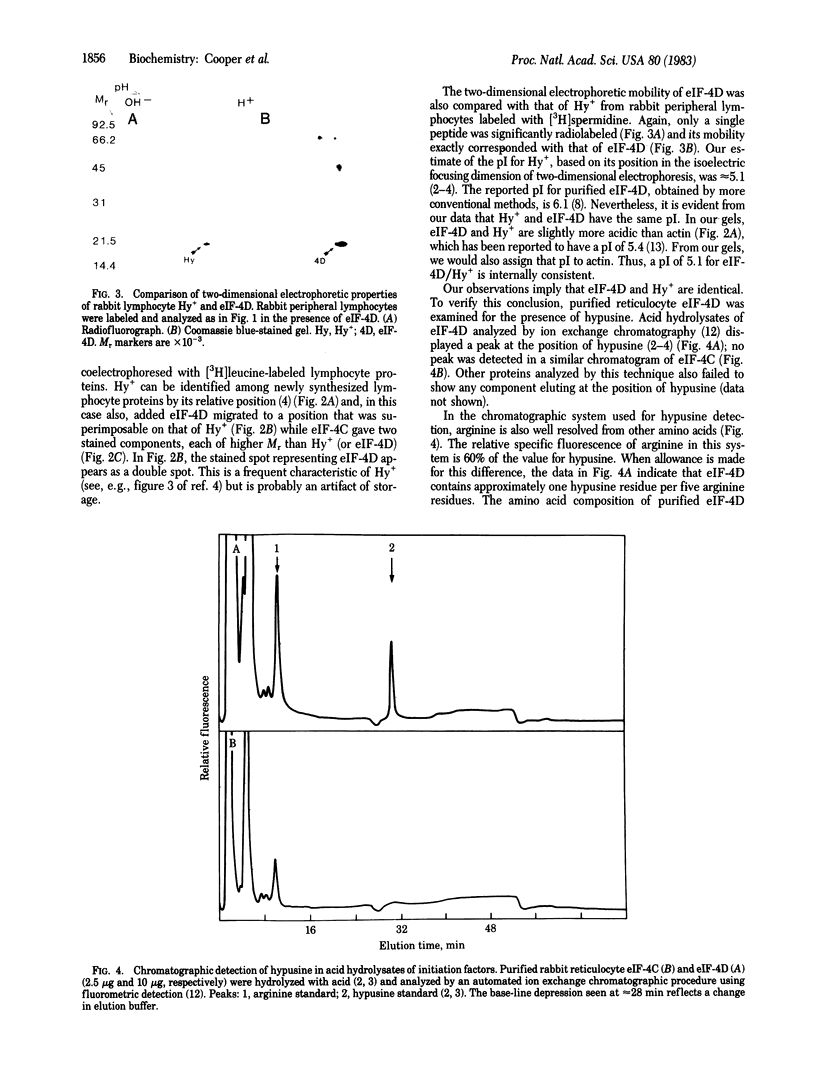
A single protein of Mr 17,000-19,000 and pI approximately equal to 5.1, found in all animal cells we have studied to date, undergoes post-translational modification in growing cells to form the unusual amino acid hypusine. Because of the association of this modification with the increasing rate of protein synthesis during lymphocyte growth stimulation, its subcellular distribution, and its widespread occurrence and structural conservation among animal cells, we considered the possibility that this protein might be a translation initiation factor. Purified rabbit reticulocyte factors (eukaryote initiation factors) eIF-4C and eIF-4D were chosen for study because of their Mr (17,000-19,000) and acidic pI. The hypusine-containing protein and purified eIF-4D showed identity of electrophoretic mobility in both isoelectric focusing and NaDodSO4/polyacrylamide gel electrophoresis dimensions, while eIF-4C was clearly nonidentical. Purified eIF-4D contained approximately 1 mol of hypusine per mol of protein. Since only one protein has thus far been observed to contain hypusine, we conclude that eIF-4D is the hypusine-containing protein. On the basis of relative synthesis among lymphocyte proteins and detection by Coomassie blue staining, we also conclude that eIF-4D is a major cell protein. It is possible that the activity of this factor is modulated by It is possible that the activity of this factor is modulated by post-translational hypusine formation, which may play a role in regulation of protein synthesis during lymphocyte growth stimulation.
Full text
PDF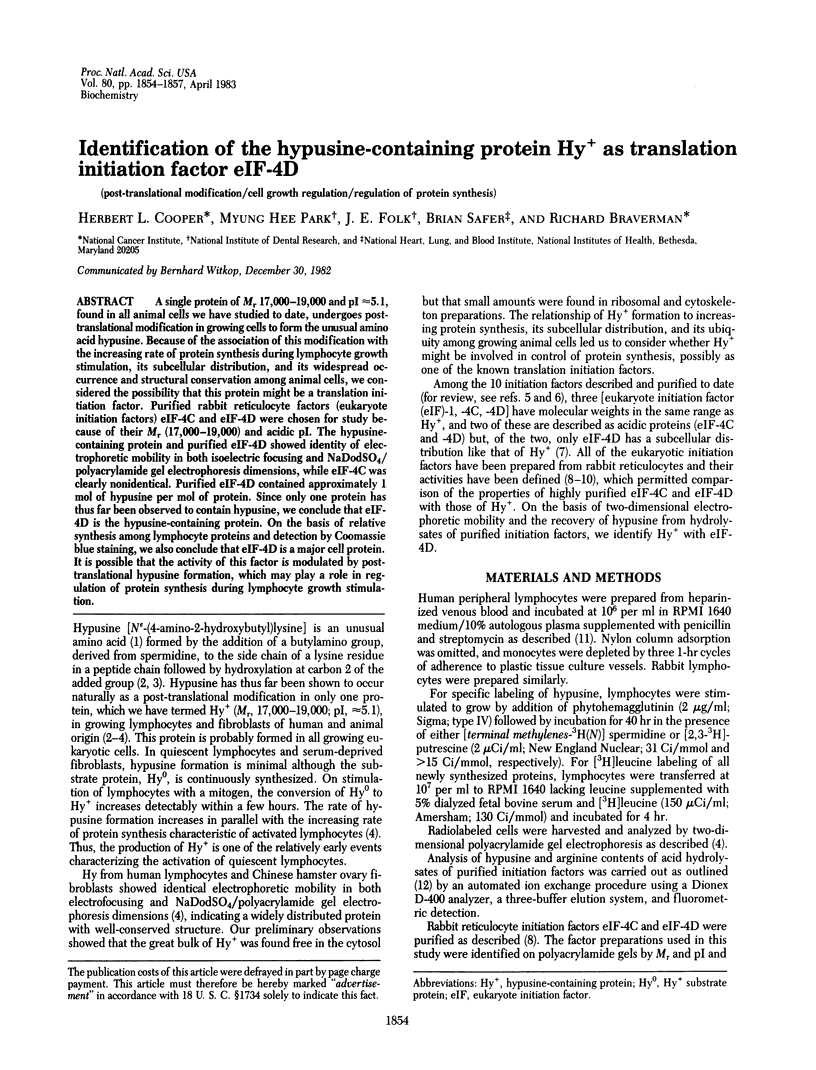

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper H. L., Park M. H., Folk J. E. Posttranslational formation of hypusine in a single major protein occurs generally in growing cells and is associated with activation of lymphocyte growth. Cell. 1982 Jul;29(3):791–797. doi: 10.1016/0092-8674(82)90441-x. [DOI] [PubMed] [Google Scholar]
- Cooper H. L. Purification of lymphocytes from peripheral blood. Methods Enzymol. 1974;32:633–636. doi: 10.1016/0076-6879(74)32065-4. [DOI] [PubMed] [Google Scholar]
- Folk J. E., Park M. H., Chung S. I., Schrode J., Lester E. P., Cooper H. L. Polyamines as physiological substrates for transglutaminases. J Biol Chem. 1980 Apr 25;255(8):3695–3700. [PubMed] [Google Scholar]
- Garrels J. I., Gibson W. Identification and characterization of multiple forms of actin. Cell. 1976 Dec;9(4 Pt 2):793–805. doi: 10.1016/0092-8674(76)90142-2. [DOI] [PubMed] [Google Scholar]
- Jagus R., Anderson W. F., Safer B. The regulation of initiation of mammalian protein synthesis. Prog Nucleic Acid Res Mol Biol. 1981;25:127–185. doi: 10.1016/s0079-6603(08)60484-5. [DOI] [PubMed] [Google Scholar]
- Kemper W. M., Berry K. W., Merrick W. C. Purification and properties of rabbit reticulocyte protein synthesis initiation factors M2Balpha and M2Bbeta. J Biol Chem. 1976 Sep 25;251(18):5551–5557. [PubMed] [Google Scholar]
- Lester E. P., Lemkin P., Lipkin L., Cooper H. L. A two-dimensional electrophoretic analysis of protein synthesis in resting and growing lymphocytes in vitro. J Immunol. 1981 Apr;126(4):1428–1434. [PubMed] [Google Scholar]
- Lester E. P., Lemkin P., Lipkin L., Cooper H. L. Computer-assisted analysis of two-dimensional electrophoreses of human lymphoid cells. Clin Chem. 1980 Sep;26(10):1392–1402. [PubMed] [Google Scholar]
- Merrick W. C. Assays for eukaryotic protein synthesis. Methods Enzymol. 1979;60:108–123. doi: 10.1016/s0076-6879(79)60011-3. [DOI] [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. Identification of hypusine, an unusual amino acid, in a protein from human lymphocytes and of spermidine as its biosynthetic precursor. Proc Natl Acad Sci U S A. 1981 May;78(5):2869–2873. doi: 10.1073/pnas.78.5.2869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M. H., Cooper H. L., Folk J. E. The biosynthesis of protein-bound hypusine (N epsilon -(4-amino-2-hydroxybutyl)lysine). Lysine as the amino acid precursor and the intermediate role of deoxyhypusine (N epsilon -(4-aminobutyl)lysine). J Biol Chem. 1982 Jun 25;257(12):7217–7222. [PubMed] [Google Scholar]
- Safer B., Adams S. L., Kemper W. M., Berry K. W., Lloyd M., Merrick W. C. Purification and characterization of two initiation factors required for maximal activity of a highly fractionated globin mRNA translation system. Proc Natl Acad Sci U S A. 1976 Aug;73(8):2584–2588. doi: 10.1073/pnas.73.8.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiba T., Mizote H., Kaneko T., Nakajima T., Kakimoto Y. Hypusine, a new amino acid occurring in bovine brain. Isolation and structural determination. Biochim Biophys Acta. 1971 Sep 21;244(3):523–531. doi: 10.1016/0304-4165(71)90069-9. [DOI] [PubMed] [Google Scholar]
- Thomas A. A., Benne R., Voorma H. O. Initiation of eukaryotic protein synthesis. FEBS Lett. 1981 Jun 15;128(2):177–185. doi: 10.1016/0014-5793(81)80076-2. [DOI] [PubMed] [Google Scholar]
- Thomas A., Goumans H., Amesz H., Benne R., Voorma H. O. A comparison of the initiation factors of eukaryotic protein synthesis from ribosomes and from the postribosomal supernatant. Eur J Biochem. 1979 Aug 1;98(2):329–337. doi: 10.1111/j.1432-1033.1979.tb13192.x. [DOI] [PubMed] [Google Scholar]





