Abstract
Background/Objectives
Colonic fermentation of dietary fibre produces short-chain fatty-acids (SCFA) acetate, propionate and butyrate, which may protect against type 2 diabetes by reducing serum free-fatty acids (FFA). Since hyperinsulinemia is associated with insulin resistance and increased diabetes risk, the main objective was to compare markers of colonic fermentation after acute inulin ingestion in subjects with normal (< 40pmol/L, NI) and high (≥ 40pmol/L, HI) plasma-insulin.
Subjects/Methods
Overnight fasted NI (n = 9) and HI (n = 9) subjects were studied for 4 h on 2 separate days after consuming 300 ml drinks containing 75 g glucose (Glucose) or 75 g glucose plus 24 g inulin (Inulin) using a randomized, single-blind, cross-over design.
Results
Inulin elicited a higher breath hydrogen and methane AUC but the increases in SCFA responses were not statistically significant. Overall mean serum-acetate over the 4 h study period was higher in NI than HI subjects (44.3±6.9 vs 22.5±3.7 μmol/L, p = 0.001). The rate of rebound of FFA was reduced by Inulin, with FFA at 4hr being less after Inulin than Glucose, regardless of insulin status (0.310±0.028 vs 0.432±0.042 mEq/L, p = 0.008).
Conclusions
This suggests that inulin increases short-term markers for colonic fermentation but a longer study period may be necessary to observe differences in SCFA production. The reason for the lower serum-acetate in HI is unclear but may be due to reduced absorption, increased clearance or decreased endogenous production. This suggests the need to compare acetate kinetics in normal and hyperinsulinemic subjects.
Keywords: Colonic fermentation, short chain fatty acids, acetate, inulin, hyperinsulinemia, free fatty acids
INTRODUCTION
The escalating rate of obesity worldwide (Caballero, 2007; Popkin and Gordon-Larsen, 2004; World Health Organisation, 2000) is associated with an increasing prevalence of type 2 diabetes (T2DM) (Wild et al., 2004). One mechanism by which obesity may contribute to the pathogenesis of T2DM is that the increased flux of FFAs from lipolysis in visceral adipose tissue causes insulin resistance and impairs pancreatic β-cell function (Prentki and Nolan, 2006; Greenberg and McDaniel, 2002; McGarry, 2002).
Epidemiological studies have shown that a high intake of dietary fibre, especially insoluble fibre from whole grains (nonviscous, slowly fermentable fibre), is associated with a lower risk for type 2 diabetes (de Munter et al, 2007; Schulze et al., 2007; Salmeron et al., 1997; Liu et al., 1999) and increased insulin sensitivity (Liese et al., 2005). The mechanisms by which insoluble fibres reduce diabetes risk are largely unknown, but, maybe via colonic fermentation. Improved insulin sensitivity has been observed after short-term consumption of purified insoluble fibres which appear to be independent of the rate of colonic fermentation (Weickert et al., 2005). Other factors may therefore also be involved, an accelerated GIP response after insoluble fibre enrichment was described as a potential mechanism linking cereal fibre intake to the reduced risk of diabetes (Weickert et al, 2005). Colonic fermentation of dietary fibre may exert an insulin-sensitizing effect through the production of the short chain fatty acids (SCFAs), acetate, propionate and butyrate. SCFA may modulate insulin sensitivity by reducing fatty acid flux. Oral ingestion (Crouse et al., 1968) and rectal infusion of acetate (Wolever et al., 1989) leads to reduced serum free-fatty acid (FFA) concentrations and feeding fermentable carbohydrate also reduces serum FFA concentrations (Jenkins et al., 1991; Ferchaud-Rocher et al., 2005; Brighenti et al., 2006; Tarini and Wolever, 2010).
It is known that both production and absorption rates of colonic SCFA differ between individuals, and that glucose tolerance status affects serum SCFA concentrations in humans who are insulin resistant but nondiabetic (Wolever et al., 1997). This suggests that insulin resistance may alter SCFA metabolism. Studies have not been done that specifically look at markers of colonic fermentation (ie. breath hydrogen and methane and SCFA concentrations) and FFA concentrations in insulin resistant subjects. Serum SCFA concentrations in normal and hyperinsulinemic humans after ingestion of fibre has not been compared before. Therefore, the main objective of this preliminary study was to determine if differences exist in breath hydrogen and methane and the SCFA response after acute ingestion of the non-digestible oligosaccharide inulin in healthy humans without T2DM who are insulin resistant compared with those who are insulin sensitive.
METHODS
Male or non-pregnant, non-lactating females aged 18–65 with BMI ≥ 20 and ≤ 35kg/m2 were recruited from a pool of subjects previously involved in similar studies. Subjects were excluded for any of the following reasons: history of diabetes, cardiovascular disease or bowel, kidney or liver disease; use of medications which affect blood glucose or insulin sensitivity (such as diuretics); use of antibiotics within 3 months of starting the study; or following any unusual dietary practices. Eligible subjects were then screened with a fasting blood sample; subjects were excluded for any of the following reasons: serum glucose ≥ 7.0 mmol/L, triglycerides ≥ 4.0 mmol/L, hematocrit (HCT) < 0.345 (the lower limit of normal) or aspartate transaminase > 1.5 times the upper limit of normal. Eligible subjects were divided prospectively to obtain a group (n = 9) with normal fasting insulin (FSI < 40pmol/L) and a group (n = 9) with high fasting insulin (FSI ≥ 40pmol/L). Subjects were selected based on FSI because of the positive association between FSI and insulin resistance (Yeni-Komshian et al., 2000), and because 40 pmol/L represents approximately the 66th percentile for healthy subjects, based on previous studies in our lab. Ethical approval for the study was obtained from the Research Ethics Boards of St. Michael’s Hospital, Toronto and the University of Toronto. Subjects gave written informed consent to participate in the study.
All tests were conducted at the Clinical Nutrition and Risk Factor Modification Centre (CNRFMC) of St. Michael’s Hospital, Toronto. Subjects were studied on two mornings, separated by 13 ± 2 days, after 10 – 14-h overnight fasts. Tests were administered in a random order. Subjects were asked to refrain from alcohol consumption and any abnormally strenuous exercise in the 24 h period prior to all test days.
On the day of the test, subjects collected fasting breath samples. An indwelling catheter was inserted into a superficial forearm vein and kept patent with normal saline and fasting blood samples were collected. The subjects then consumed a test drink containing 75 g glucose (Glucose) (Grain Process Enterprises Ltd, Scarborough, ON) or 75 g glucose + 24 g Oliggo-Fiber® Instant Inulin (Cargill Inc, Wayzata, MN) (Inulin) dissolved in 300 mL of water. They consumed the test drink over 5 min, and were given an additional 200 mL of water to drink over 15 min. Blood samples were collected at 30, 60, 90, 120, 180, and 240 min following consumption of the test drink for the measurement of glucose, insulin, C-peptide, free fatty acids and SCFA. Breath samples, for analysis of breath hydrogen and methane concentrations, were collected every half hour for 4 h following consumption of the test drink.
Whole blood for glucose, insulin, C-peptide, FFA and SCFA measurements were collected in red top Vacutainer™ tubes (Becton Dickinson, Franklin Lakes, NJ). Serum glucose was measured by a glucose oxidase method (SYNCHRON LX Systems, Beckman Coulter, Brea, CA) (inter-assay CV 1.9%), insulin using the Beckman Access Ultrasensitive Insulin method (Beckman Instruments, Fullerton, CA) (inter-assay CV 2.5 to 4.3%) and c-peptide by a highly specific double-antibody RIA (Siemens Medical Solutions Diagnostics, Los Angeles, CA) (inter- assay CV ≤10%). Blood samples for serum FFA and SCFA were allowed to clot at room temperature, centrifuged at 600 × g for 15 min at 4 °C, and the serum aliquoted and stored at −70 °C before analysis. FFA were measured by an enzymatic technique that used acylCoA oxidase(Wako Diagnostics, Wako Chemicals, USA, Inc) (inter-assay CV of <1.5%).
SCFA were measured by gas chromatography after micro filtration and vacuum distillation. An 1.2–1.5 mL aliquot of serum was filtered through a micropartition system with a 30 kDa MWCO Vivaspin RC (VS02H22) filters (Sartorius Inc., Mississauga, ON) by centrifugation at 5000 × g at 4 °C for 90 min. The protein-free filtrate was stored at −20 °C before vacuum distillation (Tollinger et al., 1979). Distillation was performed by using a 225 μL sample of protein-free serum to which was added a 25 μL internal standard solution consisting of 1.25 mM valeric acid and 1.06 M formic acid. An automatic sampler (HP 7673; Hewlett- Packard, Mississauga, ON) was used to inject 1 μL aliquots of sample into a gas chromatograph (HP 5890 Series II; Hewlett-Packard) equipped with a direct-cool, on-column inlet, an Agilent HP-FFAP column (30m × 0.53mm × 1.0 μm film), Agilent 19095F-123 (Agilent Technologies Canada Inc., Mississauga, ON), and a flame ionization detector.
Subjects collected breath samples using the Easy Sampler ™ with tube holder (Quintron Instrument Company, Milwaukee, WI). Methane and hydrogen were measured by gas chromatography (Quintron Microlyzer, Model SC, Milwaukee, WI) in breath samples and simultaneously obtained room air. Breath hydrogen and methane concentrations reported were adjusted by subtracting the hydrogen and methane of room air from that of each breath sample collected.
Statistical analysis was performed with SPSS version 17 for WINDOWS (SPSS Inc., Chicago, IL) using the General Linear Model (GLM) repeated-measures analysis of variance (ANOVA) examining for the main effects of group, treatment and time, and interactions between these three effects. Since there were only two treatments and two groups, post hoc tests were not performed in SPSS. Post hoc analysis using Bonferroni post-tests was done using GraphPad Prism 5 for Windows, Version 5.02 (GraphPad Software Inc., La Jolla, CA) when main effects were identified by ANOVA. Differences with P-values ≤ 0.05 were considered to be statistically significant.
Since a significant time × treatment × group interaction was not observed for any variable, data for study variables are presented in two ways. Figures 1 & 2 compare postprandial responses between NI and HI subjects; the values at each time point represent the mean response for Glucose and Inulin. Figures 3 & 4 compare postprandial responses between Glucose and Inulin; the values at each time point represent the mean for all subjects. A main effect of group was observed for acetate, insulin and c-peptide.
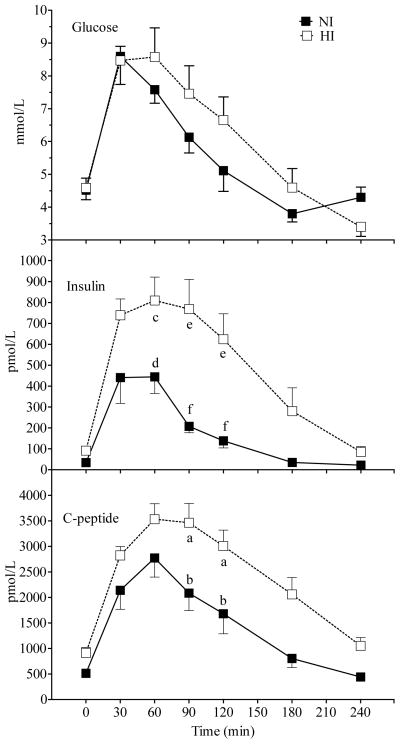
Figure 1.
Mean (± SEM) plasma glucose, insulin and c-peptide concentrations after Glucose and Inulin in NI and HI subjects. A significant main effect of group was observed for insulin (p = 0.003) and c-peptide (p < 0.01). A significant group × time interaction was observed for glucose (p = 0.01) and insulin (p < 0.001). Within a time point, labelled means without a common letter differ (a vs b, p <0.01; c vs d, p < 0.05; e vs f, p < 0.001; Bonferroni post-test after demonstration of significant main effect of group by ANOVA).
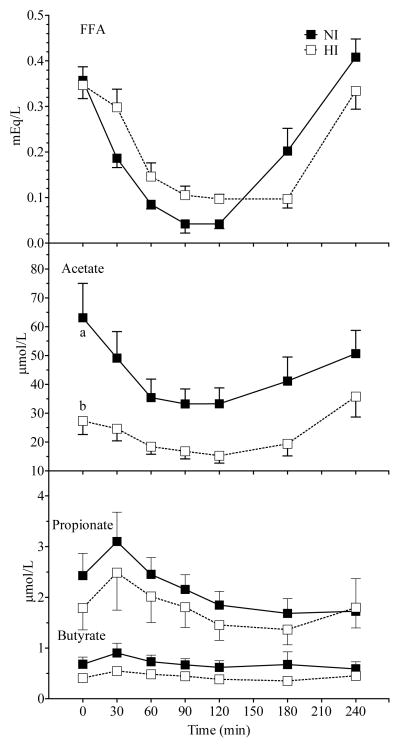
Figure 2.
Mean (± SEM) FFA, serum acetate, propionate and butyrate concentrations after Glucose and Inulin in NI and HI subjects. A significant main effect of group was observed for acetate (p = 0.01). A significant group × time interaction was observed for FFA (p = 0.001). Within a time point, labelled means without a common letter differ (a vs b, p <0.01; Bonferroni post-test after demonstration of significant main effect of group by ANOVA).
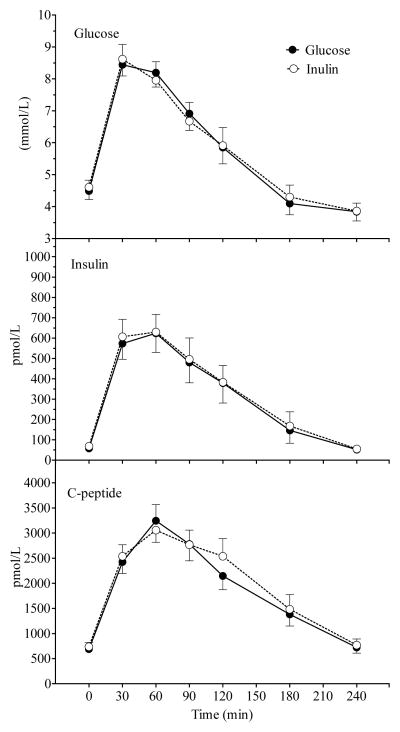
Figure 3.
Mean (± SEM) plasma glucose, insulin and c-peptide concentrations after Glucose and Inulin in all 18 subjects. No significant main effect of treatment was observed for any variable.
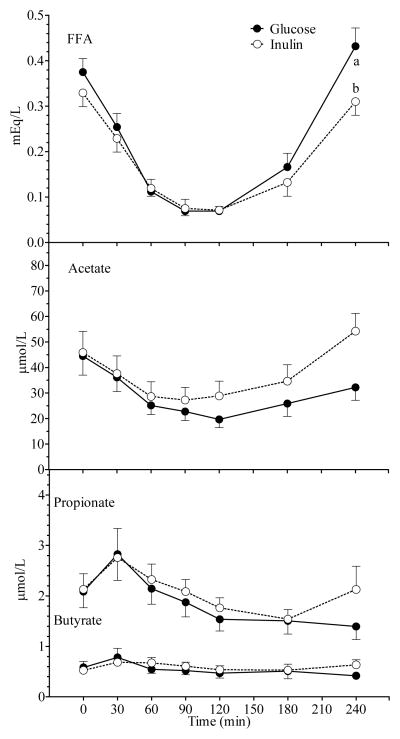
Figure 4.
Mean (± SEM) FFA, serum acetate, propionate and butyrate concentrations after Glucose and Inulin in all 18 subjects. A significant main effect of treatment was observed for FFA (p = 0.03) along with a significant time × treatment interaction (p < 0.001). A significant time × treatment interaction was also observed acetate (p = 0.02). Within a time point, labelled means without a common letter differ (a vs b, p <0.01; Bonferroni post-test after demonstration of significant main effect of treatment by ANOVA).
Incremental areas under the curve (AUC), ignoring the area beneath the baseline, were also calculated geometrically for the 0–4h period, using a Microsoft Office Excel 2003 (Microsoft Corp) spreadsheet. Statistical analysis was done on the AUC using analysis of variance (ANOVA) examining for the main effects of group and treatment and interactions between these two effects. Differences with P-values ≤ 0.05 (2-tailed) were considered to be statistically significant. The results are expressed as means ± SEM.
RESULTS
We studied 11 women and 7 men with a mean (± SEM) age of 36.4 ± 9.0 y and BMI 26.9 ± 3.7 kg/m2. Subjects with HI had significantly higher waist circumference, serum insulin, triglycerides and total cholesterol/HDL ratio and a significantly lower HDL concentration than NI subjects (Table 1). Inulin was well tolerated and participants reported no adverse reactions after consumption. The mean fasting concentrations for serum SCFA, breath gases, plasma glucose, insulin, C-peptide, and FFA did not differ between the two test days.
Table 1.
Characteristics of the normal and hyperinsulinaemic subjects at the screening visit1
| Normal | Hyperinsulinemics | P-value | |
|---|---|---|---|
| Age (y) | 35.9 ± 3.8 | 36.9 ± 2.2 | 0.8 |
| M:F | 4 : 5 | 3 : 6 | |
| BMI (kg/m2) | 25.4 ± 1.5 | 28.4 ± 0.7 | 0.1 |
| Waist Circumference (cm) | 87.7 ± 2.7 | 95.1 ± 1.4 | 0.03 |
| Systolic blood pressure (mm Hg) | 109 ± 3 | 118 ± 3 | 0.06 |
| Diastolic blood pressure (mm Hg) | 68 ± 3 | 77 ± 4 | 0.08 |
| Glucose (mmol/L) | 5.0 ± 0.1 | 5.1 ± 0.2 | 0.65 |
| Insulin (pmol/L) | 29.4 ± 2.0 | 76.7 ± 12.0 | 0.004 |
| HCT (L/L) | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.91 |
| AST (U/L) | 18.9 ± 1.3 | 21.9 ± 1.6 | 0.16 |
| CRP (mg/L) | 2.5 ± 1.8 | 2.7 ± 0.7 | 0.91 |
| Cholesterol (mmol/L) | 4.7 ± 0.2 | 5.3 ± 0.4 | 0.23 |
| Triglycerides (mmol/L) | 0.91 ± 0.12 | 1.78 ± 0.3 | 0.02 |
| HDL (mmol/L) | 1.42 ± 0.06 | 1.08 ± 0.08 | 0.004 |
| TC/HDL | 3.33 ± 0.18 | 4.93 ± 0.31 | 0.001 |
| LDL (mmol/L) | 2.84 ± 0.21 | 3.39 ± 0.30 | 0.16 |
Values are means ±SEM.
All measured variables except methane varied significantly with time. Responses in NI differed from those in HI with a significant main effect of group for insulin, c-peptide (Figure 1) and acetate (Figure 2) and a significant time×group interaction for glucose, insulin, and FFA (Figure 1 & 2). Incremental AUC for insulin and c-peptide were significantly greater in HI compared to NI subjects (Table 2). Serum insulin was significantly higher in HI than NI at 60 (p < 0.05; Bonferroni correction), 90 and 120 min (p < 0.001; Bonferroni correction), and c-peptide higher in HI than NI at 90 and 120 min (p <0.01; Bonferroni correction; Figure 1). The rise in serum glucose from 0–30 min was similar in HI and NI, but its fall was delayed by 30–60 min in HI compared to NI. Similarly the fall and subsequent rebound of FFA was delayed by about 30 min in HI compared to NI. However, despite the significant time×group interaction none of the differences in glucose or FFA between HI and NI was significant at any individual time point (Figure 1 & 2).
Table 2.
Incremental areas under the curve for glucose, insulin, C-peptide, FFA and short chain fatty acids (SCFAs) after glucose and inulin in normal (NI) and hyperinsulinemic (HI) subjects.
| Treatment | Group | P-value | ||||
|---|---|---|---|---|---|---|
| NI | HI | Group | Treatment | Group × Treatment | ||
| Glucose (mmol × min·L−1) | Glucose Inulin |
279 ± 103 201 ± 50 |
361 ± 78 400 ± 88 |
0.095 | 0.813 | 0.481 |
| Insulin (nmol × min·L−1) | Glucose Inulin |
35.1 ± 7.0 32.8 ± 6.0 |
94.3 ± 19.0 98.5 ± 17.7 |
< 0.001 | 0.949 | 0.817 |
| C-peptide (nmol × min·L −1) | Glucose Inulin |
233 ± 51 230 ± 51 |
358 ± 47 393 ± 48 |
< 0.01 | 0.745 | 0.707 |
| FFA (mEq × min·L−1) | Glucose Inulin |
−48.8 ± 8.4 −40.5 ± 10.2 |
−42.0 ± 7.7 −41.3 ± 10.5 |
0.811 | .0916 | 0.875 |
| Acetate (μmol × min·L−1) | Glucose Inulin |
−5577 ± 1989 −4238 ± 2090 |
−2054 ± 692 −796 ± 436 |
0.027 | 0.393 | 0.979 |
| Propionate (μmol × min·L−1) | Glucose Inulin |
−124 ± 58 −34 ± 43 |
0 ± 21 −24 ± 18 |
0.091 | 0.390 | 0.149 |
| Butyrate (μmol × min·L−1) | Glucose Inulin |
−24 ± 18 27 ± 25 |
0 ± 6 4 ± 7 |
0.986 | 0.098 | 0.142 |
| Hydrogen (ppm × min) | Glucose Inulin |
−595 ± 405 983 ± 1314 |
−546 ± 224 1442 ± 523 |
0.735 | 0.023 | 0.785 |
| Methane (ppm × min) | Glucose Inulin |
−88 ± 154 846 ± 666 |
−538 ± 409 659 ± 426 |
0.486 | 0.025 | 0.773 |
Note: Values are means ± SEM for n = 18 subjects.
Fasting (p <0.01; Bonferroni correction) and postprandial serum acetate was significantly higher in NI compared to HI subjects (Figure 2). Overall the mean serum-acetate concentration over the 4 h study period was higher in NI than HI subjects (43.7 ± 6.6 vs 22.5 ± 3.7 μmol/L, p = 0.02) and the acetate AUC was significantly greater in NI compared to HI subjects (p < 0.05, Table 2).
A significant time × treatment × group interaction was not observed for any variable which indicates that Inulin had a similar effect on all measured variables in NI and HI subjects. There were no significant differences in glucose, insulin, C-peptide, FFA and SCFA AUC between the Glucose and Inulin treatments in both groups (Table 2). In all subjects combined, Inulin had no significant effect on plasma glucose, insulin and c-peptide responses compared to Glucose (Figure 3). There was a significant main effect of treatment and a significant time × treatment interaction for FFA (Figure 4). Serum FFA rebounded significantly more slowly after Inulin than Glucose (0.0021 ± 0.0002 vs 0.0033 ± 0.0004 mEq/L/min, p = 0.006), and serum-FFA was significantly lower 4 h after Inulin than Glucose (0.310 ± 0.028 vs 0.432 ± 0.042 mEq/L, p < 0.01; Bonferroni correction). There was also a significant time × treatment interaction (p=0.001) for serum acetate with acetate falling initially after both test meals, but then tending to rise more quickly and to a greater extent after Inulin compared to Glucose, however the difference in serum acetate between Inulin and Glucose was not significant at any point in time (Figure 4); in addition there was no significant difference in acetate AUC. Serum propionate and butyrate concentrations tended to be higher 240 min after Inulin than Glucose, but the differences were not significant.
Breath hydrogen and methane responses did not differ significantly between NI and HI subjects. The breath hydrogen and methane responses were significantly higher after Inulin than Glucose in both groups (significant time × treatment interaction, p < 0.001 and p = 0.002, respectively. Breath hydrogen and methane AUC were also significantly greater after Inulin than Glucose in both groups (Table 2).
DISCUSSION
The significance of colonic fermentation of dietary fibre and the role of SCFA in enhancing insulin sensitivity and reducing the risk of T2DM has been the subject of studies in normal (Robertson et al., 2003, 2005) and hyperinsulinemic humans (Freeland et al., 2009). But, studies so far have not compared colonic fermentation of dietary fibre in normal and hyperinsulinemic humans. The main objective of this preliminary study was to compare markers of colonic fermentation ie. breath hydrogen and methane and serum SCFA responses in subjects with normal plasma insulin (NI) and those with high plasma insulin (HI) concentrations after 75 g glucose (Glucose) and 75 g glucose plus 24 g inulin (Inulin). The results showed that inulin had similar effects on breath hydrogen and methane and serum SCFA concentrations in NI compared to HI subjects, but that fasting and postprandial serum acetate concentrations were significantly higher in NI compared to HI subjects.
Although breath hydrogen and methane and serum SCFA are short-term markers of colonic fermentation, the breath gases are known to increase 1–2 hours after inulin ingestion, whereas serum SCFA take longer to respond. Our results are consistent with this in that we observed significant increases in breath hydrogen and methane after inulin, whereas tendency toward increases in serum SCFA responses was no statistically significant. The latter indicates that a longer study period may be required to observe changes in SCFA concentrations. In an earlier study in normals over a 6 h period, 24 g inulin plus 56 g high fructose corn syrup significantly increased postprandial acetate, propionate and butyrate responses (Tarini and Wolever, 2010), with acetate concentrations peaking at 4.5 h over the 6 h study period, propionate at 5 h and butyrate at ~5.5 h (Tarini and Wolever, 2010). The longer study period, and the fact that subjects consumed a standard lunch at 4 h may explain the discrepancy with our results. The SCFA response after Inulin did not differ between the two groups suggesting that exogenous production of SCFA may be similar in NI and HI subjects. Inulin is a prebiotic as it selectively stimulates growth and/or activity of bifidobacteria (Gibson et al., 1995). A possible criticism of this study is that inulin is mainly fermented by bifidobacteria and may not involve other gut microbial species in its colonic fermentation. However, a study in batch cultures which confirmed the bifidogenic effect of inulin also indicated that other intestinal microbial groups grew on its carbon sources (Rossi et al., 2005). It was observed that bifidobacteria grew by cross-feeding on the mono- and oligosaccharides produced by primary inulin intestinal degraders. Therefore, the colonic fermentation of inulin may be a complex process involving many metabolic pathways, carried out by numerous species which are interdependent on each other.
Interestingly, lower serum acetate concentrations were seen in HI compared to NI subjects. Previous work has not linked hyperinsulinemia to low acetate concentrations. Fasting serum acetate is increased in T2DM (Akanji et al., 1989, Todesco et al., 1993; Wolever et al., 1995), but increased concentrations of fasting serum acetate were not observed in obese or impaired glucose tolerant subjects (Wolever et al., 1997). Theoretically, a lower acetate concentration in HI subjects may be explained by decreased production (endogenous or exogenous) or decreased absorption or increased clearance. Our results showed that serum acetate after Inulin was similar in NI and HI subjects, this suggests that the lower acetate concentration in HI subjects may result from a decreased endogenous production of acetate. The significantly lower fasting acetate concentration in HI subjects also points to reduced endogenous production as, after an overnight fast, serum acetate is mainly derived from endogenous sources. In NI subjects, the acetate nadir was reached significantly more quickly at 77 ± 13 min compared to 133 ± 16 min in HI subjects (p = 0.02). This sharp fall in acetate in NI subjects may be explained by the suppressive effect of insulin on fat oxidation and, therefore, endogenous acetate release and the rebound may be due to the loss of this suppressive effect of insulin. But, in HI subjects a similar scenario was not observed as hyperinsulinemia may alter endogenous acetate production. Additionally, the delayed fall in serum acetate in HI subjects was paralleled by a delayed fall in FFA which suggests that endogenous acetate production associated with fat oxidation is normal in the NI group but it may be altered in the HI group.
Endogenous acetate metabolism is facilitated by two enzymes, acetyl-CoA synthetase (ACAS) and acetyl-CoA hydrolase. Mammals possess two isoforms of ACAS, one cytosolic and the other mitochondrial. The activity of the cytosolic enzyme (ACAS1), found predominantly in the liver, activates acetate to supply cells with acetyl-CoA for lipid synthesis (Luong et al., 2000). ACAS1 activity responds to changes in nutritional and hormonal status and in vitro studies have shown that it is regulated by insulin (Del Boca and Flatt, 1969; Knowles et al., 1974; Sone et al., 2002). Fasting reduces transcription levels for ACAS1, while refeeding restores them. The ACAS gene was downregulated in streptozotocin-induced diabetic mice and was restored after insulin replacement, suggesting that diabetic status and insulin also regulate this gene (Sone et al., 2002). Studies measuring acetate and glucose turnover in insulin resistant obese and diabetic subjects have also shown that acetate metabolism is sensitive to insulin action (Piloquet et al., 2002). It is unclear if the insulin resistance in the HI subjects in our study contributed to the altered acetate metabolism in this group possibly through the actions of insulin on ACAS1 gene expression.
Lowering postprandial FFA improves insulin sensitivity and insulin secretion (Boden, 2002; Boden and Shulman 2002). The hypothesis that SCFA modulate insulin sensitivity through alterations in fatty acid flux (Wolever et al., 1989) has been tested in several studies (Robertson et al., 2003, 2005), but their results are equivocal. It has been suggested that acetate reduces serum FFA by suppressing adipose tissue lipolysis (Akanji et al., 1989). In addition, there is evidence that acetate produced as a byproduct of microbial fermentation of dietary fibre in the human colon reduces plasma FFA concentrations and improves insulin sensitivity (Jenkins et al., 1991; Ferchaud-Rocher et al., 2005; Brighenti et al., 2006; Tarini and Wolever, 2010). Thus high concentrations of colonic acetate may have beneficial effects in the peripheral circulation on adipose tissue lipolysis and may lead to a decrease in insulin resistance by lowering FFA concentrations. Conversely, low concentrations of SCFA or the inability of SCFA to regulate FFA may contribute to the development of obesity and insulin resistance.
In an earlier study (Tarini and Wolever, 2010), feeding healthy subjects 24 g of inulin significantly increased SCFA responses and this was associated with a reduced postprandial FFA concentration. In the present study a significant main effect of treatment and a significant time × treatment interaction was observed for FFA, but, FFA AUC did not significantly differ after Inulin between NI and HI subjects. In the earlier study (Tarini and Wolever, 2010), FFA response was measured over a 6h period, therefore, a longer study period may be required to observe changes in FFA response after inulin.
In conclusion, this study shows that acute inulin ingestion increases short-term markers for colonic fermentation in NI and HI subjects as evidenced by increases in breath hydrogen and methane. But a longer study period may be necessary to observe significant increases in the serum SCFA response. The lower serum acetate concentration in HI subjects may be related to an altered endogenous acetate metabolism in this group. But, more studies are needed to see if differences in SCFA absorption and clearance between the two groups are also involved.
Acknowledgments
Supported by a grant from the Canadian Institutes for Health Research, Institute of Nutrition, Metabolism and Diabetes
Footnotes
CONFLICT OF INTEREST
The authors declare no conflict of interest.
LITERATURE CITED
- Akanji AO, Humphreys S, Thursfield V, Hockaday TD. The relationship of plasma acetate with glucose and other blood intermediary metabolites in non-diabetic and diabetic subjects. Clin Chim Acta. 1989;185:25–34. doi: 10.1016/0009-8981(89)90127-7. [DOI] [PubMed] [Google Scholar]
- Boden G. Interaction between free fatty acids and glucose metabolism. Curr Opin Nutr Metab Care. 2002;5:545–549. doi: 10.1097/00075197-200209000-00014. [DOI] [PubMed] [Google Scholar]
- Boden G, Shulman GI. Free fatty acids in obesity and type 2 diabetes: defining their role in the development of insulin resistance and B-cell dysfunction. Eur J Clin Invest. 2002;32:S14–S23. doi: 10.1046/j.1365-2362.32.s3.3.x. [DOI] [PubMed] [Google Scholar]
- Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83:817–822. doi: 10.1093/ajcn/83.4.817. [DOI] [PubMed] [Google Scholar]
- Caballero B. The global epidemic of obesity: an overview. Epidemiol Rev. 2007;29:1–5. doi: 10.1093/epirev/mxm012. [DOI] [PubMed] [Google Scholar]
- Crouse JR, Gerson CD, Decarli LM, Lieber S. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J Lipid Res. 1968;9:509–512. [PubMed] [Google Scholar]
- Del Boca J, Flatt JP. Fatty acid synthesis from glucose and acetate and the control of lipogenesis in adipose tissue. Eur J Biochem. 1969;11:127–134. doi: 10.1111/j.1432-1033.1969.tb00749.x. [DOI] [PubMed] [Google Scholar]
- de Munter JSL, Hu FB, Spiegelman D, Franz M, van Dam RM. Whole Grain, Bran, and Germ Intake and Risk of Type 2 Diabetes: A Prospective Cohort Study and Systematic Review. PLoS Med. 2007;4:1385–1394. doi: 10.1371/journal.pmed.0040261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferchaud-Roucher V, Pouteau E, Piloquet H, Zaïr Y, Krempf M. Colonic fermentation from lactulose inhibits lipolysis in overweight subjects. Am J Physiol Endocrinol Metab. 2005;289:E716–E720. doi: 10.1152/ajpendo.00430.2004. [DOI] [PubMed] [Google Scholar]
- Freeland KR, Wilson C, Wolever TMS. Adaption of colonic fermentation and glucagon-like peptide-1 (GLP-1) secretion with increased wheat fibre intake for 1-year in hyperinsulinaemic humans. Br J Nutr. 2009;103:82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- Gibson GR, Beatty ER, Wang X, Cummings JH. Selective stimulation of bifidobacteria in the human colon by oligofructose and inulin. Gastroenterology. 1995;108:975–982. doi: 10.1016/0016-5085(95)90192-2. [DOI] [PubMed] [Google Scholar]
- Greenberg AS, McDaniel ML. Identifying the links between obesity, insulin resistance and β-cell function: potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur J Clin Invest. 2002;32 (suppl 3):24–34. doi: 10.1046/j.1365-2362.32.s3.4.x. [DOI] [PubMed] [Google Scholar]
- Jenkins DJA, Wolever TMS, Jenkins A, Brighenti F, Vuksan V, Rao AV, et al. Specific types of colonic fermentation may raise low-density-lipoprotein-cholesterol concentrations. Am J Clin Nutr. 1991;54:141–147. doi: 10.1093/ajcn/54.1.141. [DOI] [PubMed] [Google Scholar]
- Knowles SE, Jarrett IG, Filsell OH, Ballard FJ. Production and utilization of acetate in mammals. Biochem J. 1974;142:401–411. doi: 10.1042/bj1420401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liese AD, Schulz M, Fang F, Wolever TMS, D’Agostino RB, Jr, Sparks KC. Dietary glycemic index and glycemic load, carbohydrate and fiber intake, and measures of insulin sensitivity, secretion, and adiposity in the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2005;28:2832–2838. doi: 10.2337/diacare.28.12.2832. [DOI] [PubMed] [Google Scholar]
- Liu S, Stampfer MJ, Hu FB, Giovannucci E, Rimm E, Manson JE, et al. Whole-grain consumption and risk of coronary heart disease: results from the Nurses’ Health Study. Am J Clin Nutr. 1999;70:412–419. doi: 10.1093/ajcn/70.3.412. [DOI] [PubMed] [Google Scholar]
- Luong A, Hannah V, Brown M, Goldstein J. Molecular characterization of human acetyl-CoA synthetase, an enzyme regulated by sterol regulatory element-binding proteins. J Biol Chem. 2000;275:458–466. doi: 10.1074/jbc.M004160200. [DOI] [PubMed] [Google Scholar]
- McGarry JD. Dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes. 2002;51:7–18. doi: 10.2337/diabetes.51.1.7. [DOI] [PubMed] [Google Scholar]
- Piloquet H, Ferchaud-Roucher V, Duengler F, Zair Y, Maugere P, Krempf M. Insulin effects on acetate metabolism. Am J Physiol Endocrinol Metab. 2003;285:E561–E565. doi: 10.1152/ajpendo.00042.2003. [DOI] [PubMed] [Google Scholar]
- Popkin BM, Gordon-Larsen P. The nutrition transition: worldwide obesity dynamics and their determinants. Int J Obes Relat Metab Disord. 2004;28 (suppl):S2–9. doi: 10.1038/sj.ijo.0802804. [DOI] [PubMed] [Google Scholar]
- Prentki M, Nolan CJ. Islet β cell failure in type 2 diabetes. J Clin Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson MD, Bickerton AS, Dennis AL, Vidal H, Frayn KN. Insulin-sensitizing effects of dietary resistant starch and effects on sleletal muscle and adipose tissue metabolism. Am J Clin Nutr. 2005;82:559–567. doi: 10.1093/ajcn.82.3.559. [DOI] [PubMed] [Google Scholar]
- Robertson MD, Currie JM, Morgan LM, Jewell DP, Frayn KN. Prior short-term consumption of resistant starch enhances postprandial insulin sensitivity in healthy subjects. Diabetologia. 2003;46:659–665. doi: 10.1007/s00125-003-1081-0. [DOI] [PubMed] [Google Scholar]
- Rossi M, Corradini C, Amaretti A, Nicolini M, Pompei A, Zanoni S, et al. Fermentation of fructooligosaccharides and inulin by bifidobacteria: a comparative study of pure and fecal cultures. Appl Environ Microbiol. 2005;71:6150–6158. doi: 10.1128/AEM.71.10.6150-6158.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmeron J, Manson JE, Stampfer MJ, Colditz GA, Wing AL, Willett WC. Dietary fiber, glycemic load, and risk of non-insulin-dependent diabetes mellitus in women. JAMA. 1997;277:472–477. doi: 10.1001/jama.1997.03540300040031. [DOI] [PubMed] [Google Scholar]
- Schulze MB, Schulz M, Heidemann C, Schienkiewitz A, Hoffmann K, Boeing H. Fiber and Magnesium Intake and Incidence of Type 2 Diabetes. A Prospective Study and Meta-analysis. Arch Intern Med. 2007;167:956–965. doi: 10.1001/archinte.167.9.956. [DOI] [PubMed] [Google Scholar]
- Sone H, Shimano H, Sakakura Y, Inoue N, Amemiya-Kudo M, Yahagi N, et al. Acetyl-coenzyme A synthetase is a lipogenic enzyme controlled by SREBP-1 and energy status. Am J Physiol Endocrinol Metab. 2002;282:E222–E230. doi: 10.1152/ajpendo.00189.2001. [DOI] [PubMed] [Google Scholar]
- Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- Todesco T, Zamboni M, Armellini F, Bissoli L, Turcato E, Piemonte G, et al. Plasma acetate levels in a group of obese diabetic, obese normoglycemic, and control subjects and their relationships with other blood parameters. Am J Gastroenterol. 1993;88:751–755. [PubMed] [Google Scholar]
- Tollinger CD, Vreman HJ, Wiener MW. Measurement of acetate in human blood by gas chromatography: effects of sample preparation, feeding and various diseases. Clin Chem. 1979;25:1787–1790. [PubMed] [Google Scholar]
- Weickert MO, Mohlig M, Koebnick C, Holst JJ, Namsolleck P, Ristow M, et al. Impact of cereal fibre on glucose-regulating factors. Diabetologia. 2005;48:2343–2353. doi: 10.1007/s00125-005-1941-x. [DOI] [PubMed] [Google Scholar]
- Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27:1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Josse RG, Leiter LA, Chiasson JL. Time of day and glucose tolerance status affect serum short-chain fatty acid concentrations in humans. Metabolism. 1997;46:805–811. doi: 10.1016/s0026-0495(97)90127-x. [DOI] [PubMed] [Google Scholar]
- Wolever TM, Radmard R, Chiasson J-L, Hunt JA, Josse RG, Palmason C, et al. One-year acarbose therapy raises fasting serum acetate in diabetic patients. Diabet Med. 1995;12:164–172. doi: 10.1111/j.1464-5491.1995.tb00448.x. [DOI] [PubMed] [Google Scholar]
- Wolever TMS, Brighenti F, Royall D, Jenkins AL, Jenkins DJA. Effect of rectal infusion of short chain fatty acids in human subjects. Am J Gastroenterol. 1989;84:1027–1033. [PubMed] [Google Scholar]
- World Health Organization. Obesity: preventing and managing the global epidemic: Report of a WHO Consultation. World Health Organization; Geneva: 2000. (WHO technical report series 894) [PubMed] [Google Scholar]
- Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000;23:171–175. doi: 10.2337/diacare.23.2.171. [DOI] [PubMed] [Google Scholar]