Abstract
Age remains the most powerful prognostic factor among glioblastoma (GBM) patients. Half of all patients with GBM are aged 65 years or older at the time of diagnosis, and the incidence rate of GBM in patients aged over 65 years is increasing rapidly. Median survival for elderly GBM patients is less than 6 months and reflects less favorable tumor biologic factors, receipt of less aggressive care, and comorbid disease. The standard of care for elderly GBM patients remains controversial. Based on limited data, extensive resection appears to be more beneficial than biopsy. For patients with favorable Karnofsky performance status (KPS), adjuvant radiotherapy (RT) has a demonstrated survival benefit with no observed decrement in quality of life. Concurrent and adjuvant temozolomide (TMZ) along with RT to 60 Gy have not been prospectively studied among patients aged over 70 years but should be considered for patients aged 65–70 years with excellent KPS. Based on the recent NOA-08 and Nordic randomized trials, testing for O6-methylguanine-DNA-methyltransferase (MGMT) promoter methylation should be performed routinely immediately after surgery to aid in adjuvant treatment decisions. Patients aged over 70 years with favorable KPS, or patients aged 60–70 years with borderline KPS, should be considered for monotherapy utilizing standard TMZ dosing for patients with MGMT-methylated tumors, and hypofractionated RT (34 Gy in ten fractions or 40 Gy in 15 fractions) for patients with MGMT-unmethylated tumors. The ongoing European Organisation for Research and Treatment of Cancer/National Cancer Institute of Canada trial will help clarify the role for concurrent TMZ with hypofractionated RT. For elderly patients with poor KPS, reasonable options include best supportive care, TMZ alone, hypofractionated RT alone, or whole brain RT for symptomatic patients needing to start treatment urgently. Given the balance between short survival and quality of life in this patient population, optimal management of elderly GBM patients must be made individually according to patient age, MGMT methylation status, performance score, and patient preferences.
Keywords: glioblastoma, elderly, radiotherapy, hypofractionated, temozolomide, MGMT
Introduction
Glioblastoma (GBM) is the most common malignant primary brain tumor among adults, with an incidence rate of 3.2 newly diagnosed cases per 100,000.1 However, the median age at diagnosis is approximately 65 years, and the incidence rate of GBM in patients aged over 65 years is increasing rapidly, with a doubling in incidence from 5.1 per 100,000 in the 1970s up to 10.6 per 100,000 in the 1990s among this age cohort.2 Thus, as the general population ages, GBM is becoming increasingly common among elderly patients.
Despite aggressive treatment, median survival among all GBM patients is only 12–15 months from diagnosis. Among elderly patients, median survival is markedly reduced at only 4–5 months, according to population-based studies.3,4 As will be outlined in this review, patient age has been shown in multiple series to be the single most important prognostic factor among GBM patients. Reasons for shorter survival among elderly patients may include less favorable tumor biology, receipt of less aggressive care, treatment toxicity due to less physiologic reserve, and competing comorbidities that may shorten life. Of note, definitions of the elderly vary in the GBM literature, with most of the randomized trials including patients aged 60, 65, or 70 years and older.
Given the short survival experienced by most elderly patients with GBM, appropriately balancing quality of life with quantity of life has been a major focus among both patients and clinicians as treatments are considered for newly diagnosed patients. Although elderly patients were excluded from many prior GBM clinical trials, recent trials over the past decade have sought to determine optimal GBM management specifically among the elderly, and additional trials are ongoing. Based on current evidence, there are several ways to appropriately manage GBM among the elderly, and this review will highlight these varying approaches.
Biology and prognostic factors
Growing research demonstrates that GBM among elderly patients has less favorable molecular signatures, as compared with younger patients. In general, GBM arises from two distinct pathways, including the primary GBM phenotype, which tends to arise de novo among older adults including the elderly, often harboring amplification of the epidermal growth factor receptor and deletion or mutation of phosphate and tensin homolog.5,6 This is in contrast to secondary GBM, which classically arises from lower-grade gliomas, is found more frequently among younger patients, and often has mutations of the isocitrate dehydrogenase-1 and -2 genes.7–9 In addition, there are data to suggest that genetic markers within GBM tumors have differential effects on survival based on patient age. For example, TP53 alterations have been shown to be associated with reduced survival among patients aged over 70 years, whereas the opposite effect was true for TP53 alteration among younger patients within the same study.10 Similarly, CDKN1A/p16 alterations confer a significantly worse survival among patients aged over 70 years compared with patients aged under 70 years. Moreover, although isocitrate dehydrogenase-1 mutations are known to be prognostically favorable among gliomas,11 these mutations are almost entirely absent from the elderly GBM population.12 Methylation of the O6-methylguanine-DNA-methyltransferase (MGMT) gene promoter, which is both a predictive and prognostic marker among GBM patients in general,13 does remain both predictive and prognostic among elderly patients and will be discussed later. Approximately 40%–60% of GBMs among the elderly exhibit MGMT promoter methylation.14–16 Finally, expression of vascular endothelial growth factor was found to be higher among patients aged over 55 years with recurrent GBM.17 This finding has led to recent interest in using antivascular endothelial growth factor antibody therapy with bevacizumab among elderly GBM patients, as is currently being evaluated in the Avastin Plus Radiotherapy in Elderly Patients with Glioblastoma (ARTE) randomized Phase II trial.
The classic recursive partitioning analysis (RPA) for GBM reported by Curran et al18 more than 20 years ago found that the single factor that best predicted how long patients with high-grade gliomas survive was patient age, with patients aged 50 years or older having shorter survival than those aged under 50 years. Even among GBM patients aged 50 years or older with the most favorable RPA factors, including favorable Karnofsky performance status (KPS) of 70 or higher, complete surgical resection, preserved neurocognitive function, and ability to carry on active work, the median survival was only 11.1 months with a 2-year survival of 15%. However, this analysis was performed by pooling three randomized trials that were conducted during the 1970s and 1980s, all of which excluded patients aged over 70 years, potentially limiting applicability to the contemporary management of elderly GBM patients. A more recent RPA was conducted specifically among GBM patients aged 70 years and older, drawing on patients treated up through 2007 at two institutions in the US and validated using patient data from a French consortium.19 Among these elderly patients, the most important predictor of survival was the extent of surgical resection, with patients receiving biopsy alone having the shortest survival. In this study, age was only prognostic among patients who underwent gross total resection or partial resection; in that cohort, age under 75.5 years had the longest median survival at 9.3 months, compared with 6.4 months among older patients.
Beyond patient age, extent of resection, and performance status, methylation status of the MGMT promoter appears to be an important prognostic factor that was not included in the recent RPA study cited previously. Two large European trials among elderly patients with GBM both demonstrated that independent of treatment, MGMT promoter methylation was associated with an overall survival gain of approximately 3 months, from 8.2 months median survival for MGMT-unmethylated tumors up to11.9 months for MGMT-methylated tumors in the NOA-08 trial,20 and from 6.9 months up to 9.0 months in the Nordic trial.14 Given the important prognostic information made available by knowing MGMT methylation status, in addition to its performance as a predictive marker for response to various therapies outlined in this review, many are calling for MGMT evaluation to be performed routinely on all newly diagnosed GBM patients before adjuvant treatment decisions are made, especially in the elderly.21
Standard GBM treatment: applicable to the elderly?
The standard treatment of GBM has historically relied on maximal safe surgical resection, followed by adjuvant radiotherapy (RT). RT dose-finding studies dating back to the 1960s and 1970s have supported RT dose escalation up to 60 Gy in 2 Gy daily fractions delivered over 6 weeks, which have conferred a survival advantage with acceptable toxicity.22 In 2005, the landmark European Organisation for Research and Treatment of Cancer (EORTC)/National Cancer Institute of Canada (NCIC) randomized trial by Stupp et al23 added concurrent and adjuvant temozolomide (TMZ) to RT and became the first demonstration of a convincing survival benefit with the addition of chemotherapy for GBM, extending median survival from 12.1 months to 14.6 months in that trial. However, this trial excluded patients aged over 70 years and enrolled only patients of good performance status who were on a stable corticosteroid dose, so the applicability of the results of this trial to patients aged over 70 years, or frailer patients in general, is unclear. Unfortunately, this represents a large proportion of patients with GBM, and thus it is unclear whether the survival gains seen in the trial population extend to the elderly.
A subgroup analysis from the EORTC/NCIC trial that focused on outcomes according to patient age found that patients aged over 60 years still had a survival benefit with the addition of TMZ to RT but of a slightly smaller magnitude when compared with younger patients.24 The hazard ratio (HR) for combined therapy (TMZ plus RT) was 0.7 (95% confidence interval [CI]: 0.5–0.97) for patients aged 60–70 years versus an HR of 0.6 (95% CI: 0.5–0.8) for patients aged 50 years and younger. An additional subgroup analysis from this trial found that among the 114 patients aged 61–65 years, there was only a trend for survival benefit for combined therapy (HR 0.64; P=0.096), and among the 83 patients aged 66–70 years there was no survival advantage to adding TMZ to RT (HR 0.78; P=0.34).25 Although the lack of survival advantage with the addition of TMZ for patients aged 65 years and older may be due to this being an underpowered subgroup analysis, patients of this age range did constitute nearly 15% of the EORTC/NCIC trial population (83 of 573 patients). Regardless, the possibility that the now standard GBM management used for most young patients, consisting of surgical resection followed by adjuvant RT and TMZ, may be ineffective or inappropriate for half of the GBM population – given that the median age of GBM diagnosis is approximately 65 years – is a question that remains unanswered. A small prospective single-arm study from Italy investigated 32 patients aged 70 years and older with GBM assigned to 60 Gy of RT with concurrent and adjuvant TMZ, finding median overall survival to be 10.6 months, which compares favorably with RT alone, though this had no comparator arm.26
Potential toxicity of concurrent chemoradiation in the elderly is documented in several reports, though randomized data on toxicity experienced by GBM patients aged 70 years and older are lacking. The Italian study cited previously reported a 40% rate of neurotoxicity among patients receiving combined modality therapy, though most of these events were grade 2 in severity and were reversible with steroid administration. In addition, there was a 28% rate of grade 3–4 hematologic toxicity.26 A German study of 46 GBM patients with mean age 59 years who received concurrent chemoradiation highlights that hematologic toxicity may be persistent, with the mean duration of thrombocytopenia in that series being just over 2 months.27 Another Italian report on 58 GBM patients aged 65 years or older receiving concurrent and adjuvant TMZ reported a 25% rate of grade 3–4 mental status toxicity during the adjuvant TMZ phase, at a median time of approximately 6 months after completion of chemoradiation, although it is not clear whether this was attributable to combined RT/TMZ treatment as opposed to underlying disease progression.28 Finally, a single-institution Canadian study reported a 42% rate of grade 3–4 toxicity among GBM patients aged 65 years or older receiving concurrent chemoradiation versus 0% among similar patients receiving RT alone, with a 3-month survival gain seen for concurrent chemoradiation.16 These findings highlight both the potential toxicity of combined modality and the potential survival gain among elderly patients.
Surgery
Although much of the focus on managing elderly patients with GBM appropriately centers on the optimal use of RT and TMZ, it is important to first address the role of neurosurgical resection among elderly patients. Although chronologic age is likely less important than number and severity of comorbidities, both are important when considering how aggressive a resection performed for elderly patients should be, especially among patients in their 70s and 80s, due to surgical risks and potential postoperative complications.
Among the general GBM population, no randomized trials have defined the optimal extent of resection, though on first principles most agree that the more that can be safely removed, the better the apparent outcome, with retrospective data in support of this approach.29 However, among the elderly specifically, there was a small randomized trial conducted in Finland in the pre-TMZ era among 23 patients aged over 65 years with high-grade glioma and KPS >60, in an attempt to answer the question of the relative benefits of biopsy only versus more extensive resection.30 The authors found that undergoing subtotal or gross total resection was associated with a statistically significant 3-month survival gain as compared with biopsy alone. Median survival was 5.6 months (subtotal or gross total resection) versus 2.8 months (biopsy alone) (P=0.035). An imbalance in prognostic factors between arms may have accounted for some degree of this survival advantage. Although overall survival was short in both groups, the value of this study is that it was randomized. In contrast, most of the other studies that report survival outcomes according to extent of resection, including the RPA discussed previously, are limited by their retrospective nature. Specifically, confounding bias often arises, whereby the fittest patients may have been selected by their physicians to receive more aggressive resection. A case-control study from Johns Hopkins University, Baltimore, MA, USA, assessed consecutive patients aged 65 years or older who underwent resection versus biopsy, and matched 40 patients from each group according to age, KPS, eloquent location, receipt of RT, and receipt of TMZ.31 Although there were no significant differences in perioperative outcomes, the patients undergoing resection had a significantly longer median survival (5.7 vs 4.0 months; P=0.03).
Radiotherapy
As outlined, the historical standard for GBM management has been surgical resection followed by adjuvant RT. However, many clinical trials have excluded patients aged over 70 years or had small representation among this age cohort. Survival tends to be shorter in the elderly GBM population, and RT treatment duration and toxicities may be considered onerous if not associated with significantly prolonged survival. It is in this context that the Association des Neuro-Oncologues d’Expression Française (ANOCEF) group conducted a randomized trial from 2001 to 2005 to examine whether RT was indeed superior to best supportive care following surgery.32 This study enrolled 85 patients aged 70 years or older with high-grade glioma and KPS 70 or higher, and randomly assigned them to supportive care – including steroids, anticonvulsants, physical therapy, and psychological/palliative care – or supportive care plus tumor-directed RT to 50.4 Gy over the course of 28 fractions. Approximately 50% of patients in each arm underwent biopsy only, and the median age was approximately 74 years in both arms. The trial was stopped early with a median follow-up of 5.1 months, due to the superiority of the RT arm. Specifically, a 3-month survival advantage was noted for the RT cohort, increasing median survival from 4.2 months to 7.3 months, with an HR of 0.47 (P=0.002). Importantly, there were no differences in patient performance status or health-related quality of life between arms when assessed at 1-, 2-, 3-, and 4.5-month follow-up after treatment, and in addition there was no significant quality of life decline from baseline in either arm. This suggests that active treatment of elderly GBM patients with RT can be delivered without significant toxicity and with a significant survival benefit.
Given that RT is associated with a survival advantage over supportive care alone in elderly patients with GBM, there has been interest in determining whether shorter, hypofractionated courses of RT may offer benefits comparable with longer, standard courses of RT. Roa et al33 investigated whether a 3-week hypofractionated course of RT given as 40 Gy delivered in 15 daily fractions was comparable with standard 60 Gy delivered in 30 daily fractions among GBM patients aged 60 years or older and with KPS of at least 50, none of whom received concurrent chemotherapy. The trial was closed early due to slow accrual after approximately 100 patients had enrolled at four Canadian centers from 1996 to 2001. The mean age was 71 years in the hypofractionated arm and 72 years in the standard arm. Median KPS in each arm was 70. Biopsy alone was performed among 35% of patients in the hypofractionated arm and 43% of patients in the standard arm. Although the study was limited due to underpowering, the median survival appeared similar between groups at 5.6 months for the hypofractionated arm and 5.1 months for the standard arm (P=0.57), with no significant difference in KPS throughout treatment and through the first two follow-up visits. Of note, this trial involved RT alone, and there are two ongoing trials that are evaluating whether the addition of concurrent systemic therapy to hypofractionated RT will result in improved outcomes among elderly patients with newly diagnosed GBM. The first is an EORTC/NCIC/Radiation Therapy Oncology Group randomized Phase III trial (EORTC 26062–22061), which adds concurrent and adjuvant TMZ to 40 Gy of hypofractionated RT among patients aged 65 years and older with favorable performance status. There are retrospective data to support this regimen34,35 but nothing randomized to date. The second is the Swiss ARTE randomized Phase II trial, which adds concurrent and adjuvant bevacizumab to 40 Gy of hypofractionated RT among patients aged over 65 years and does not include TMZ. These two studies will help shed light on the tolerability and efficacy of combined modality therapy in the setting of hypofractionated RT.
An RT option for elderly GBM patients with low KPS, including those who are decompensating and need to begin treatment quickly due to progressive symptoms, is conventional whole brain RT, which was assessed in a prospective single-arm study.36 Whole brain RT was delivered as 30 Gy in ten daily fractions to 29 patients with GBM aged at least 65 years or who had KPS 50 or lower and compared with historical controls treated with supportive care alone or with standard-course RT. The median survival for patients treated with whole brain RT was 6 months. Overall survival was no different between whole brain RT versus standard-course RT for patients with KPS of 50 or lower, but survival was significantly longer for patients with KPS over 50 treated with standard-course RT (P=0.006). This was not a randomized comparison but suggests that 10 days of whole brain RT is a reasonable option for patients with poor performance status and who have substantial tumor-related symptoms. However, we would note that in most cases it may be possible to start with a whole brain RT plan in urgent situations such as these, with a transition to a more conformal RT plan during the course of treatment.
Temozolomide
In light of the demonstrated antitumor activity of TMZ among patients with GBM in the EORTC/NCIC trial by Stupp et al,23 there has been subsequent interest in evaluating TMZ alone among elderly GBM patients. The ANOCEF group accordingly conducted a nonrandomized Phase II study known as the Temozolomide in Elderly Patients with KPS <70 (TAG) trial, enrolling 70 GBM patients aged at least 70 years with a KPS below 70, and treated them with TMZ alone until progression.37 TMZ was dosed per the EORTC/NCIC trial with 150 mg/m2 for 5 consecutive days, every 4 weeks, with an increase in dose to 200 mg/m2 if tolerated by the patient. At progression, supportive care was recommended, with no patients receiving RT in the trial and less than 10% receiving second-line chemotherapy. The median age was 77 years with a range of 70–87 years, 91% had undergone biopsy alone, and 83% of patients had KPS of 50 or 60. The median number of TMZ cycles received was two. Despite a low number of cycles received, the median survival was 6.3 months, which was substantially longer than the 3–4 month survival anticipated with supportive care alone. Interestingly, one-third of patients experienced an improved KPS score by at least ten points, with 26% achieving a KPS of at least 70 (capable of self-care). The authors found that patients receiving TMZ who had MGMT-methylated tumors had significantly longer survival compared with unmethylated patients (7.8 months vs 4.8 months; P=0.03). Finally, rates of grade 3 or 4 hematologic toxicity were reasonably low at 25% overall and very similar to those seen in the trial by Stupp et al.23 Other retrospective reports have also emerged on TMZ monotherapy but have now been superseded by the recent publication of two large trials directly comparing TMZ monotherapy with various RT approaches, highlighted in this review. In addition, a recent Phase II trial examining the addition of bevacizumab to TMZ, without RT, among patients aged at least 70 years with poor KPS (<70) was presented, showing no survival gains and suggesting that TMZ monotherapy may not be improved by the addition of an antiangiogenic agent.38
Radiotherapy versus temozolomide
In 2012, two important randomized Phase III trials were published that included head-to-head comparisons of RT monotherapy versus TMZ monotherapy in elderly GBM patients. The first was the German NOA-08 trial,20 and the second was the Nordic trial,14 and although these studies were carried out differently, common themes emerge that are important to our understanding of optimal management of this patient population.
The German NOA-08 trial enrolled high-grade glioma patients aged over 65 years and with KPS of at least 60, and randomized them to 60 Gy of RT alone in 1.8 or 2 Gy fractions, or to dose-dense TMZ given as 100 mg/m2 for 7 days in a row every other week.20 The study design was to test the hypothesis that dose-dense TMZ is noninferior to RT, defined as no more than a 25% difference in overall survival. There were 373 patients enrolled between 2005 and 2009 who received at least one dose of treatment and were included in the efficacy analyses. In both arms, median age was approximately 72 years, median KPS was 80, approximately 90% of patients had GBM, and approximately 40% of patients had undergone biopsy only. Unfortunately, MGMT promoter methylation status was missing in almost 45% of all patients but was known to be methylated in 16% of patients receiving TMZ and 24% of patients receiving RT. At progression, patients switched to receive RT or TMZ as salvage therapy, depending on their initial treatment arm. Median overall survival was 9.6 months for RT and 8.6 months for dose-dense TMZ, with a P-value of 0.03, consistent with noninferiority for a 25% difference between arms. Dose-dense TMZ had relatively more side effects, with higher rates of grade 3 and 4 toxicity, though no grade 5 toxicities were observed. There were no significant differences in health-related quality of life parameters between arms.
The NOA-08 authors also analyzed overall survival and event-free survival according to MGMT status and treatment arm. Among patients receiving upfront RT, MGMT methylation status was not associated with overall survival. However, among patients receiving TMZ, unmethylated patients had a significantly shorter event-free survival (HR 1.95; P=0.01) and a trend to shorter overall survival (HR 1.34; P=0.129) compared with RT. In contrast, methylated patients receiving TMZ had a significantly longer event-free survival (HR 0.53; P=0.01) and trend to longer overall survival (HR 0.69; P=0.139) compared with RT. This suggests an important predictive role for MGMT promoter methylation status in determining optimal treatment among elderly GBM patients with favorable KPS for whom monotherapy with RT or TMZ is being considered. Specifically, patients with MGMT-methylated tumors may benefit more from TMZ alone as opposed to RT alone, whereas patients with MGMT-unmethylated tumors appeared to benefit more from RT alone. Given that these findings were observed in the setting of dose-dense TMZ, which had increased toxicity, it was helpful that the Nordic trial, which utilized standard TMZ dosing, was published shortly after NOA-08 to enable comparison of results.
The Nordic trial14 enrolled GBM patients with favorable performance status aged 60 years or older from 2000 to 2004, and then patients aged 65 years or older from 2005 to 2009 after publication of the practice-changing EORTC/NCIC trial in 2005.23 Patients were randomized between three arms: 1) TMZ for six cycles, dosed according to the Stupp et al23 trial; 2) hypofractionated RT to 34 Gy in ten daily fractions; or 3) standard RT to 60 Gy in 30 daily fractions. The trial was initially powered to detect a 10% overall survival difference between arms, and 291 patients were enrolled. Median age was 70 years, and approximately 25% of all patients had undergone biopsy only. Treatment was completed by 95% of the hypofractionated RT patients versus 72% of the standard RT patients, and only 34% of patients received all six cycles of TMZ, mostly due to disease progression. The median number of cycles of TMZ received was four. At progression, approximately one-third of patients received salvage therapy. Overall, side effects were modest, with low rates of grade 3 or 4 toxicity among all arms. Median survival was 8.3 months in the TMZ group, 7.5 months in the hypofractionated RT group, and 6.0 months in the standard RT group. This was statistically significant between the TMZ and standard RT groups (HR 0.70; P=0.01) but not between TMZ and hypofractionated RT (HR 0.85; P=0.24). For patients aged over 70 years, TMZ (HR 0.35; P<0.0001) and hypofractionated RT (HR 0.59; P=0.02) were associated with significantly longer survival than standard RT. No significant survival differences were observed between TMZ and hypofractionated RT. Echoing findings from the NOA-08 trial, the Nordic authors found that patients treated with TMZ had significantly higher survival if their tumors were MGMT methylated as compared with unmethylated (P=0.02). In addition, MGMT methylation status did not impact survival for patients treated with RT.
As outlined, these two European trials have helped clarify the relative benefits of RT versus TMZ among elderly GBM patients. In particular, it appears important to routinely test all tumors for MGMT promoter methylation in an expedited way in order to make fully informed adjuvant treatment decisions.21 Patients who are being considered for monotherapy as opposed to combined chemoradiotherapy appear to have most benefit from TMZ if their tumor is MGMT methylated versus RT (hypofractionated, in particular) if MGMT is unmethylated. The ongoing EORTC 26062–22061 study mentioned previously will help shed light on whether adding TMZ to hypofractionated RT may be beneficial and whether this varies according to MGMT status. An overview of the existing randomized trials among elderly GBM patients is shown in Table 1, and a comparison of the different RT dose/fractionation regimens in the various trials is shown in Table 2.
Table 1.
Comparison of randomized trials among elderly patients with glioblastoma
| Randomized trials
|
||||
|---|---|---|---|---|
| NOA-0820 | Nordic14 | Roa et al33 | ANOCEF37 | |
| No of patients | 373 | 291 | 100 | 81 |
| Inclusion | ||||
| Age (years) | >65 | ≥60 | ≥60 | ≥70 |
| KPS | ≥60 | ECOG ≤2 | ≥50 | ≥70 |
| Histology | GBM (89%) or AA (11%) | GBM | GBM | GBM |
| Median age (years) | 72 | 70 | 71 | 74 |
| Years | 2005–2009 | 2000–2009 | 1996–2001 | 2001–2005 |
| Biopsy only | 39% | 26% | 39% | 52% |
| MS (months) | ||||
| RT 50–60 Gy | 9.6 | 6.0 | 5.1 | 7.3 |
| RT hypofx | 7.5 | 5.6 | ||
| TMZ | 8.6 | 8.3 | ||
| Supportive care | 4.2 | |||
| Comment | TMZ noninferior | RT 60 Gy inferior | RT hypofx noninferior | Supportive care inferior |
Abbreviations: AA, anaplastic astrocytoma; ANOCEF, Association des Neuro-Oncologues d’Expression Française; ECOG, Eastern Cooperative Oncology Group; GBM, glioblastoma; hypofx, hypofractionated; KPS, Karnofsky performance status; MS, median survival; RT, radiotherapy; TMZ, temozolomide.
Table 2.
Comparison of radiotherapy regimens in various glioblastoma trials
| RT regimen and trial(s) | Equivalent RT dose in 2 Gy fractionsa |
|---|---|
| 60 Gy/30 fractions | 60 Gy |
| Stupp et al23 | |
| NOA-0820 | |
| Nordic14 | |
| 50.4 Gy/28 fractions | 49.6 Gy |
| ANOCEF37 | |
| 40 Gy/15 fractions | 42.3 Gy |
| Roa et al33 | |
| 34 Gy/10 fractions | 38.0 Gy |
| Nordic14 | |
| 30 Gy/10 fractions | 32.5 Gy |
| Bauman et al36 | |
Note: Assuming an alpha/beta ratio of 10 Gy for estimating radiotherapeutic effect on tumor.
Abbreviations: ANOCEF, Association des Neuro-Oncologues d’Expression Française; RT, radiotherapy.
In addition, these trials have helped clarify the toxicities attributable specifically to RT in the short term and long term, which is an important consideration among elderly GBM patients, with the preponderance of the data suggesting that RT is generally well tolerated among this population. As noted previously, the ANOCEF investigators found no differences in KPS or health-related quality of life between patients who received 50.4 Gy of RT versus those who received no RT, up to nearly 5 months after treatment.32 In the NOA-08 trial, the investigators observed grade 3–4 fatigue among 20% of patients in the 60 Gy RT alone arm as compared with 24% in the TMZ alone arm in the trial overall, with a slight increase in fatigue during the end of the RT course, which then returned to baseline levels.20 This is echoed by the Nordic trial results, which found fatigue increasing in the RT arms at 6 weeks compared with in the TMZ alone arm but without longer-term follow-up data, due to low compliance.14 In NOA-08, 13% of patients had grade 3–4 seizures and 25% of patients had grade 3–4 neurologic symptoms in the RT arm, as compared with 17% and 36% in the TMZ arm, respectively. Cognitive function in NOA-08 was found to be similar between arms and compared with baseline in the first post-treatment assessment according to mini-mental state examination score, though as the authors note, this is a relatively crude test for cognition. The authors also reported that there were no significant differences between arms with regard to communication deficits out to 12 months after randomization. The Nordic investigators observed a mild decline in cognitive function in the 60 Gy arm compared with in the TMZ arm at the 6-week time point, but unfortunately no late outcomes were available. Based on the existing randomized data, there do not appear to be significant cognitive toxicities associated with RT alone in the elderly, though further study is warranted.
Treatment patterns and outcomes
Several population-based studies have investigated the treatment patterns and outcomes among elderly GBM patients over the last 20–30 years. Patients aged 65 years and older have been found in several of these series to have a median survival of only 4–5 months from diagnosis.3,4 The existing literature suggests that elderly patients are significantly less likely to receive effective therapies, including surgery, RT, and chemotherapy. Reports based on patients in the US have found that surgical resection was performed in 61%–78% of patients aged 65 years and older from the early 1990s through the mid-to-late 2000s,3,4,39,40 whereas a report from Switzerland found that only 47% of patients aged over 65 years had received resection at diagnosis,41 with the majority receiving biopsy only. The likelihood of undergoing resection has been shown to decrease with increasing age, with patients of age group 65–69 years having a 71% resection rate compared with 69% for those aged 70–74 years, 62% for those aged 80–84 years, 46% for those aged 80–84 years, and 31% for those aged 85 years and older, which was highly significant (P<0.0001) even after adjusting for confounding factors.3 This same trend was repeated almost identically for receipt of RT within 3 months of diagnosis or receipt of chemotherapy within 3 months of diagnosis, when analyzed according to increasing age, in the same US population-based report,3 as well as in other reports in the US and Canada.4,42 Among GBM patients aged over 65 years, factors that have been reported to be associated with decreased use of RT, apart from increasing age, include marital status, surgical resection, and year of diagnosis, with a slight overall decrease in RT utilization observed over time.39
Most patterns-of-care studies demonstrate survival increases of several months among elderly GBM patients who do receive surgery, RT, and/or chemotherapy, yet given the retrospective study design of most of these investigations, it is not clear whether the increased survival reflects treatment efficacy versus better patient performance status and physician selection for these more aggressive therapies. Since publication in 2005 of the EORTC/NCIC randomized trial adding TMZ for GBM,23 there have been no population-based studies directly comparing RT with RT plus TMZ for the elderly. However, US data suggest that survival has increased in the TMZ era for elderly GBM patients, with a similar proportional survival increase observed for patients aged 65–79 years as compared with younger patients, though without any survival increase observed among patients aged 80 and older.43 Overall, the authors found that median overall survival increased from 8.5 months to 10.5 months between the 1993–1995 era and the 2005–2007 era (P<0.0001) among patients aged 65–79 years, compared with the increase from 11.5 months to 15.5 months seen among patients aged 45–64 years (P<0.0001). This report did not contain direct information about TMZ chemotherapy itself, and although the same group has recently investigated the actual benefit from TMZ using US Veterans Health Administration data, the new study did not perform subgroup analyses according to patient age.44
Although the randomized trials of TMZ or RT discussed here have generally not found major differences in toxicity or health-related quality of life between different treatment arms among elderly GBM patients,14,20,33 there still remains a critical balance between the potential survival gains of aggressive treatments and treatment- or disease-associated morbidity, especially in the setting of short overall survival. Highlighting this balance, a 2001 report on GBM patients in Canada found that 45% of patients aged 60–69 years spent at least half of their remaining survival after diagnosis as an inpatient, which increased to 59% for those aged 70–79 years and 76% for those aged 80 years or older.42 A more recent study among over 5,000 GBM patients aged 65 years and older in the US found that despite median survival being only 4.9 months, 21% of all patients were hospitalized for at least 30 cumulative days between diagnosis and death, and 22% of all patients spent at least one-quarter of their remaining lives as an inpatient, with this risk increasing with increasing age.45 Thus, as management decisions are made regarding elderly patients with newly diagnosed GBM, honest discussions between physicians and patients and their families are crucial so that treatment options are discussed with regard to not only disease outcomes but also quality of life and patient preferences.
Summary and recommendations
In conclusion, GBM is an aggressive disease among the elderly, with median survival under 6 months. Elderly patients already represent half of the GBM population, a proportion that will continue to increase as the population ages and GBM incidence among the elderly climbs.2 Even in the modern era, increasing age remains the most powerful negative prognostic factor in GBM, which appears related to less favorable tumor biology, receipt of less aggressive care, and comorbid disease among the elderly.
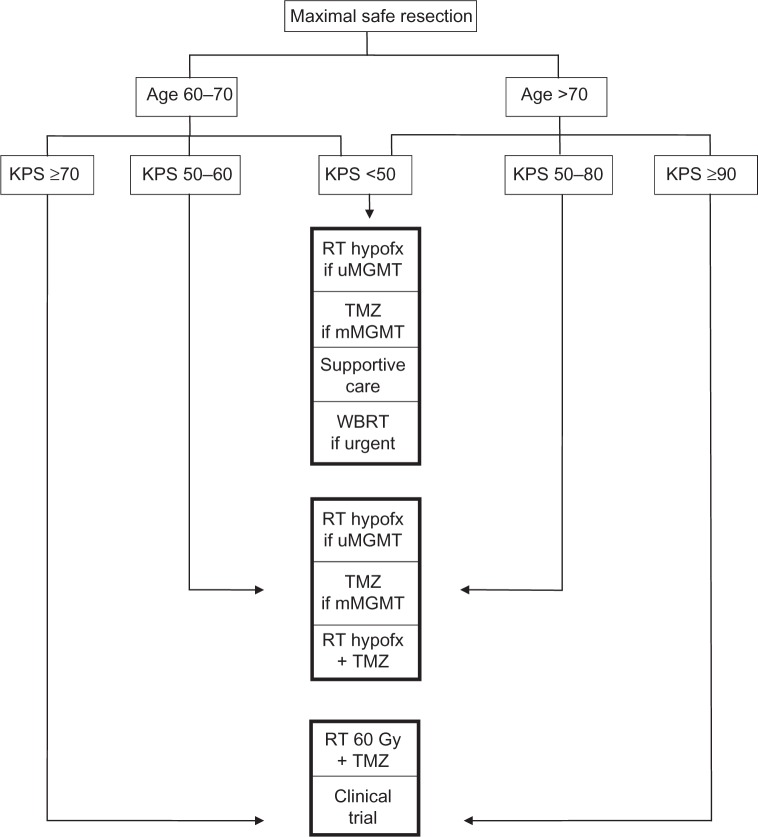
The standard of care for elderly GBM patients remains controversial and undefined. The treatment standard for younger adult patients with favorable KPS, consisting of concurrent chemoradiation followed by adjuvant TMZ per the Stupp et al23 regimen, showed no significant benefit for patients aged over 65 years in a subgroup analysis from the trial, but this analysis was not prespecified.23,25 There are retrospective data16,28 and a single-arm prospective study26 suggesting efficacy of combined modality therapy with RT and TMZ among elderly patients aged over 70 years, but this is not universally accepted, as this is not based on randomized data. Thus, for patients aged 65–70 years with very favorable performance status, we recommend maximal safe resection followed by consideration of concurrent TMZ-based chemoradiation or a clinical trial if eligible.
For patients aged over 70 years with favorable KPS, there is a clearly defined survival benefit to longer-course RT to 50 Gy as compared with best supportive care,32 so patients with favorable KPS should certainly be offered some form of tumor-directed treatment. Roa et al33 demonstrated possible noninferiority of shorter-course RT in an underpowered study of hypofractionated RT to 40 Gy compared with standard-course 60 Gy among patients aged 60 years or older with KPS of at least 50, which is being utilized with increasing frequency among older patients with favorable or borderline performance status. The NOA-08 trial (age >65 years) and Nordic trial (age ≥60 years) both evaluated TMZ monotherapy as compared with standard-course RT in GBM patients with favorable performance status, and the Nordic trial also compared these with hypofractionated RT.14,20 Both trials found survival in the range of 8 months for the TMZ only and hypofractionated RT arms, and the NOA-08 trial found increased toxicity for dose-dense TMZ. These two studies both support the routine testing of MGMT promoter methylation at the time of diagnosis, especially if considering using monotherapy as opposed to concurrent chemoradiation. Specifically, standard-dose TMZ monotherapy appears preferable for MGMT-methylated patients, and hypofractionated RT appears preferable for MGMT-unmethylated patients (either 34 Gy in ten fractions as per the Nordic trial or 40 Gy in 15 fractions as per Roa et al33). The question of whether to add TMZ to hypofractionated RT will hopefully be answered in the ongoing EORTC/NCIC/Trans Tasman Radiation Oncology Group randomized trial. Thus, for patients aged over 70 years with favorable performance status, or for patients aged 60 years or older with borderline performance status, we recommend maximal safe resection, MGMT testing, and then consideration of monotherapy with either TMZ or hypofractionated RT, or hypofractionated RT concurrently with TMZ.34,35 The Stupp et al23 regimen did not include patients aged over 70 years, but this treatment may still be considered for very fit patients; unfortunately, none of the recent randomized trials in elderly GBM patients has included this regimen as a comparator arm.
Finally, for elderly GBM patients with poorer performance status, reasonable options include best supportive care, including corticosteroids and anticonvulsant medications; TMZ monotherapy,37 especially if MGMT methylated; hypofractionated RT,31 especially if MGMT unmethylated; or whole brain RT36 if quite symptomatic with a need to start therapy quickly. The use of MGMT status to help inform treatment decisions is based on the findings from the Nordic and NOA-08 randomized trials, though is not based on current level I evidence. In addition, as the KPS scale defines patients who are “disabled” or who require “considerable assistance” to have a KPS score of 40–50, and given that GBM patients may have neurologic symptoms, such as paresis, that cause disability but may or may not be permanent, it is important to realize that the poor performance status GBM population represents a heterogeneous group with regard to patient trajectory and recovery from a symptom standpoint. In general, poor performance status at baseline predicts not only shortened survival but also more difficulty with treatment tolerance. Thus, active treatments should be considered carefully in this population, with ability to transition care goals if patients are struggling with treatment.
The landscape of treatment options for elderly GBM patients has changed substantially over the past decade and even in the past 2 years, with further information still amassing in ongoing clinical trials in this population, as outlined here. Based on the available evidence, Figure 1 shows suggested management options based on patient age and KPS. Until further treatment advances are made for GBM in general, utilizing the current therapeutic options of surgery, RT, and TMZ appropriately according to patient age, performance status, and patient preferences represents optimal management.
Figure 1.
Suggested treatment options for elderly glioblastoma patients.
Abbreviations: GBM, glioblastoma; hypofx, hypofractionated; KPS, Karnofsky performance status; MGMT, O6-methylguanine-DNA-methyltransferase; mMGMT, MGMT promoter methylated; RT, radiotherapy; TMZ, temozolomide; uMGMT, MGMT promoter unmethylated; WBRT, whole brain radiotherapy.
Footnotes
Disclosure
Nils D Arvold reports no conflicts of interest in this work. David A Reardon has served as a consultant and received honoraria from Apogenix, Stemline Therapeutics, Asmgen, Momenta Pharmaceuticals, EMD Serono, Genentech/Roche, Merck/Schering, and Novartis.
References
- 1.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005–2009. Neuro Oncol. 2012;14(Suppl 5):v1–v49. doi: 10.1093/neuonc/nos218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chakrabarti I, Cockburn M, Cozen W, Wang YP, Preston-Martin S. A population-based description of glioblastoma multiforme in Los Angeles County, 1974–1999. Cancer. 2005;104(12):2798–2806. doi: 10.1002/cncr.21539. [DOI] [PubMed] [Google Scholar]
- 3.Iwamoto FM, Reiner AS, Panageas KS, Elkin EB, Abrey LE. Patterns of care in elderly glioblastoma patients. Ann Neurol. 2008;64(6):628–634. doi: 10.1002/ana.21521. [DOI] [PubMed] [Google Scholar]
- 4.Barnholtz-Sloan JS, Maldonado JL, Williams VL, et al. Racial/ethnic differences in survival among elderly patients with a primary glioblastoma. J Neurooncol. 2007;85(2):171–180. doi: 10.1007/s11060-007-9405-4. [DOI] [PubMed] [Google Scholar]
- 5.Goodenberger ML, Jenkins RB. Genetics of adult glioma. Cancer Genet. 2012;205(12):613–621. doi: 10.1016/j.cancergen.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 6.Jha P, Suri V, Singh G, et al. Characterization of molecular genetic alterations in GBMs highlights a distinctive molecular profile in young adults. Diagn Mol Pathol. 2011;20(4):225–232. doi: 10.1097/PDM.0b013e31821c30bc. [DOI] [PubMed] [Google Scholar]
- 7.Yan H, Parsons DW, Jin G, et al. IDH1 and IDH2 mutations in gliomas. N Engl J Med. 2009;360(8):765–773. doi: 10.1056/NEJMoa0808710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ichimura K, Pearson DM, Kocialkowski S, et al. IDH1 mutations are present in the majority of common adult gliomas but rare in primary glioblastomas. Neuro Oncol. 2009;11(4):341–347. doi: 10.1215/15228517-2009-025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Nobusawa S, Watanabe T, Kleihues P, Ohgaki H. IDH1 mutations as molecular signature and predictive factor of secondary glioblastomas. Clin Cancer Res. 2009;15(19):6002–6007. doi: 10.1158/1078-0432.CCR-09-0715. [DOI] [PubMed] [Google Scholar]
- 10.Batchelor TT, Betensky RA, Esposito JM, et al. Age-dependent prognostic effects of genetic alterations in glioblastoma. Clin Cancer Res. 2004;10(1 Pt 1):228–233. doi: 10.1158/1078-0432.ccr-0841-3. [DOI] [PubMed] [Google Scholar]
- 11.Sanson M, Marie Y, Paris S, et al. Isocitrate dehydrogenase 1 codon 132 mutation is an important prognostic biomarker in gliomas. J Clin Oncol. 2009;27(25):4150–4154. doi: 10.1200/JCO.2009.21.9832. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann C, Hentschel B, Wick W, et al. Patients with IDH1 wild type anaplastic astrocytomas exhibit worse prognosis than IDH1-mutated glioblastomas, and IDH1 mutation status accounts for the unfavorable prognostic effect of higher age: implications for classification of gliomas. Acta Neuropathol. 2010;120(6):707–718. doi: 10.1007/s00401-010-0781-z. [DOI] [PubMed] [Google Scholar]
- 13.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003. doi: 10.1056/NEJMoa043331. [DOI] [PubMed] [Google Scholar]
- 14.Malmström A, Grønberg BH, Marosi C, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomised, phase 3 trial. Lancet Oncol. 2012;13(9):916–926. doi: 10.1016/S1470-2045(12)70265-6. [DOI] [PubMed] [Google Scholar]
- 15.Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, Batchelor TT. MGMT methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology. 2009;73(18):1509–1510. doi: 10.1212/WNL.0b013e3181bf9907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sijben AE, McIntyre JB, Roldan GB, et al. Toxicity from chemoradiotherapy in older patients with glioblastoma multiforme. J Neurooncol. 2008;89(1):97–103. doi: 10.1007/s11060-008-9593-6. [DOI] [PubMed] [Google Scholar]
- 17.Nghiemphu PL, Liu W, Lee Y, et al. Bevacizumab and chemotherapy for recurrent glioblastoma: a single-institution experience. Neurology. 2009;72(14):1217–1222. doi: 10.1212/01.wnl.0000345668.03039.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Curran WJ, Jr, Scott CB, Horton J, et al. Recursive partitioning analysis of prognostic factors in three Radiation Therapy Oncology Group malignant glioma trials. J Natl Cancer Inst. 1993;85(9):704–710. doi: 10.1093/jnci/85.9.704. [DOI] [PubMed] [Google Scholar]
- 19.Scott JG, Bauchet L, Fraum TJ, et al. Recursive partitioning analysis of prognostic factors for glioblastoma patients aged 70 years or older. Cancer. 2012;118(22):5595–5600. doi: 10.1002/cncr.27570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wick W, Platten M, Meisner C, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomised, phase 3 trial. Lancet Oncol. 2012;13(7):707–715. doi: 10.1016/S1470-2045(12)70164-X. [DOI] [PubMed] [Google Scholar]
- 21.Weller M, Stupp R, Hegi ME, et al. Personalized care in neuro-oncology coming of age: why we need MGMT and 1p/19q testing for malignant glioma patients in clinical practice. Neuro Oncol. 2012;14(Suppl 4):iv100–iv108. doi: 10.1093/neuonc/nos206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker MD, Strike TA, Sheline GE. An analysis of dose-effect relationship in the radiotherapy of malignant gliomas. Int J Radiat Oncol Biol Phys. 1979;5(10):1725–1731. doi: 10.1016/0360-3016(79)90553-4. [DOI] [PubMed] [Google Scholar]
- 23.Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–996. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 24.Stupp R, Hegi ME, Mason WP, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–466. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 25.Laperriere N, Weller M, Stupp R, et al. Optimal management of elderly patients with glioblastoma. Cancer Treat Rev. 2013;39(4):350–357. doi: 10.1016/j.ctrv.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 26.Minniti G, De Sanctis V, Muni R, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma in elderly patients. J Neurooncol. 2008;88(1):97–103. doi: 10.1007/s11060-008-9538-0. [DOI] [PubMed] [Google Scholar]
- 27.Niewald M, Berdel C, Fleckenstein J, Licht N, Ketter R, Rübe C. Toxicity after radiochemotherapy for glioblastoma using temozolomide: a retrospective evaluation. Radiat Oncol. 2011;6:141. doi: 10.1186/1748-717X-6-141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brandes AA, Franceschi E, Tosoni A, et al. Temozolomide concomitant and adjuvant to radiotherapy in elderly patients with glioblastoma: correlation with MGMT promoter methylation status. Cancer. 2009;115(15):3512–3518. doi: 10.1002/cncr.24406. [DOI] [PubMed] [Google Scholar]
- 29.Sanai N, Polley MY, McDermott MW, Parsa AT, Berger MS. An extent of resection threshold for newly diagnosed glioblastomas. J Neurosurg. 2011;115(1):3–8. doi: 10.3171/2011.2.jns10998. [DOI] [PubMed] [Google Scholar]
- 30.Vuorinen V, Hinkka S, Färkkilä M, Jääskeläinen J. Debulking or biopsy of malignant glioma in elderly people: a randomised study. Acta Neurochir. 2003;145(1):5–10. doi: 10.1007/s00701-002-1030-6. [DOI] [PubMed] [Google Scholar]
- 31.Chaichana KL, Garzon-Muvdi T, Parker S, et al. Supratentorial glioblastoma multiforme: the role of surgical resection versus biopsy among older patients. Ann Surg Oncol. 2011;18(1):239–245. doi: 10.1245/s10434-010-1242-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Keime-Guibert F, Chinot O, Taillandier L, et al. Radiotherapy for glioblastoma in the elderly. N Engl J Med. 2007;356:1527–1535. doi: 10.1056/NEJMoa065901. [DOI] [PubMed] [Google Scholar]
- 33.Roa W, Brasher PM, Bauman G, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583–1588. doi: 10.1200/JCO.2004.06.082. [DOI] [PubMed] [Google Scholar]
- 34.Minniti G, Lanzetta G, Scaringi C, et al. Phase II study of short-course radiotherapy plus concomitant and adjuvant temozolomide in elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2012;83(1):93–99. doi: 10.1016/j.ijrobp.2011.06.1992. [DOI] [PubMed] [Google Scholar]
- 35.Reyngold M, Lassman AB, Chan TA, Yamada Y, Gutin PH, Beal K. Abbreviated course of radiation therapy with concurrent temozolomide for high-grade glioma in patients of advanced age or poor functional status. J Neurooncol. 2012;110(3):369–374. doi: 10.1007/s11060-012-0972-7. [DOI] [PubMed] [Google Scholar]
- 36.Bauman GS, Gaspar LE, Fisher BJ, Halperin EC, Macdonald DR, Cairncross JG. A prospective study of short-course radiotherapy in poor prognosis glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 1994;29(4):835–839. doi: 10.1016/0360-3016(94)90573-8. [DOI] [PubMed] [Google Scholar]
- 37.Gállego Pérez-Larraya J, Ducray F, Chinot O, et al. Temozolomide in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2011;29(22):3050–3055. doi: 10.1200/JCO.2011.34.8086. [DOI] [PubMed] [Google Scholar]
- 38.Reyes-Botero G, Honnorat L, Chinot OL, et al. Temozolomide plus bevacizumab in elderly patients with newly diagnosed glioblastoma and poor performance status: an ANOCEF phase II trial. J Clin Oncol. 2013;(Suppl):2020. doi: 10.1634/theoncologist.2017-0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Walker GV, Li J, Mahajan A, et al. Decreasing radiation therapy utilization in adult patients with glioblastoma multiforme. Cancer. 2012;118(18):4538–4544. doi: 10.1002/cncr.27439. [DOI] [PubMed] [Google Scholar]
- 40.Scott J, Tsai YY, Chinnaiyan P, Yu HH. Effectiveness of radiotherapy for elderly patients with glioblastoma. Int J Radiat Oncol Biol Phys. 2011;81(1):206–210. doi: 10.1016/j.ijrobp.2010.04.033. [DOI] [PubMed] [Google Scholar]
- 41.Kita D, Ciernik IF, Vaccarella S, et al. Age as predictive factor in glioblastomas: population-based study. Neuroepidemiology. 2009;33(1):17–22. doi: 10.1159/000210017. [DOI] [PubMed] [Google Scholar]
- 42.Paszat L, Laperriere N, Groome P, Schulze K, Mackillop W, Holowaty E. A population-based study of glioblastoma multiforme. Int J Radiat Oncol Biol Phys. 2001;51(1):100–107. doi: 10.1016/s0360-3016(01)01572-3. [DOI] [PubMed] [Google Scholar]
- 43.Darefsky AS, King JT, Jr, Dubrow R. Adult glioblastoma multiforme survival in the temozolomide era: a population-based analysis of Surveillance, Epidemiology, and End Results registries. Cancer. 2012;118(8):2163–2172. doi: 10.1002/cncr.26494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dubrow R, Darefsky AS, Jacobs DI, et al. Time trends in glioblastoma multiforme survival: the role of temozolomide. Neuro-Oncol. 2013;15(12):1750–1761. doi: 10.1093/neuonc/not122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Arvold ND, Wang Y, Zigler C, Schrag D, Dominici F. Hospitalization burden and survival among elderly patients with glioblastoma. Neuro Oncol. 2013;15(Suppl 3):iii226–iii234. doi: 10.1093/neuonc/nou060. [DOI] [PMC free article] [PubMed] [Google Scholar]