Abstract
Background/Objectives
Colonic fermentation of dietary fiber may improve insulin sensitivity via the metabolic effects of short chain fatty acids (SCFA) in reducing free fatty acids (FFA). The main objectives of this study were to compare peripheral uptake of acetate (AC) in participants with normal (< 40pmol/L, NI) and high (≥ 40pmol/L, HI) plasma-insulin and the ability of AC to reduce FFA in both groups.
Subject/Methods
Overnight fasted NI (n = 9) and HI (n = 9) participants were given an intravenous (IV) infusion of 140 mmol/L sodium acetate at 3 different rates over 90 minutes. The total amount of AC infused was 51.85 mmols.
Results
Acetate clearance in NI participants was not significantly different than that in HI participants (2.11 ± 0.23 vs 2.09 ± 0.24 ml/min). FFA fell in both groups, but rebounded to a greater extent in NI than HI participants (time × group interaction, P = 0.001). Significant correlations between insulin resistance (IR) indices (HOMA-IR, Matsuda and Insulinogenic Index) vs FFA rebound during IV AC infusion were also observed.
Conclusions
These findings suggest that AC uptake is similar in both groups. Participants with lower plasma insulin and lower IR indices had a greater FFA rebound. These results support the hypothesis that increasing AC concentrations in the systemic circulation may reduce lipolysis and plasma FFA concentrations and thus improve insulin sensitivity. More in-depth studies are needed to look at the effects of SCFA on FFA metabolism in insulin resistant participants.
Keywords: Humans, acetate, FFA, insulin sensitivity
Introduction
Much epidemiologic evidence has accumulated to suggest that whole-grain cereals can protect against obesity and type 2 diabetes mellitus (T2DM) (1, 2, 3, 4). One potential mechanism by which cereal fiber is protective is through its colonic effects via short chain fatty acid (SCFA) production and the resulting decrease in plasma free fatty acid (FFA) concentrations. The SCFA, acetate, propionate and butyrate are the major byproducts of the microbial fermentation of unabsorbed dietary carbohydrate in the human colon (5). Approximately 100–450 mmol of SCFA are produced daily in the colon from exogenous sources (6). SCFA are also endogenously produced, as a result of increased fat oxidation (7, 8) and branched chain amino acid and methionine metabolism (9, 10).
The SCFA produced in the colon are readily absorbed and transported to the liver, where they combine with the endogenously produced SCFA from substrate metabolism and reach the systemic circulation (11). It has been hypothesised that SCFA may play a role in enhancing peripheral insulin sensitivity (12). Production and absorption rates of colonic SCFA differ between individuals, and the metabolism of SCFA may be abnormal in certain metabolic states. Previous studies have shown that glucose tolerance status affects serum SCFA concentrations in humans who are insulin resistant but nondiabetic (13). This suggests that insulin resistance may alter SCFA metabolism. In a previous study lower fasting serum acetate (AC) concentrations were observed in participants with high fasting insulin (HI) compared to those with normal insulin concentrations (NI) (14). It was hypothesized that this may be due to reduced absorption, increased uptake or decreased endogenous production of AC in HI participants. Therefore, this study is aimed at comparing AC uptake in NI and HI participants.
Insulin resistance and T2DM are also associated with elevated FFA concentrations (15). Oral ingestion (16) and rectal infusion of AC (17) leads to reduced serum free-fatty acid (FFA) concentrations and feeding fermentable carbohydrate also reduces serum FFA concentrations (18, 19, 20, 21). Similar results were observed after inulin ingestion in NI and HI participants in a recent study (14). But, studies so far have not looked at FFA concentrations after intravenous (IV) infusion of AC in NI and HI participants.
The purpose of this study therefore is to compare peripheral uptake of AC in participants with normal and high fasting insulin concentrations and the ability of IV infusion of AC to reduce FFA in the two groups.
Methods
Male or non-pregnant, non-lactating females aged 18–65 with BMI ≥ 20 and ≤ 5kg/m2 were recruited from people previously involved in similar studies and their friends. Participants were excluded for any of the following reasons: history of diabetes, cardiovascular disease or bowel, kidney or liver disease; use of medications which affect blood glucose or insulin sensitivity (such as diuretics); use of antibiotics within 3 months of starting the study; or following any unusual dietary practices. Eligible participants were then screened with a fasting blood sample; participants were excluded for any of the following reasons: serum glucose ≥ 7.0 mmol/L, triglycerides ≥ 4.0 mmol/L, hemoglobin < the lower limit of normal or aspartate transaminase > 1.5 times the upper limit of normal. Eligible participants were divided prospectively to obtain a group (n=9) with normal fasting serum insulin (FSI<40pmol/L) and a group (n=9) with high fasting serum insulin (FSI≥40pmol/L). Participants were selected based on FSI because of the positive association between FSI and insulin resistance (22), and because 40pmol/L represents approximately the 66%ile for healthy participants in our clinical experience. Ethical approval for the study was obtained from the Research Ethics Boards of St. Michael’s Hospital, Toronto and the University of Toronto. Participants gave written informed consent to participate in the study.
All tests were conducted at the Clinical Nutrition and Risk Factor Modification Centre (CNRFMC) of St. Michael’s Hospital, Toronto. Participants were studied in the morning after 10 – 14 h overnight fasts. Participants were asked to refrain from alcohol consumption and any abnormally strenuous exercise in the 24 h period prior to the test day. Participants were also asked to consume a low fiber diet for the 24 h period prior to the test day in order to minimise concentrations of systemic acetate originating from colonic fermentation. The following foods were provided to the participants: Eggs, yogurt, cheddar cheese, canned tuna, chicken, white bread, crackers and apple juice. Participants were also allowed to supplement the diet provided with tea, coffee, juices, carbonated drinks, spreads, margarine and oils in cooking. Participants were instructed not to consume whole grain breads and cereals, vegetables and fruits.
On the day of the test, participants collected 2 fasting breath samples. An indwelling IV catheter was inserted into a superficial forearm vein and kept patent with normal saline and fasting blood samples were collected. A peripheral IV line was inserted into a vein on the dorsum of the subject’s other hand and participants were then given an IV infusion of acetic acid over a 90 minute period. The IV solution contained 140 mmol/L sodium acetate and was freshly prepared by the St Michael’s Hospital Research Pharmacy, Toronto, on the morning of the test. The infusion was given using a Baxter Flo-Gard 6201 infusion pump (Baxter Healthcare Corp, Deerfield, IL). The pump was programmed to deliver the infusion in 3 different steps. The initial infusion rate was 1.73 mL/min from 0–30 min; the rate was increased to 3.53 mL/min from 30–60 min; and further increased to 7.08 mL/min from 60–90 min. The infusion rates and volumes administered are shown in Table 1. On a typical western diet the average intake of dietary fibre is ~20g/day this corresponds to a colonic SCFA production of ~300mmol/24h of which ~60% (180mmol) is acetate. The total amount of AC infused was 51.85 mmols. This is equivalent to roughly one-third the amount of AC produced daily in the human colon on a typical western diet. Additional blood samples were collected at 20, 25, and 30 minutes after the start of each infusion rate, for a total of 12 blood samples (including the 3 fasting samples). These samples were analyzed for SCFA and FFA.
Table 1.
Infusion Rates and Volumes for Intravenous Acetate Infusion.
| Time | Infusion Rate | Amount of acetate infused per 30 minutes (mmol) | ||
|---|---|---|---|---|
|
| ||||
| mL/min | mL/30 min | mmols/min | mmols | |
| 0 – 30 min | 1.73 | 51.9 | 0.24 | 7.27 |
| 30 – 60 min | 3.53 | 105.9 | 0.49 | 14.83 |
| 60 – 90 min | 7.08 | 212.5 | 0.99 | 29.75 |
| Total over 90 min | N/A | 370.3 | N/A | 51.85 |
All participants also had an oral glucose tolerance test (OGTT) performed after an overnight fast. The participants consumed a test drink containing 75 g glucose (Grain Process Enterprises Ltd, Scarborough, ON) dissolved in 300 mL of water. They consumed the test drink over 5 min, and were given an additional 200 mL of water to drink over 15 min. Blood samples were collected at 0 min and at 30, 60, 90, 120, 180, and 240 min following consumption of the test drink for the measurement of glucose, insulin, free fatty acids. The OGTT done by the participants has been reported in a different form in a previously published study (14).
Whole blood for FFA, SCFA, glucose and insulin measurements were collected in red top Vacutainer™ tubes containing no substrates (Becton Dickinson, Franklin Lakes, NJ). Blood samples were allowed to clot at room temperature, centrifuged at 600×g for 15 min at 4°C, and the serum aliquoted and stored at −70 °C before analysis. FFA were measured by an enzymatic technique that used acylCoA oxidase (Wako Diagnostics, Wako Chemicals, USA, Inc) (inter-assay CV of <1.5%). SCFA were measured by gas chromatography after micro filtration and vacuum distillation as previously described (14). Serum glucose was measured by a glucose oxidase method (SYNCHRON LX Systems, Beckman Coulter, Brea, CA) (inter-assay CV 1.9%), insulin using the Beckman Access Ultrasensitive Insulin method (Beckman Instruments, Fullerton, CA) (inter-assay CV 2.5 to 4.3%) and c-peptide by a highly specific double-antibody RIA (Siemens Medical Solutions Diagnostics, Los Angeles, CA) (inter-assay CV ≤ 10%).
Participants collected breath samples using the Easy Sampler ™ with tube holder (Quintron Instrument Company, Milwaukee, WI). Methane and hydrogen were measured by gas chromatography (Quintron Microlyzer, Model SC, Milwaukee, WI) in breath samples and simultaneously obtained room air. Breath hydrogen and methane concentrations reported are corrected by subtracting the hydrogen and methane concentrations of room air from that of each breath sample collected. Breath methane and hydrogen concentrations were measured because they are indicators of colonic fermentation.
The clearance rate (ml/min) for AC was calculated from the ratio of AC infusion rate (mmol/min) to steady-state serum AC concentrations (μmol/L). Clearance rate was calculated at each infusion rate and the mean is reported. Three indirect indexes for the assessment of IR were calculated from the oral glucose tolerance test (OGTT). Homeostasis model assessment of insulin resistance (HOMA-IR) (23) was calculated from fasting values of glucose and insulin. The insulin sensitivity index of Matsuda (ISI Matsuda) is an OGTT index calculated using formulas adapted from euglycemic-hyperinsulinemic clamp studies (24). The insulinogenic index was calculated as the ratio of the increment in insulin concentration to the increment in glucose concentration.
Statistical analysis was performed with SPSS version 17 for WINDOWS (SPSS Inc., Chicago, IL) using the General Linear Model (GLM) repeated-measures analysis of variance (ANOVA) examining for the main effects of group and time, and interactions between these two effects. Since there were only two groups, post hoc tests were not performed in SPSS. Post hoc analysis using Bonferroni post-tests was done using GraphPad Prism 5 for Windows, Version 5.02 (GraphPad Software Inc., La Jolla, CA) when main effects were identified by ANOVA. The association between two variables was tested by linear regression using GraphPad Prism 5 for Windows, Version 5.02 (GraphPad Software Inc., La Jolla, CA). Differences with P-values ≤ 0.05 (2-tailed) were considered to be statistically significant. The results are expressed as means ± SEM.
Results
We studied 11 women and 7 men with a mean (± SEM) age of 36.4 ± 9.0 y and BMI 26.9 ± 3.7 kg/m2. Participants with HI had significantly higher waist circumference, serum insulin, triglycerides and total cholesterol/HDL ratio and a significantly lower HDL concentration than NI participants (Table 2). Mean fasting breath hydrogen (4.4 ± 0.8) and methane (6.9 ± 1.6) concentrations in all participants indicate low levels of colonic fermentation. Both groups of participants tolerated the IV acetic acid infusion well.
Table 2.
Characteristics of the normal and hyperinsulinemic participants at the screening visit.
| Normal | Hyperinsulinemics | P-value | |
|---|---|---|---|
| Age (y) | 35.9 ± 3.8 | 36.9 ± 2.2 | 0.8 |
| M:F | 4 : 5 | 3 : 6 | |
| Weight (kg) | 71.9 ± 4.9 | 74.3 ± 3.3 | 0.69 |
| BMI (kg/m2) | 25.4 ± 1.5 | 28.4 ± 0.7 | 0.1 |
| Waist Circumference (cm) | 87.7 ± 2.7 | 95.1 ± 1.4 | 0.03 |
| Systolic blood pressure (mm Hg) | 109 ± 3 | 118 ± 3 | 0.06 |
| Diastolic blood pressure (mm Hg) | 68 ± 3 | 77 ± 4 | 0.08 |
| Glucose (mmol/L) | 5.0 ± 0.1 | 5.1 ± 0.2 | 0.65 |
| Insulin (pmol/L) | 29.4 ± 2.0 | 76.7 ± 12.0 | 0.004 |
| HCT (L/L) | 0.39 ± 0.01 | 0.39 ± 0.01 | 0.91 |
| AST (U/L) | 18.9 ± 1.3 | 21.9 ± 1.6 | 0.16 |
| CRP (mg/L) | 2.5 ± 1.8 | 2.7 ± 0.7 | 0.91 |
| Cholesterol (mmol/L) | 4.7 ± 0.2 | 5.3 ± 0.4 | 0.23 |
| Triglycerides (mmol/L) | 0.91 ± 0.12 | 1.78 ± 0.3 | 0.02 |
| HDL (mmol/L) | 1.42 ± 0.06 | 1.08 ± 0.08 | 0.004 |
| TC/HDL | 3.33 ± 0.18 | 4.93 ± 0.31 | 0.001 |
| LDL (mmol/L) | 2.84 ± 0.21 | 3.39 ± 0.30 | 0.16 |
Data are presented as mean ± SEM. Abbreviations: HCT, hematocrit; AST, aspartate transaminase; CRP, C-reactive protein; TC, total cholesterol; HDL, high density lipoprotein; LDL, low density lipoprotein.
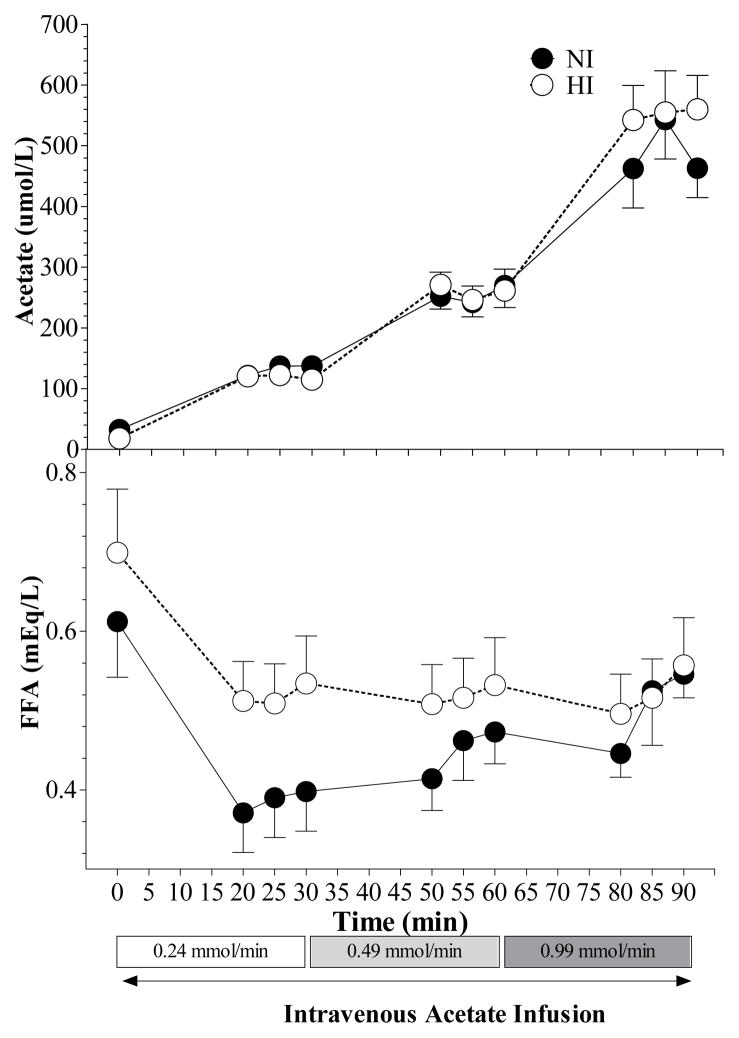
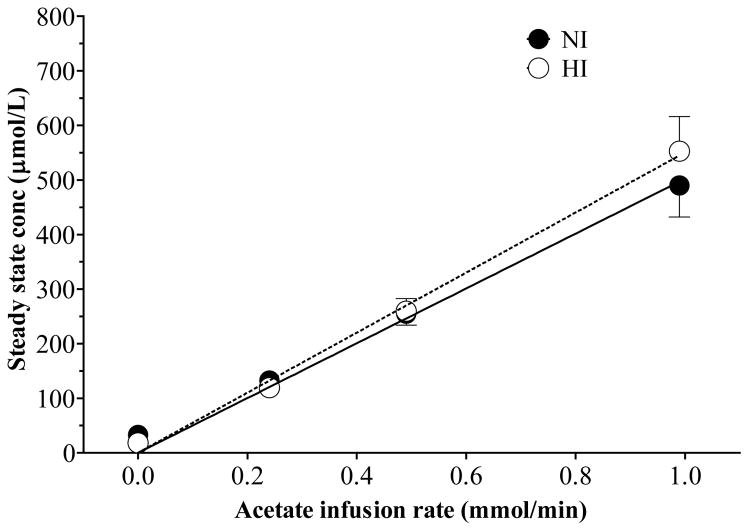
A steady-state concentration was achieved for AC after 20 min at each infusion rate in participants with normal and high insulin (Figure 1). Acetate clearance in participants with normal insulin was not significantly different than that in participants with high insulin (2.11 ± 0.23 vs 2.09 ± 0.24 ml/min). The steady-state plasma concentration of AC was directly proportional to the infusion rate in both groups of participants (NI, r2 = 0.999, P = 0.0003; HI, r2 = 0.996, P = 0.002) (Figure 2), so AC clearance did not vary by infusion rate in both groups.
Figure 1.
Mean (± SEM) serum acetate and FFA concentration during the intravenous infusion of 140mmol/L of sodium acetate for 90 min in participants with normal insulin (NI) and high insulin (HI) concentrations. No significant differences in plasma acetate concentrations were observed between the two groups. A significant time × group interaction was observed (p = 0.001) for plasma FFA.
Figure 2.
Linear regression analysis between acetate infusion rate vs steady state acetate concentration for NI (r2 = 0.999, P = 0.0003) and HI (r2 = 0.996, P = 0.002) participants. The regression lines are depicted in the graph.
FFA responses varied significantly with time in NI and HI participants (p< 0.001, Figure 1). Also, the FFA responses in NI differed from those in HI and a significant time×group interaction (p = 0.001) was observed (Figure 1). However, despite the significant time×group interaction none of the differences in FFA between HI and NI was significant at any individual time point (Figure 1). Insufficient plasma sample was available for analysis in 2 participants in the NI group, therefore, FFA responses are based on n = 7 in this group.
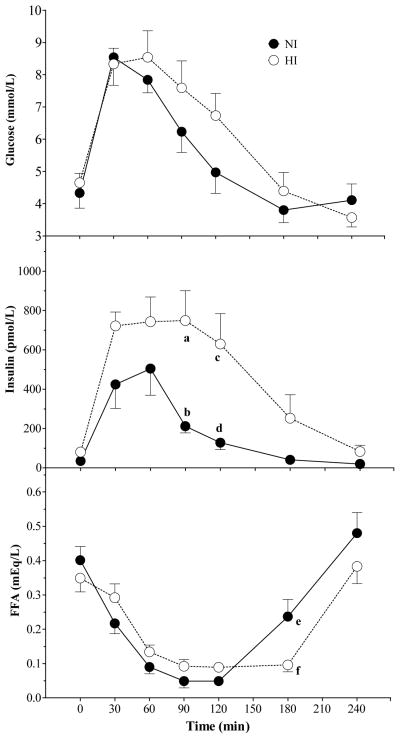
Glucose, insulin and FFA responses after OGTT varied significantly with time (P < 0.001). Responses in NI differed from those in HI with a significant main effect of group for insulin (P = 0.03) and a significant time×group interaction for insulin (P = 0.01) and FFA (P = 0.02) (Figure 3). Serum insulin was significantly higher in HI than NI at 90 and 120 min (P < 0.001 and P < 0.01, respectively) (Figure 3) and FFA was significantly lower in HI than NI at 180 min (P < 0.05) (Figure 3).
Figure 3.
Mean (± SEM) plasma glucose, insulin and FFA concentrations after oral glucose tolerance test (OGTT) in NI and HI subjects. A significant main effect of time was observed for glucose, insulin and FFA (P < 0.001). A significant main effect of group was observed for insulin (P = 0.03). A significant group × time interaction was observed insulin (P = 0.01) and FFA (P = 0.02). Within a time point, labelled means without a common letter differ (a vs b, P <0.001; c vs d, P < 0.01; e vs f, P < 0.05; Bonferroni post-test after demonstration of significant main effect of group by ANOVA).
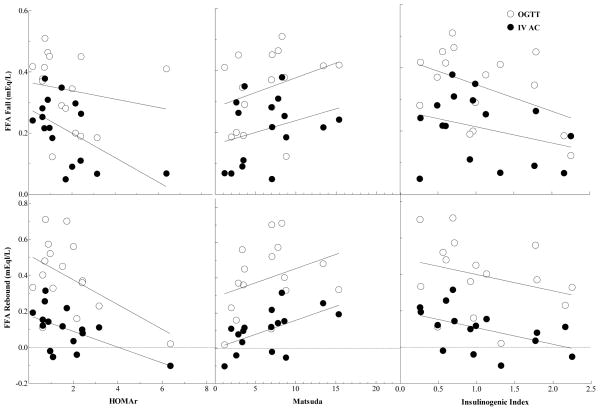
Acetate clearance, FFA fall and rebound after OGTT and IV AC infusion were correlated with Insulin resistance (IR) indexes, HOMA-IR, Matsuda and Insulinogenic indexes. Acetate clearance was not related to measures of IR. HOMA-IR was not correlated with FFA fall after OGTT but was negatively correlated with FFA fall after IV AC infusion (r = −0.565, P = 0.02) (Figure 4). HOMA-IR was also negatively correlated with FFA rebound after OGTT (r = −0.515, P = 0.04) and IV AC infusion (r = −0.564, P = 0.02) (Figure 4). Matsuda index was not correlated with FFA fall after OGTT and IV AC infusion. Matsuda index was also not correlated with FFA rebound after OGTT but was positively correlated with FFA rebound after IV AC infusion (r = 0.557, P = 0.03) (Figure 4). Insulinogenic index was not correlated with FFA fall after OGTT and IV AC infusion. Insulinogenic index was also not correlated with FFA rebound after OGTT but was negatively correlated with FFA rebound after IV AC infusion (r = −0.500, P = 0.05) (Figure 4).
Figure 4.
Relationships between HOMA-IR, Matsuda and Insulinogenic Index vs FFA fall and rebound after oral glucose tolerance test (OGTT) and intravenous acetate infusion (IV AC) in NI and HI participants. Correlation coefficients for HOMA-IR vs FFA fall: Glucose, NS; Acetate, r = −0.565 (P = 0.02). HOMA-IR vs FFA rebound: Glucose, r = −0.515 (P = 0.04); Acetate, r = −0.564 (P = 0.02). Matsuda Index vs FFA fall: Glucose, NS; Acetate, NS. Matsuda Index vs FFA rebound: Glucose, NS; Acetate, r = 0.557 (P = 0.03). Insulinogenic Index vs FFA fall: Glucose, NS; Acetate, NS. Insulinogenic Index vs FFA rebound: Glucose, NS; Acetate, r = −0.500 (P = 0.05).
Discussion
Acetate metabolism and kinetics in healthy and T2DM participants has been studied previously by various investigators (25, 26, 27). But, studies so far have not compared AC uptake in NI and HI human participants. The main objective of this study was to compare peripheral AC uptake in participants with normal and high fasting insulin concentrations and the ability of AC to reduce FFA in the two groups. The results of this study showed that AC uptake in NI participants was not significantly different than that in HI participants. Our results may also point to FFA metabolism being impaired in insulin-resistant participants after IV AC infusion.
Previous studies have shown that participants with T2DM tend to have an altered AC metabolism (28, 29) and elevated plasma AC concentrations (30). Intravenous AC infusions to measure AC utilization showed that AC was metabolised at a slower rate in participants with T2DM when compared to healthy participants (27). Acetate metabolism is impaired in participants with T2DM due to an increased availability of acetyl-CoA from glucose and non-esterified fatty acids (8). But, participants with impaired glucose tolerance had significantly lower serum AC concentrations over a 12 h period when compared to young normal participants (13). In a recent study, fasting (27.3 ± 4.7 vs 63.1 ± 11.9 μmol/L, p < 0.01) and overall mean serum AC over the 4 h study period (22.5±3.7 vs 44.3±6.9 μmol/L, p = 0.001) was lower in HI than NI participants (14). In the present study using the same group of participants, no significant differences were seen in fasting serum AC concentrations and AC uptake between NI and HI participants. The participants in this study were on a low fiber diet the day before in order to minimise AC production from exogenous sources. Endogenous AC production may thus be similar in the two groups. Therefore, the possibility exists that the lower serum AC concentrations in HI participants seen in the previous study (14) may be related to differences in AC absorption between the two groups. Future studies need to look at AC absorption in NI and HI participants.
Insulin reduces FFA concentration through its anti-lipolytic effects (inhibition of hormone-sensitive lipase) on adipose tissue (31). Excessive adipose tissue energy storage results in increased fatty acid flux to other tissues and increased triacylglycerol storage in peripheral tissues, which promotes insulin resistance. Lowering elevated FFA could reduce insulin resistance. In this study, intravenous infusion of AC suppressed plasma FFA in NI and HI participants. This increase in AC availability to the peripheral tissues has important metabolic consequences because AC inhibits lipolysis. Acute alcohol intake has anti-lipolytic properties which decrease plasma FFA concentrations since AC is a metabolite of alcohol degradation. Ethanol consumption leads to a decrease in FFA turnover (32, 33) and concentrations (16, 34, 35, 36). In vitro (37) and in vivo studies have shown that the SCFA, AC, when infused intravenously in humans suppresses adipose tissue lipolysis which results in decreased FFA concentrations (8, 27) and feeding fermentable carbohydrate also reduces serum FFA concentrations (18, 19, 20). High concentrations of colonic AC that suppress adipose tissue lipolysis may therefore be beneficial and may lead to a decrease in insulin resistance by lowering FFA concentrations. High FFA concentrations cause insulin resistance and β-cell dysfunction therefore, low concentrations of SCFA or the inability of SCFA to regulate FFA may contribute to the development of obesity, insulin resistance and diabetes.
During the OGTT, the rebound in FFA concentrations occurred sooner in NI participants, ie. after 120 min compared to 180 mins in HI participants. The significant correlations between IR indices (HOMA-IR, Matsuda and Insulinogenic Index) vs FFA rebound during IV AC infusion suggest that participants who were more insulin resistant had a lesser FFA rebound. These observations are consistent with metabolic inflexibility in the insulin resistant participants in this study. Metabolic flexibility is the ability to adjust fuel oxidation to fuel availability. It’s the capacity to switch from predominantly lipid oxidation and high rates of fatty acid uptake during fasting conditions to the suppression of lipid oxidation and increased glucose uptake, oxidation and storage under insulin-stimulated conditions (38). Metabolic inflexibility is defined as an impaired capacity to increase fat oxidation upon increased fatty acid availability and to switch between fat and glucose as the primary fuel source after a meal. Normally, healthy muscle switches rapidly from predominantly fat oxidation during fasting to predominantly carbohydrate oxidation in the postprandial state. The uptake of plasma FFA into skeletal muscle was reduced during fasting in participants with impaired glucose tolerance (39). In obese, insulin-resistant participants, fat oxidation in skeletal muscle was reduced during fasting and after insulin stimulation, fat oxidation was less suppressed (40). In the present study during IV AC infusion the slower FFA rebound in participants with higher IR indices may point to an altered FFA metabolism and may be due to the presence of metabolic inflexibility in insulin resistant participants. The inability of SCFA to regulate FFA metabolism in insulin resistant participants who show signs of metabolic inflexibility needs to be further studied.
The rates at which we infused acetate, 0.24, 0.49 and 0.99 mmol/L, are likely to be physiologically relevant. It has been estimated that the amount of fermentable carbohydrate entering the colon daily is between 30 to 80g which would yield 300–800mmol of SCFA, or 180 to 540mmol acetate. If fermentation occurred at a constant rate throughout the day, it would yield 0.125 to 0.56 mmol/min acetate. However, since the rate of delivery of carbohydrate to the colon varies by about 30-fold throughout the day, with peaks occurring after meals (41), the rate of fermentation likely varies throughout the day as reflected in variable serum SCFA concentrations which also peak after meals (13).
One limitation of the study is that we did not use a control IV infusion of saline. However, in previous studies participants were given oral water or rectal saline infusion on different occasions; neither of these control treatments elicited any biologically or statistically significant change in serum acetate or FFA over 90min (17). We could also be criticized for not adjusting the infusion rates of acetate for the body weight of the participants. However, this objection is partly overcome by the facts that we obtained plasma steady-state acetate concentrations after each of 3 infusion rates, and that the steady-state concentration was linearly related to the infusion rate. In addition, it is not clear whether it would be more appropriate to adjust for total body weight, or on a measure of body composition since energy requirements (and hence intake) are related to lean body mass while FFA release would presumably be related to fat mass.
In conclusion, this study shows that AC uptake is similar in NI and HI participants. Our results also confirm previous findings that AC suppresses FFA concentrations. The slower FFA rebound observed in more insulin resistant participants after IV AC infusion may point to FFA metabolism being impaired in this group. Thus, the effects of SCFA on FFA metabolism in more insulin resistant participants maybe be different compared to more insulin sensitive participants and this needs to be further studied. Finally, this study supports the hypothesis that increasing AC concentrations in the systemic circulation may reduce lipolysis and plasma FFA concentrations and improve insulin sensitivity.
Acknowledgments
Supported by grant no. OOP-64648 from the Canadian Institutes for Health Research (CIHR), Institute of Nutrition, Metabolism and Diabetes.
Footnotes
Conflict of Interest
The authors declare no conflict of interest.
References
- 1.van de Vijver LPL, van den Bosch LMC, van den Brandt PA, Goldbohm RA. Whole-grain consumption, dietary fibre intake and body mass index in the Netherlands cohort study. Eur J Clin Nutr. 2009;63:31–38. doi: 10.1038/sj.ejcn.1602895. [DOI] [PubMed] [Google Scholar]
- 2.Lutsey PL, Jacobs DR, Kori S, Mayer-Davis E, Shea S, Steffen LM, et al. Whole grain intake and its cross-sectional association with obesity, insulin resistance, inflammation, diabetes and subclinical CVD: The MESA Study. Br J Nutr. 2007;98:397–405. doi: 10.1017/S0007114507700715. [DOI] [PubMed] [Google Scholar]
- 3.Newby P, Maras J, Bakun P, Muller D, Ferruci L, Tucker KL. Intake of whole grains, refined grains, and cereal fiber measured with 7-d diet records and associations with risk factors for chronic disease. Am J Clin Nutr. 2007;86:1745–1753. doi: 10.1093/ajcn/86.5.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr. 2002;76:390–398. doi: 10.1093/ajcn/76.2.390. [DOI] [PubMed] [Google Scholar]
- 5.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Macfarlane GT, Gibson GR. Microbiological aspects of the production of short-chain fatty acids in the large bowel. In: Cummings JH, Rombeau JL, Sakata T, editors. Physiological and clinical aspects of short-chain fatty acids. Cambridge University Press; Cambridge, UK: 1995. pp. 87–105. [Google Scholar]
- 7.Scheppach W, Pomare WE, Elia M, Cummings JH. The contribution of the large intestine to blood acetate in man. Clin Sci. 1991;80:177–182. doi: 10.1042/cs0800177. [DOI] [PubMed] [Google Scholar]
- 8.Akanji AO, Humphreys S, Thursfield V, Hockaday TD. The relationship of plasma acetate with glucose and other blood intermediary metabolites in non-diabetic and diabetic participants. Clin Chim Acta. 1989;185:25–34. doi: 10.1016/0009-8981(89)90127-7. [DOI] [PubMed] [Google Scholar]
- 9.Brindle PA, Schooley DA, Tsai LW, Baker FC. Comparitive metabolism of branched-chain aminoacids to precursors of juvenile hormone biogenesis in corpora allata of lepidopterous versus nonlepidopterous insects. J Biol Chem. 1988;263:10653–10657. [PubMed] [Google Scholar]
- 10.Walter JH, Thompson GN, Leonard JV, Hetherington CS, Bartlett K. Measurement of propionate turnover in vivo using sodium (2H5) propionate and sodium (13C) propionate. Clin Chim Acta. 1989;182:141–150. doi: 10.1016/0009-8981(89)90073-9. [DOI] [PubMed] [Google Scholar]
- 11.Cummings JH, Pomare EW, Branch WJ, Naylor CPE, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Robertson MD. Metabolic cross talk between the colon and the periphery: implications for insulin sensitivity. Proc Nutr Soc. 2007;66:351–361. doi: 10.1017/S0029665107005617. [DOI] [PubMed] [Google Scholar]
- 13.Wolever TMS, Josse RG, Leiter LA, Chiasson JL. Time of day and glucose tolerance status affect serum short-chain fatty acid concentrations in humans. Metabolism. 1997;46:805–811. doi: 10.1016/s0026-0495(97)90127-x. [DOI] [PubMed] [Google Scholar]
- 14.Fernandes J, Vogt J, Wolever TMS. Inulin increases short-term markers for colonic fermentation similarly in healthy and hyperinsulinaemic humans. Eu J Clin Nutr. 2011;65:1279–1286. doi: 10.1038/ejcn.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crouse JR, Gerson CD, DeCarli LM, Lieber CS. Role of acetate in the reduction of plasma free fatty acids produced by ethanol in man. J Lipid Res. 1968;9:509–512. [PubMed] [Google Scholar]
- 17.Wolever TMS, Spadafora P, Eshuis H. Interaction between colonic acetate and propionate in humans. Am J Clin Nutr. 1991;53:681–687. doi: 10.1093/ajcn/53.3.681. [DOI] [PubMed] [Google Scholar]
- 18.Jenkins DJA, Wolever TMS, Jenkins A, Brighenti F, Vuksan V, Rao AV, et al. Specific types of colonic fermentation may raise low-density-lipoprotein-cholesterol concentrations. Am J Clin Nutr. 1991;54:141–147. doi: 10.1093/ajcn/54.1.141. [DOI] [PubMed] [Google Scholar]
- 19.Ferchaud-Roucher V, Pouteau E, Piloquet H, Zaïr Y, Krempf M. Colonic fermentation from lactulose inhibits lipolysis in overweight participants. Am J Physiol Endocrinol Metab. 2005;289:E716–E720. doi: 10.1152/ajpendo.00430.2004. [DOI] [PubMed] [Google Scholar]
- 20.Brighenti F, Benini L, Del Rio D, Casiraghi C, Pellegrini N, Scazzina F, et al. Colonic fermentation of indigestible carbohydrates contributes to the second-meal effect. Am J Clin Nutr. 2006;83:817–822. doi: 10.1093/ajcn/83.4.817. [DOI] [PubMed] [Google Scholar]
- 21.Tarini J, Wolever TMS. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy participants. Appl Physiol Nutr Metab. 2010;35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 22.Yeni-Komshian H, Carantoni M, Abbasi F, Reaven GM. Relationship between several surrogate estimates of insulin resistance and quantification of insulin-mediated glucose disposal in 490 healthy nondiabetic volunteers. Diabetes Care. 2000;23:171–175. doi: 10.2337/diacare.23.2.171. [DOI] [PubMed] [Google Scholar]
- 23.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and b cell function from fasting plasma glucose and insulin concentration in man. Diabetologia. 1985;28:412–419. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 24.Matsuda M, DeFronzo RA. Insulin sensitivity indices obtained from oral glucose tolerance testing: comparison with the euglycemic insulin clamp. Diabetes Care. 1999;22:1462–1470. doi: 10.2337/diacare.22.9.1462. [DOI] [PubMed] [Google Scholar]
- 25.Mittendorfer B, Sidossis S, Walser E, Chinkes DL, Wolfe RR. Regional acetate kinetics and oxidation in human volunteers. Am J Physiol. 1998;274(Endocril Metab 37):E978–E983. doi: 10.1152/ajpendo.1998.274.6.E978. [DOI] [PubMed] [Google Scholar]
- 26.Pouteau E, Piloquet H, Maugeais P, Champ M, Dumon H, Nguyen P, et al. Kinetic aspects of acetate metabolism in healthy humans using (1-13C) acetate. Am J Physiol. 1996;271(Endocrinol. Metab. 34):E58–E64. doi: 10.1152/ajpendo.1996.271.1.E58. [DOI] [PubMed] [Google Scholar]
- 27.Akanji AO, Hockaday TDR. Acetate tolerance and the kinetics of acetate utilization in diabetic and nondiabetic participants. Am J Clin Nutr. 1990;51:112–118. doi: 10.1093/ajcn/51.1.112. [DOI] [PubMed] [Google Scholar]
- 28.Seufert CD, Graf M, Janson G, Kuhn A, Söling HD. Formation of free acetate by isolated perfused livers from normal, starved and diabetic rats. Biochem Biophys Res Commun. 1974;57:901–909. doi: 10.1016/0006-291x(74)90631-7. [DOI] [PubMed] [Google Scholar]
- 29.Buckley BM, Williamson DH. Origin of blood acetate in the rat. Biochem J. 1977;166:539–545. doi: 10.1042/bj1660539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith RF, Humphreys S, Hockaday TDR. The measurement of plasma acetate by a manual or automated technique in diabetic and non-diabetic participants. Ann Clin Biochem. 1986;23:285–291. doi: 10.1177/000456328602300307. [DOI] [PubMed] [Google Scholar]
- 31.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 32.Wolfe BM, Havel JR, Marliss EB, Kane JP, Seymour J, Ahuja SP. Effects of a 3-day fast and of ethanol on splanchnic metabolism of FFA, amino acids, and carbohydrates in healthy young men. J Clin Invest. 1976;57:329–40. doi: 10.1172/JCI108284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jones DP, Perman ES, Lieber CS. Free fatty acid turnover and triglyceride metabolism after ethanol ingestion in man. J Lab Clin Med. 1965;66:804–813. [PubMed] [Google Scholar]
- 34.Siler SC, Neese RA, Hellerstein MK. De novo lipogenesis, lipid kinetics, and whole-body lipid balances in humans after acute alcohol consumption. Am J Clin Nutr. 1999;70:928–936. doi: 10.1093/ajcn/70.5.928. [DOI] [PubMed] [Google Scholar]
- 35.Yki-Järvinen H, Koivisto VA, Ylikahri R, Taskinen MR. Acute effects of ethanol and acetate on glucose kinetics in normal participants. Am J Physiol. 1988;254:E175–80. doi: 10.1152/ajpendo.1988.254.2.E175. [DOI] [PubMed] [Google Scholar]
- 36.Abramson EA, Arky RA. Acute antilipolytic effects of ethyl alcohol and acetate in man. J Lab Clin Med. 1968;72:105–117. [PubMed] [Google Scholar]
- 37.Nilsson NO, Belfrage P. Effects of acetate, acetaldehyde, and ethanol on lipolysis in isolated rat adipocytes. J Lipid Res. 1978;19:737–741. [PubMed] [Google Scholar]
- 38.Kelley DE, Mandarino LJ. Fuel selection in human skeletal system in insulin resistance: a reexamination. Diabetes. 2000;49:677–683. doi: 10.2337/diabetes.49.5.677. [DOI] [PubMed] [Google Scholar]
- 39.Turpeinen AK, Takala TO, Nuutila P, Axelin T, Luotolahti M, Haaparanta M, et al. Impaired free fatty acid uptake in skeletal muscle but not in myocardium in patients with impaired glucose tolerance: studies with pet and 14(r,s)-(18f)fluoro-6-thia-heptadecanoic acid. Diabetes. 1999;48:1245–1250. doi: 10.2337/diabetes.48.6.1245. [DOI] [PubMed] [Google Scholar]
- 40.Kelley DE, Goodpaster B, Wing RR, Simoneau JA. Skeletal muscle fatty acid metabolism in association with insulin resistance, obesity, and weight loss. Am J Physiol. 1999;277:E1130–E1141. doi: 10.1152/ajpendo.1999.277.6.E1130. [DOI] [PubMed] [Google Scholar]
- 41.Flourié B, Leblond A, Flourent C, Rautureau M, Bisalli A, Rambaud J-C. Starch malabsorption and breath gas excretion in healthy subjects consuming low- and high-starch diets. Gastroenterology. 1988;95:356–63. doi: 10.1016/0016-5085(88)90491-x. [DOI] [PubMed] [Google Scholar]