Abstract
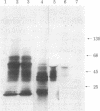
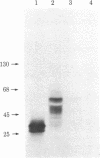
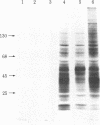
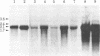
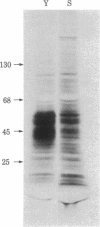
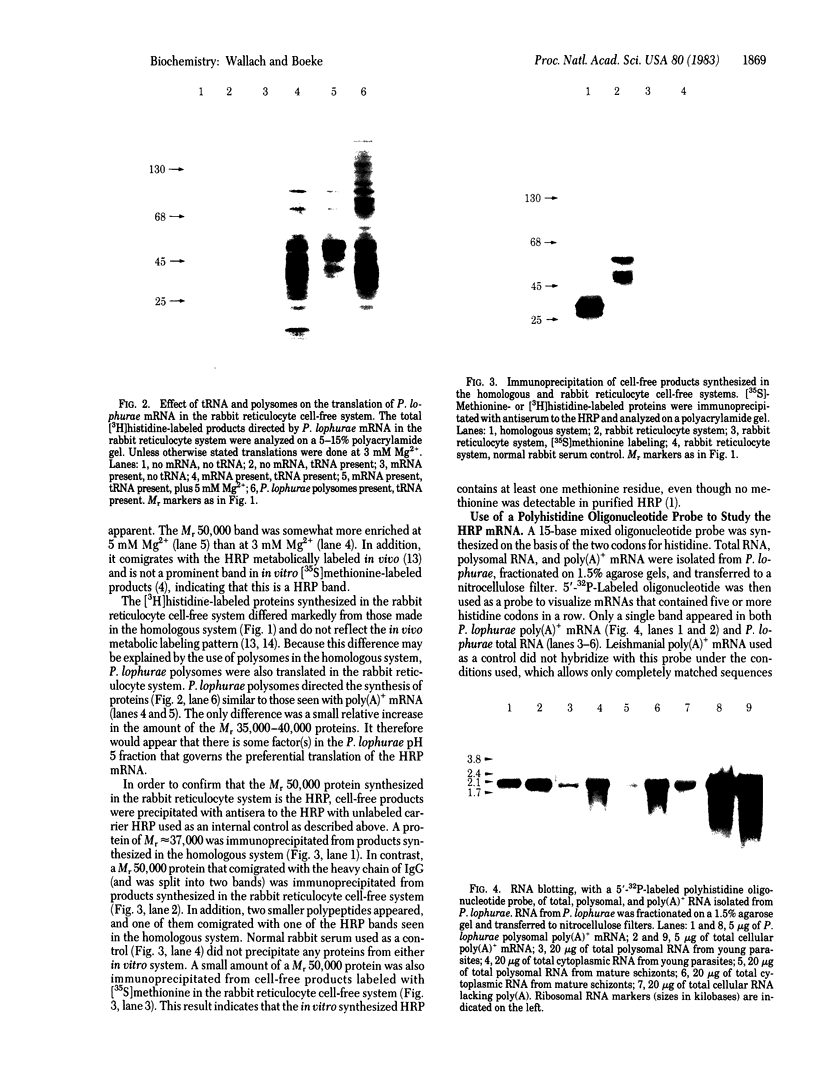
The histidine-rich protein (HRP) of the avian malaria parasite Plasmodium lophurae contains 70% histidine. It is found in dense cytoplasmic granules and during the erythrocytic cycle it accumulates to represent 10% of the dry weight of the parasite. In the present work the HRP mRNA was studied by in vitro translation and by the use of a polyhistidine oligonucleotide probe. The HRP mRNA contains 2,000-2,100 nucleotides encoding a protein with an apparent molecular weight of 50,000. In addition a HRP of molecular weight 35,000-40,000 is also produced in vitro, probably as a result of proteolytic cleavage of the molecular weight 50,000 polypeptide which corresponds to in vivo labeled and purified HRP. The HRP represents a much larger proportion of the in vitro products synthesized in the homologous cell-free system compared to the rabbit reticulocyte system, and it reflects more closely the pattern of protein synthesis seen in vivo. In addition, HRP mRNA is more abundant in polysomes isolated from young parasites than in polysomes from mature schizonts. These results indicate that the HRP accumulates as a result of amplified translation of its mRNA at certain stages of its erythrocytic cycle.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erickson A. H., Blobel G. Early events in the biosynthesis of the lysosomal enzyme cathepsin D. J Biol Chem. 1979 Dec 10;254(23):11771–11774. [PubMed] [Google Scholar]
- Gyang F. N., Poole B., Trager W. Peptidases from Plasmodium falciparum cultured in vitro. Mol Biochem Parasitol. 1982 Apr;5(4):263–273. doi: 10.1016/0166-6851(82)90034-2. [DOI] [PubMed] [Google Scholar]
- Kilejian A. A unique histidine-rich polypeptide from the malaria parasite, Plasmodium lophurae. J Biol Chem. 1974 Jul 25;249(14):4650–4655. [PubMed] [Google Scholar]
- Kilejian A. Does a histidine-rich protein from Plasmodium lophurae have a function in merozoite penetration? J Protozool. 1976 May;23(2):272–277. doi: 10.1111/j.1550-7408.1976.tb03768.x. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Histidine-rich protein as a model malaria vaccine. Science. 1978 Sep 8;201(4359):922–924. doi: 10.1126/science.567375. [DOI] [PubMed] [Google Scholar]
- Kilejian A. Homology between a histidine-rich protein from Plasmodium lophurae and a protein associated with the knob-like protrusions on membranes of erythrocytes infected with Plasmodium falciparum. J Exp Med. 1980 Jun 1;151(6):1534–1538. doi: 10.1084/jem.151.6.1534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilejian A., Liao T. H., Trager W. On primary structure and biosynthesis of histidine-rich polypeptide from malarial parasite Plasmodium lophurae. Proc Natl Acad Sci U S A. 1975 Aug;72(8):3057–3059. doi: 10.1073/pnas.72.8.3057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystosek A., Cawthon M. L., Kabat D. Improved methods for purification and assay of eukaryotic messenger ribonucleic acids and ribosomes. Quantitative analysis of their interaction in a fractionated reticulocyte cell-free system. J Biol Chem. 1975 Aug 10;250(15):6077–6084. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Nienhuis A. W., Falvey A. K., Anderson W. F. Preparation of globin messenger RNA. Methods Enzymol. 1974;30:621–630. doi: 10.1016/0076-6879(74)30060-2. [DOI] [PubMed] [Google Scholar]
- Rondinelli E., Soares M. C., de Castro J. F., de Castro F. T. Characterization of messenger RNA populations of Crithidia fasciculata. Arch Biochem Biophys. 1981 Jul;209(2):349–355. doi: 10.1016/0003-9861(81)90291-5. [DOI] [PubMed] [Google Scholar]
- TRAGER W. Folinic acid and non-dialyzable materials in the nutrition of malaria parasites. J Exp Med. 1958 Nov 1;108(5):753–772. doi: 10.1084/jem.108.5.753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallach M. Efficient extraction and translation of Plasmodium falciparum messenger RNA. Mol Biochem Parasitol. 1982 Dec;6(6):335–342. doi: 10.1016/0166-6851(82)90023-8. [DOI] [PubMed] [Google Scholar]
- Wallach M., Fong D., Chang K. P. Post-transcriptional control of tubulin biosynthesis during leishmanial differentiation. Nature. 1982 Oct 14;299(5884):650–652. doi: 10.1038/299650a0. [DOI] [PubMed] [Google Scholar]
- Wallach M., Kilejian A. The importance of tRNA for the in vitro cell-free translation of messenger RNA isolated from the malaria parasite Plasmodium lophurae. Mol Biochem Parasitol. 1982 Apr;5(4):245–261. doi: 10.1016/0166-6851(82)90033-0. [DOI] [PubMed] [Google Scholar]