Abstract
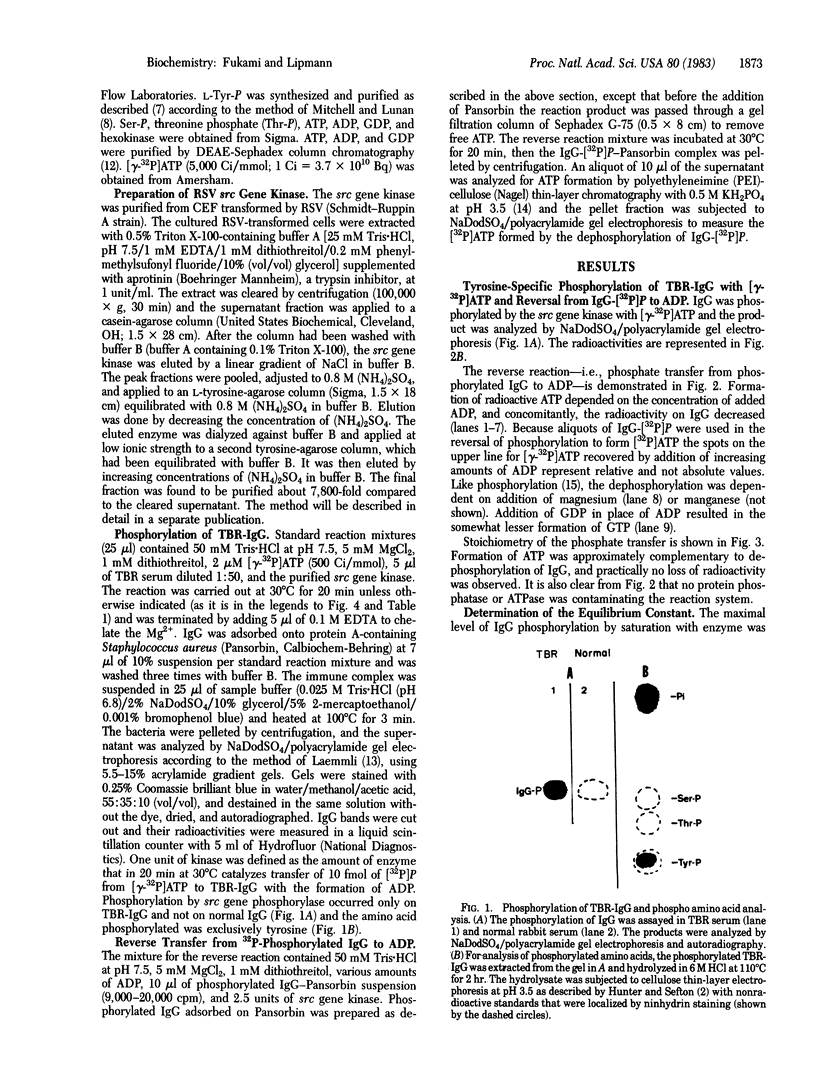
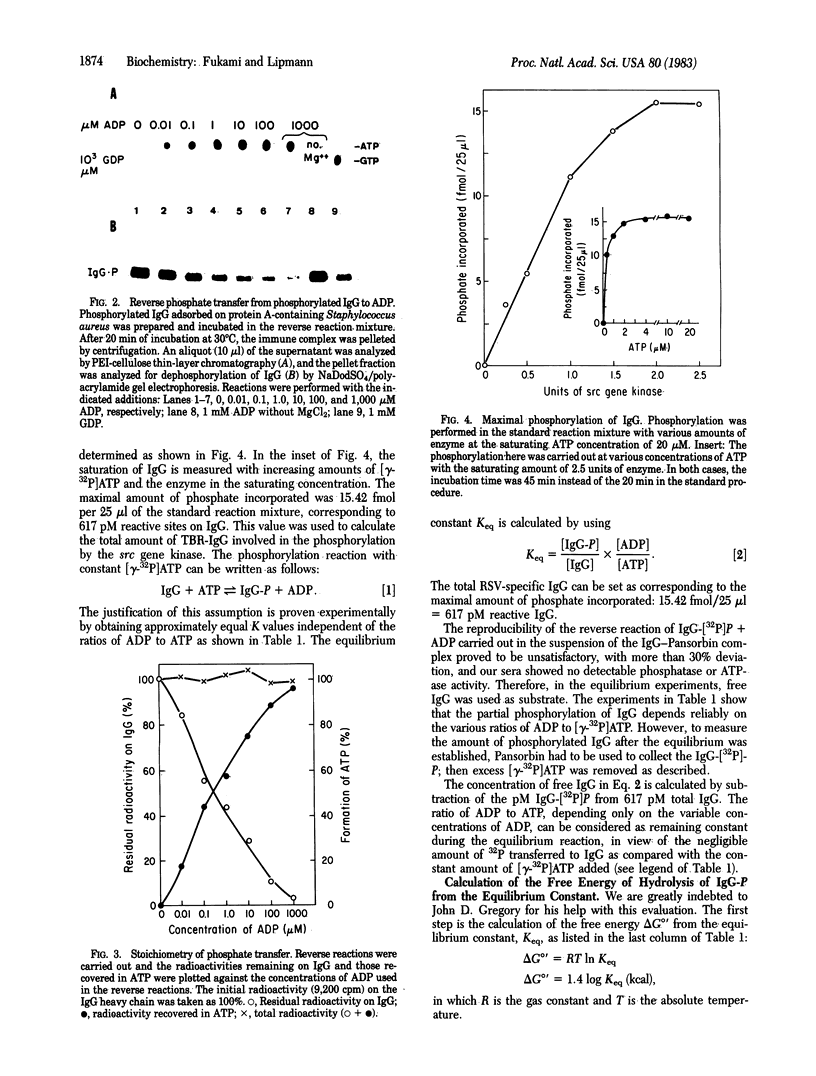
To determine the equilibrium constant of the reaction between ATP and protein-bound tyrosine we used as catalyst the highly purified Rous sarcoma src gene transcript. J. M. Sturtevant had earlier found (personal communication) that free tyrosine O-phosphate, upon hydrolysis with alkaline phosphatase in a calorimeter (37 degrees C, pH 9), yielded a delta H degrees of -2.8 kcal/mol (1 kcal = 4.18 kJ), less than half of that found in ATP hydrolysis. Experience with protein-bound serine phosphate (in phosvitin) had shown it to be energy rich [Rabinowitz, M. & Lipmann, F. (1960) J. Biol. Chem. 235, 1043-1050]. We wondered if the same is true for tyrosine phosphate when it is protein bound. From the equilibrium constant of 2.62 (at pH 6.5 and 5 mM Mg2+), we calculate a delta G degrees' of -9.48 kcal/mol for hydrolysis of protein-bound tyrosine phosphate, assuming an approximate delta G degrees' of -10 kcal/mol for hydrolysis of ATP. The experiments show that protein-bound tyrosine phosphate is energy rich, like serine phosphate in phosvitin.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberty R. A. Standard Gibbs free energy, enthalpy, and entropy changes as a function of pH and pMg for several reactions involving adenosine phosphates. J Biol Chem. 1969 Jun 25;244(12):3290–3302. [PubMed] [Google Scholar]
- Atmar V. J., Kuehn G. D. Phosphorylation of ornithine decarboxylase by a polyamine-dependent protein kinase. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5518–5522. doi: 10.1073/pnas.78.9.5518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Cashel M., Lazzarini R. A., Kalbacher B. An improved method for thin-layer chromatography of nucleotide mixtures containing 32P-labelled orthophosphate. J Chromatogr. 1969 Mar 11;40(1):103–109. doi: 10.1016/s0021-9673(01)96624-5. [DOI] [PubMed] [Google Scholar]
- Chan D. C., Piette L. H. Effect of Tyrosyl modifications on nucleosome reconstitution: a spin-labeling study. Biochemistry. 1982 Jun 8;21(12):3028–3035. doi: 10.1021/bi00541a034. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L., Collett M. S., Erikson E., Purchio A. F. Evidence that the avian sarcoma virus transforming gene product is a cyclic AMP-independent protein kinase. Proc Natl Acad Sci U S A. 1979 Dec;76(12):6260–6264. doi: 10.1073/pnas.76.12.6260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami Y., Lipmann F. Purification of a specific reversible tyrosine-O-phosphate phosphatase. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4275–4279. doi: 10.1073/pnas.79.14.4275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein phosphorylated by the RSV transforming function. Cell. 1980 Dec;22(3):647–648. doi: 10.1016/0092-8674(80)90539-5. [DOI] [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huttner W. B. Sulphation of tyrosine residues-a widespread modification of proteins. Nature. 1982 Sep 16;299(5880):273–276. doi: 10.1038/299273a0. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lerch K., Muir L. W., Fischer E. H. Purification and properties of a yeast protein kinase. Biochemistry. 1975 May 6;14(9):2015–2023. doi: 10.1021/bi00680a032. [DOI] [PubMed] [Google Scholar]
- MITCHELL H. K., LUNAN K. D. TYROSINE-O-PHOSPHATE IN DROSOPHILA. Arch Biochem Biophys. 1964 Jul 20;106:219–222. doi: 10.1016/0003-9861(64)90179-1. [DOI] [PubMed] [Google Scholar]
- Mantel M., Holzer H. Reversibility of the ATP:glutamine synthetase adenylyltransferase reaction. Proc Natl Acad Sci U S A. 1970 Mar;65(3):660–667. doi: 10.1073/pnas.65.3.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RABINOWITZ M., LIPMANN F. Reversible phosphate transfer between yolk phosphoprotein and adenosine triphosphate. J Biol Chem. 1960 Apr;235:1043–1050. [PubMed] [Google Scholar]
- Radke K., Martin G. S. Transformation by Rous sarcoma virus: effects of src gene expression on the synthesis and phosphorylation of cellular polypeptides. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5212–5216. doi: 10.1073/pnas.76.10.5212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richert N. D., Davies P. J., Jay G., Pastan I. H. Characterization of an immune complex kinase in immunoprecipitates of avian sarcoma virus-transformed fibroblasts. J Virol. 1979 Sep;31(3):696–706. doi: 10.1128/jvi.31.3.696-706.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy J. In vitro degradation of guanosine 5'-diphosphate, 3'-diphosphate. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5529–5533. doi: 10.1073/pnas.74.12.5529. [DOI] [PMC free article] [PubMed] [Google Scholar]


