Abstract
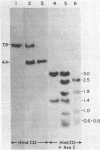
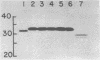
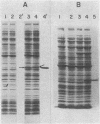
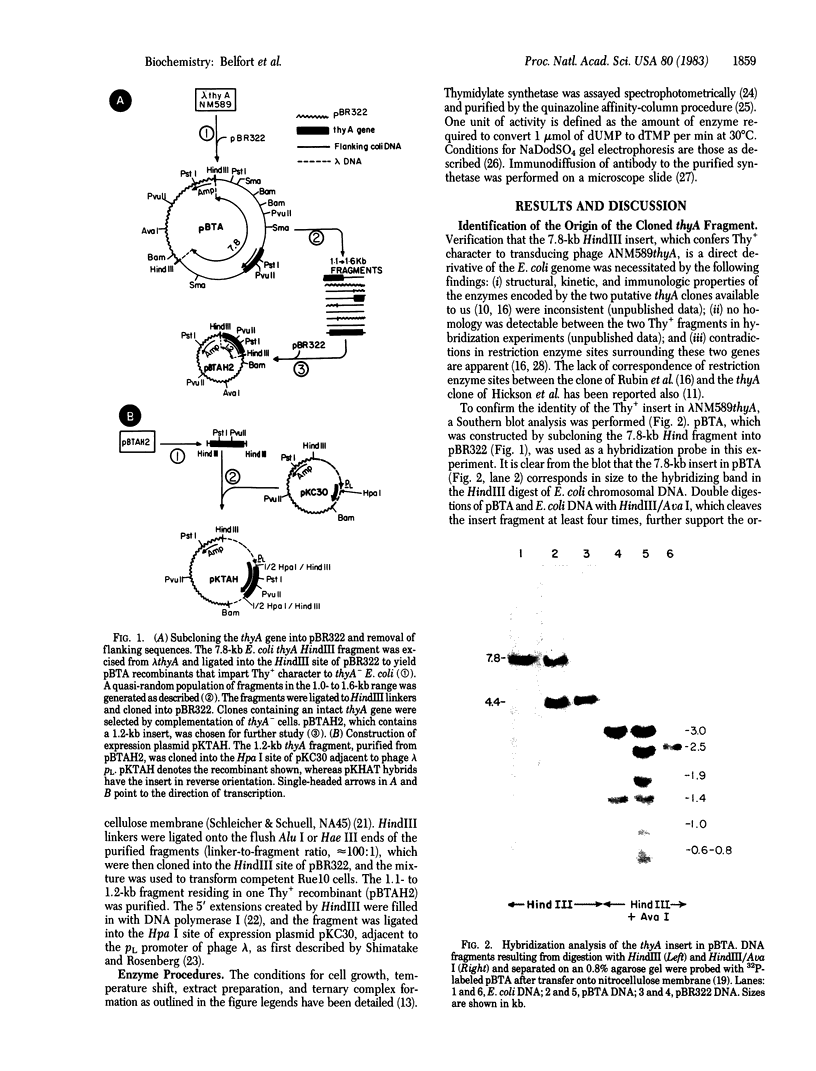
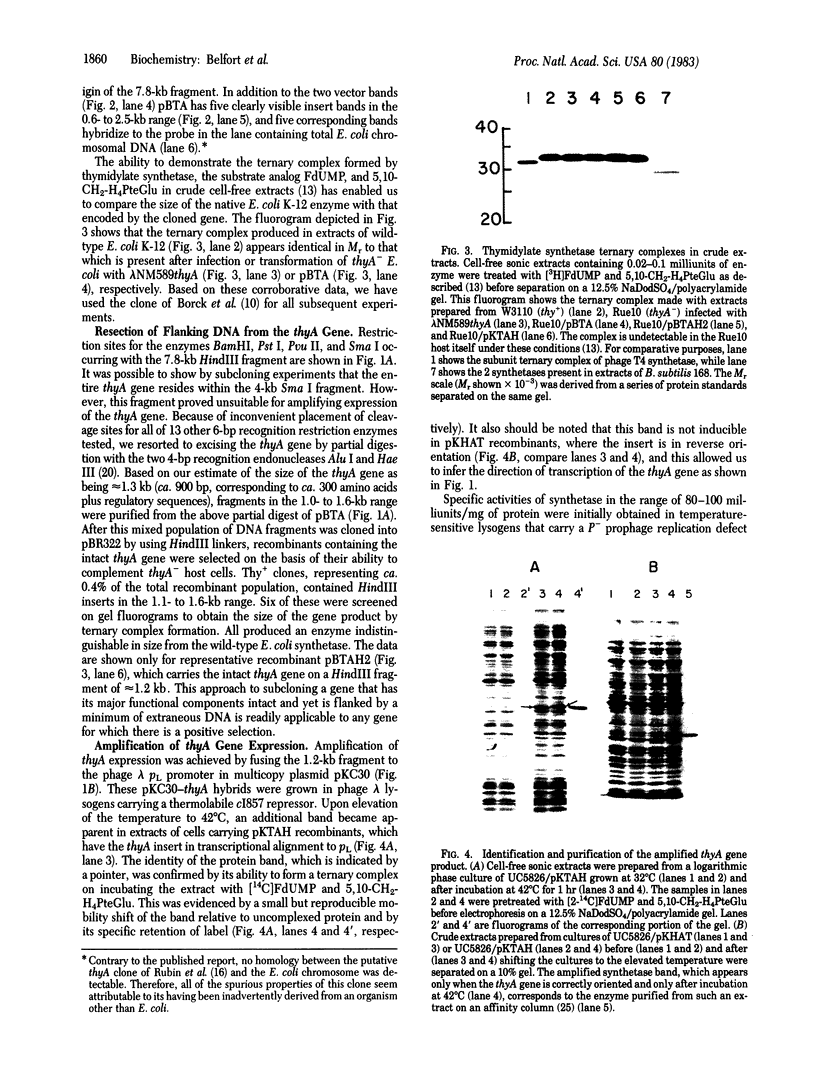
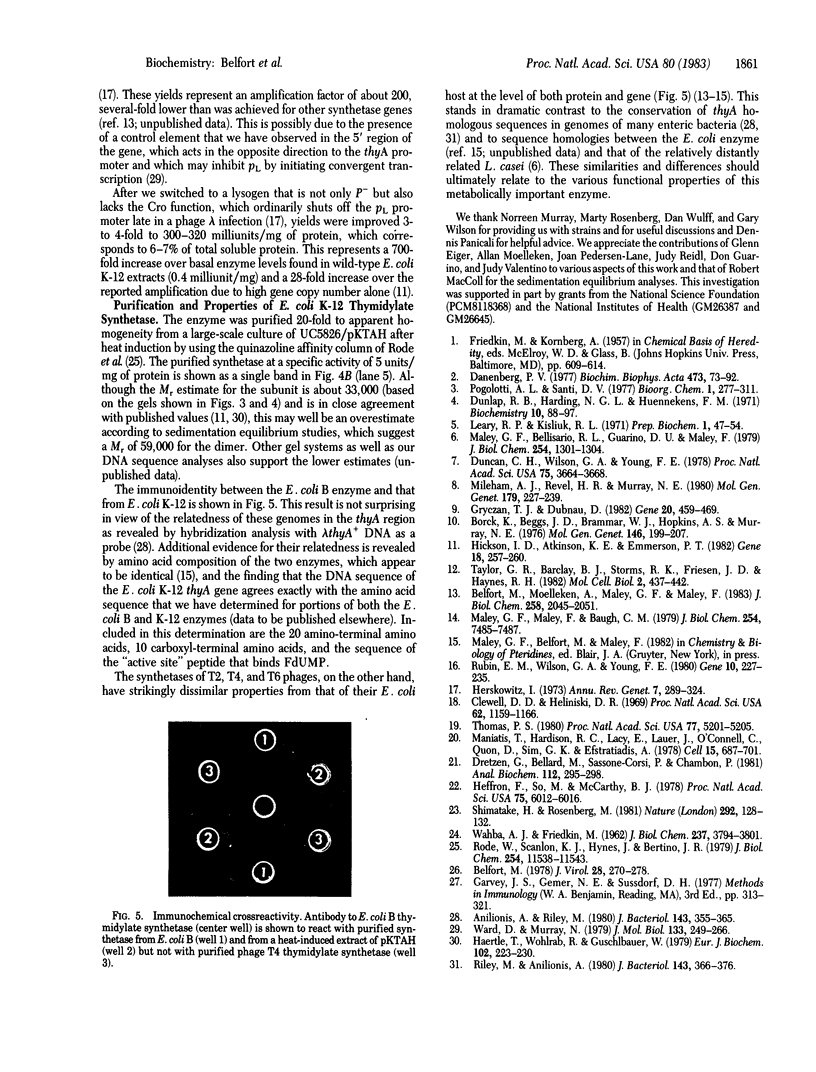
The 7.8-kilobase HindIII insert in phage lambda NM589thyA [Borck, K., Beggs, J.D., Brammar, W.J., Hopkins, A.S. & Murray, N. (1976) Mol. Gen. Genet. 146, 199] was confirmed as originating from Escherichia coli by hybridization analysis and was shown to encode the thymidylate synthetase (5,-10-methylenetetrahydrofolate:dUMP C-methyltransferase EC 2.1.1.45) of E. coli K-12 by using biochemical, structural, and immunologic criteria. The 7.8-kilobase insert was reduced in size to a quasi-random population of DNA subfragments by partial digestion with the 4-base-pair recognition enzymes Alu I and Hae III. A clone containing a 1.1- to 1.2-kilobase fragment that encompassed the gene was obtained from this mixture by selecting for Thy+ recombinants. Fusion of this DNA fragment to the phage lambda rho L promoter in plasmid pKC30 revealed the direction of transcription of the thyA gene, and, in a phage lambda lysogen containing a thermolabile repressor, intracellular synthetase levels were increased about 700-fold. The enzyme was purified to homogeneity from this source by affinity chromatography, and some of its properties are described.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anilionis A., Riley M. Conservation and variation of nucleotide sequences within related bacterial genomes: Escherichia coli strains. J Bacteriol. 1980 Jul;143(1):355–365. doi: 10.1128/jb.143.1.355-365.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M. Anomalous behavior of bacteriophage lambda polypeptides in polyacrylamide gels: resolution, identification, and control of the lambda rex gene product. J Virol. 1978 Oct;28(1):270–278. doi: 10.1128/jvi.28.1.270-278.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belfort M., Moelleken A., Maley G. F., Maley F. Purification and properties of T4 phage thymidylate synthetase produced by the cloned gene in an amplification vector. J Biol Chem. 1983 Feb 10;258(3):2045–2051. [PubMed] [Google Scholar]
- Borck K., Beggs J. D., Brammar W. J., Hopkins A. S., Murray N. E. The construction in vitro of transducing derivatives of phage lambda. Mol Gen Genet. 1976 Jul 23;146(2):199–207. doi: 10.1007/BF00268089. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danenberg P. V. Thymidylate synthetase - a target enzyme in cancer chemotherapy. Biochim Biophys Acta. 1977 Dec 23;473(2):73–92. doi: 10.1016/0304-419x(77)90001-4. [DOI] [PubMed] [Google Scholar]
- Dretzen G., Bellard M., Sassone-Corsi P., Chambon P. A reliable method for the recovery of DNA fragments from agarose and acrylamide gels. Anal Biochem. 1981 Apr;112(2):295–298. doi: 10.1016/0003-2697(81)90296-7. [DOI] [PubMed] [Google Scholar]
- Duncan C. H., Wilson G. A., Young F. E. Mechanism of integrating foreign DNA during transformation of Bacillus subtilis. Proc Natl Acad Sci U S A. 1978 Aug;75(8):3664–3668. doi: 10.1073/pnas.75.8.3664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlap R. B., Harding N. G., Huennekens F. M. Thymidylate synthetase from amethopterin-resistant Lactobacillus casei. Biochemistry. 1971 Jan 5;10(1):88–97. doi: 10.1021/bi00777a014. [DOI] [PubMed] [Google Scholar]
- Gryczan T. J., Dubnau D. Direct selection of recombinant plasmids in Bacillus subtilis. Gene. 1982 Dec;20(3):459–469. doi: 10.1016/0378-1119(82)90215-3. [DOI] [PubMed] [Google Scholar]
- Haertlé T., Wohlrab F., Guschlbauer W. Thymidylate synthetase from Escherichia coli K12. Purification, and dependence of kinetic properties on sugar conformation and size of the 2' substituent. Eur J Biochem. 1979 Dec;102(1):223–230. doi: 10.1111/j.1432-1033.1979.tb06283.x. [DOI] [PubMed] [Google Scholar]
- Heffron F., So M., McCarthy B. J. In vitro mutagenesis of a circular DNA molecule by using synthetic restriction sites. Proc Natl Acad Sci U S A. 1978 Dec;75(12):6012–6016. doi: 10.1073/pnas.75.12.6012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskowitz I. Control of gene expression in bacteriophage lambda. Annu Rev Genet. 1973;7:289–324. doi: 10.1146/annurev.ge.07.120173.001445. [DOI] [PubMed] [Google Scholar]
- Hickson I. D., Atkinson K. E., Emmerson P. T. Molecular cloning and amplification of the gene for thymidylate synthetase of E. coli. Gene. 1982 Jun;18(3):257–260. doi: 10.1016/0378-1119(82)90163-9. [DOI] [PubMed] [Google Scholar]
- Leary R. P., Kisliuk R. L. Crystalline thymidylate synthetase from dichloromethotrexate resistant Lactobacillus casei. Prep Biochem. 1971 Jan;1(1):47–54. doi: 10.1080/00327487108081929. [DOI] [PubMed] [Google Scholar]
- Maley G. F., Bellisario R. L., Guarino D. U., Maley F. The primary structure of Lactobacillus casei thymidylate synthetase. III. The use of 2-(2-nitrophenylsulfenyl)-3-methyl-3-bromoindolenine and limited tryptic peptides to establish the complete amino acid sequence of the enzyme. J Biol Chem. 1979 Feb 25;254(4):1301–1304. [PubMed] [Google Scholar]
- Maley G. F., Maley F., Baugh C. M. Differential inhibition of host and viral thymidylate synthetases by folylpolyglutamates. J Biol Chem. 1979 Aug 25;254(16):7485–7487. [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Mileham A. J., Revel H. R., Murray N. E. Molecular cloning of the T4 genome; organization and expression of the frd--DNA ligase region. Mol Gen Genet. 1980;179(2):227–239. doi: 10.1007/BF00425449. [DOI] [PubMed] [Google Scholar]
- Riley M., Anilionis A. Conservation and variation of nucleotide sequences within related bacterial genomes: enterobacteria. J Bacteriol. 1980 Jul;143(1):366–376. doi: 10.1128/jb.143.1.366-376.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode W., Scanlon K. J., Hynes J., Bertino J. R. Purification of mammalian tumor (L1210) thymidylate synthetase by affinity chromatography on stable biospecific adsorbent. Stabilization of the enzyme with neutral detergents. J Biol Chem. 1979 Nov 25;254(22):11538–11543. [PubMed] [Google Scholar]
- Rubin E. M., Wilson G. A., Young F. E. Expression of thymidylate synthetase activity in Bacillus subtilis upon integration of a cloned gene from Escherichia coli. Gene. 1980 Aug;10(3):227–235. doi: 10.1016/0378-1119(80)90052-9. [DOI] [PubMed] [Google Scholar]
- Simatake H., Rosenberg M. Purified lambda regulatory protein cII positively activates promoters for lysogenic development. Nature. 1981 Jul 9;292(5819):128–132. doi: 10.1038/292128a0. [DOI] [PubMed] [Google Scholar]
- Taylor G. R., Barclay B. J., Storms R. K., Friesen J. D., Haynes R. H. Isolation of the thymidylate synthetase gene (TMP1) by complementation in Saccharomyces cerevisiae. Mol Cell Biol. 1982 Apr;2(4):437–442. doi: 10.1128/mcb.2.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas P. S. Hybridization of denatured RNA and small DNA fragments transferred to nitrocellulose. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5201–5205. doi: 10.1073/pnas.77.9.5201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WAHBA A. J., FRIEDKIN M. The enzymatic synthesis of thymidylate. I. Early steps in the purification of thymidylate synthetase of Escherichia coli. J Biol Chem. 1962 Dec;237:3794–3801. [PubMed] [Google Scholar]
- Ward D. F., Murray N. E. Convergent transcription in bacteriophage lambda: interference with gene expression. J Mol Biol. 1979 Sep 15;133(2):249–266. doi: 10.1016/0022-2836(79)90533-3. [DOI] [PubMed] [Google Scholar]