Abstract
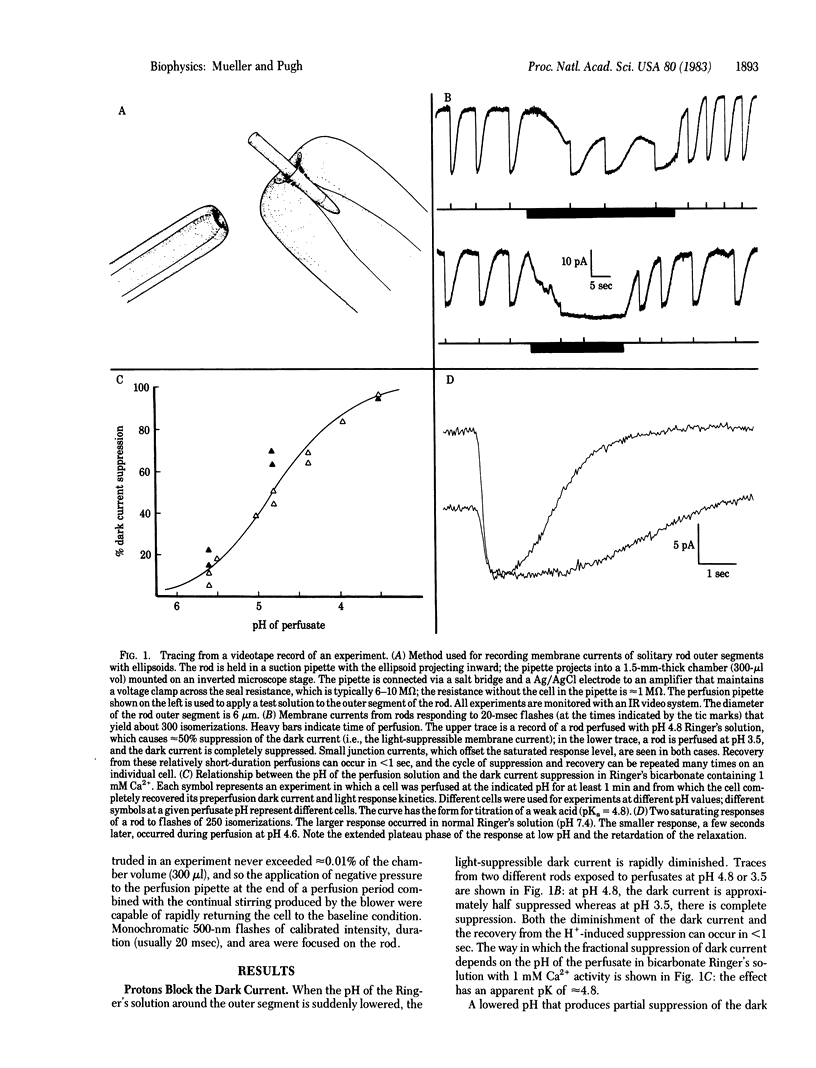
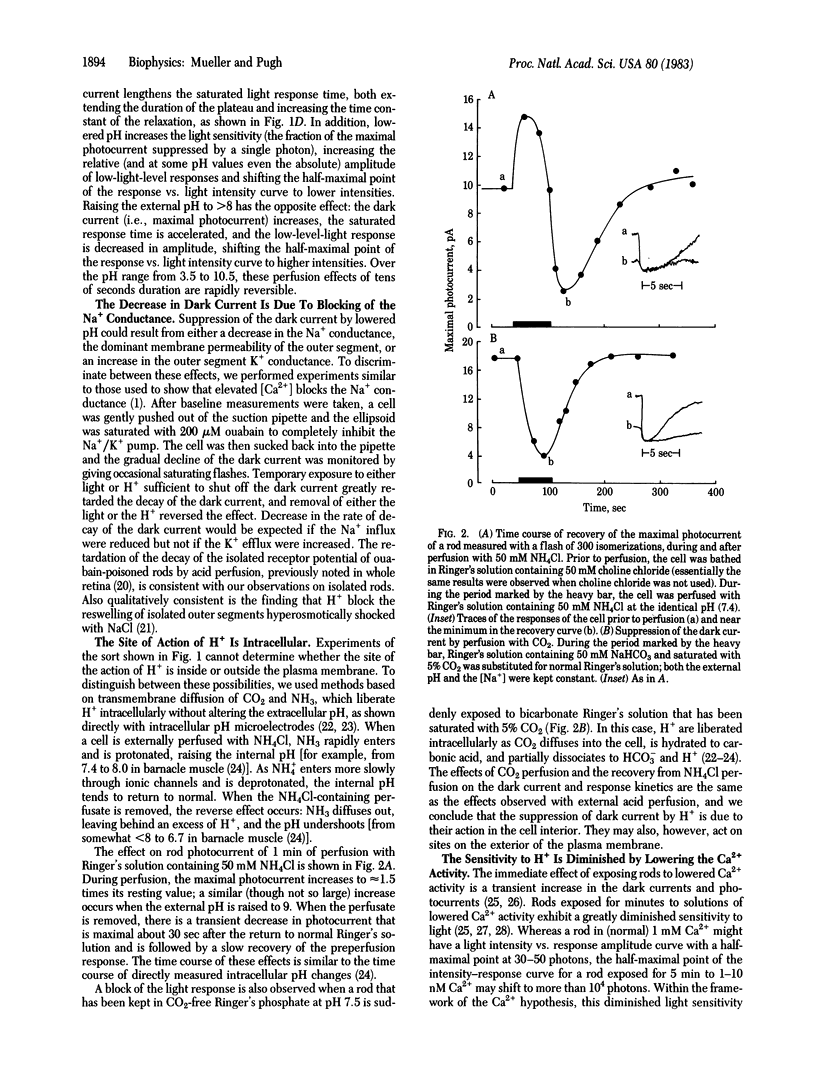
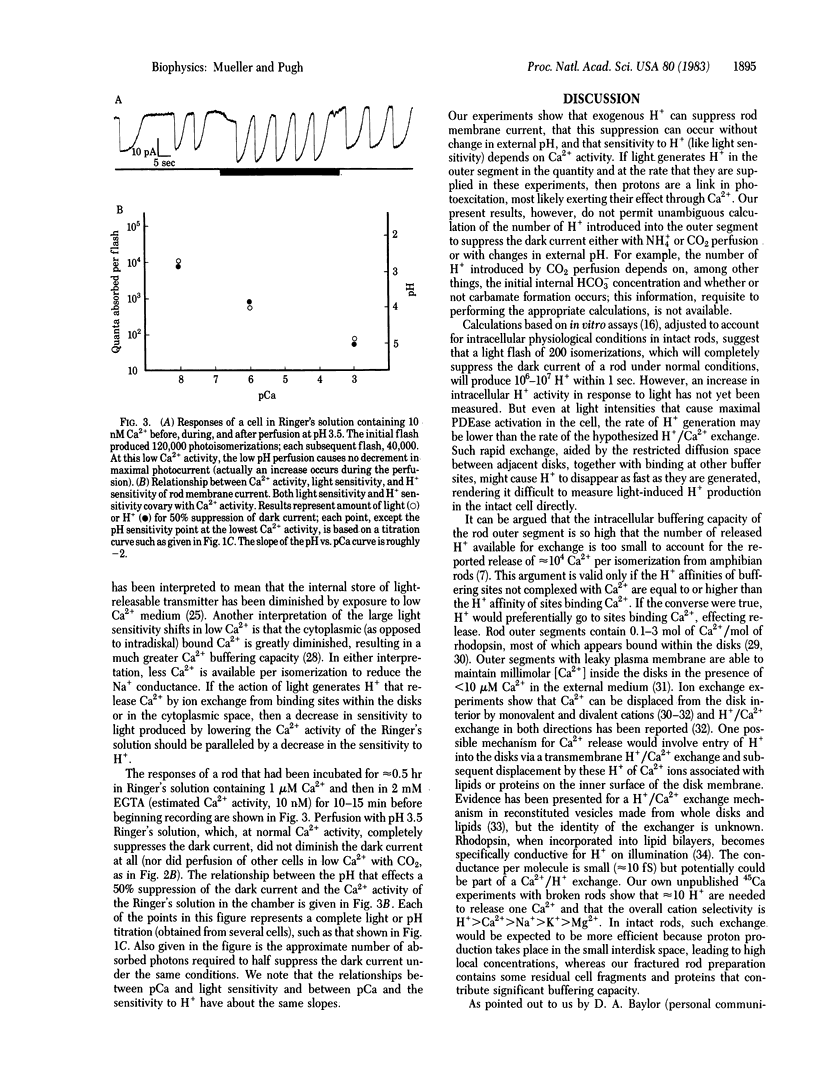
Membrane currents of isolated frog rods were recorded with the suction pipette technique and tested by perfusion techniques for their sensitivity to H+. The following facts have been established. (i) Increased [H+] suppresses the Na+ conductance of the outer segment rapidly and reversibly. (ii) H+ acts in the rod interior. (iii) The [H+] necessary to cause a 50% decrement in Na+ conductance is inversely related to the [Ca2+] over 5 orders of magnitude. (iv) The sensitivity to H+ and the sensitivity to light, as a function of [Ca2+], have the same slope. Thus, H+ act like light in effecting membrane current suppression but behave as if their effect is mediated through Ca2+. Based on these results and properties of rod disk membrane phosphodiesterase, we propose that protons produced in the light-activated hydrolysis of cGMP liberate Ca2+ from the disks by ion exchange.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baehr W., Devlin M. J., Applebury M. L. Isolation and characterization of cGMP phosphodiesterase from bovine rod outer segments. J Biol Chem. 1979 Nov 25;254(22):11669–11677. [PubMed] [Google Scholar]
- Bastian B. L., Fain G. L. The effects of low calcium and background light on the sensitivity of toad rods. J Physiol. 1982 Sep;330:307–329. doi: 10.1113/jphysiol.1982.sp014343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor D. A., Lamb T. D., Yau K. W. The membrane current of single rod outer segments. J Physiol. 1979 Mar;288:589–611. [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. E., Coles J. A., Pinto L. H. Effects of injections of calcium and EGTA into the outer segments of retinal rods of Bufo marinus. J Physiol. 1977 Aug;269(3):707–722. doi: 10.1113/jphysiol.1977.sp011924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen A. I. New evidence supporting the linkage to extracellular space of outer segment saccules of frog cones but not rods. J Cell Biol. 1968 May;37(2):424–444. doi: 10.1083/jcb.37.2.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleischman D., Denisevich M. Guanylate cyclase of isolated bovine retinal rod axonemes. Biochemistry. 1979 Nov 13;18(23):5060–5066. doi: 10.1021/bi00590a006. [DOI] [PubMed] [Google Scholar]
- Fung B. K., Hurley J. B., Stryer L. Flow of information in the light-triggered cyclic nucleotide cascade of vision. Proc Natl Acad Sci U S A. 1981 Jan;78(1):152–156. doi: 10.1073/pnas.78.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gedney C., Ostroy S. E. Hydrogen ion effects of the vertebrate photoreceptor. The pK's of ionizable groups affecting cell permeability. Arch Biochem Biophys. 1978 May;188(1):105–113. doi: 10.1016/0003-9861(78)90362-4. [DOI] [PubMed] [Google Scholar]
- Gold G. H., Korenbrot J. I. Light-induced calcium release by intact retinal rods. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5557–5561. doi: 10.1073/pnas.77.9.5557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govardovskii V. I., Berman A. L. Light-induced changes of cyclic GMP content in frog retinal rod outer segments measured with rapid freezing and microdissection. Biophys Struct Mech. 1981;7(3):125–130. doi: 10.1007/BF00539174. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Robinson W. E., Yoshikami S. Ionic aspects of excitation in rod outer segments. Ciba Found Symp. 1975;(31):169–189. doi: 10.1002/9780470720134.ch10. [DOI] [PubMed] [Google Scholar]
- Hagins W. A., Yoshikami S. Proceedings: A role for Ca2+ in excitation of retinal rods and cones. Exp Eye Res. 1974 Mar;18(3):299–305. doi: 10.1016/0014-4835(74)90157-2. [DOI] [PubMed] [Google Scholar]
- Kaupp U. B., Schnetkamp P. P., Junge W. Rapid calcium release and proton uptake at the disk membrane of isolated cattle rod outer segments. 1. Stoichiometry of light-stimulated calcium release and proton uptake. Biochemistry. 1981 Sep 15;20(19):5500–5510. doi: 10.1021/bi00522a024. [DOI] [PubMed] [Google Scholar]
- Kilbride P., Ebrey T. G. Light-initiated changes of cyclic guanosine monophosphate levels in the frog retina measured with quick-freezing techniques. J Gen Physiol. 1979 Sep;74(3):415–426. doi: 10.1085/jgp.74.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwok-Keung Fung B., Stryer L. Photolyzed rhodopsin catalyzes the exchange of GTP for bound GDP in retinal rod outer segments. Proc Natl Acad Sci U S A. 1980 May;77(5):2500–2504. doi: 10.1073/pnas.77.5.2500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr ATP mediates rapid reversal of cyclic GMP phosphodiesterase activation in visual receptor membranes. Nature. 1980 Oct 23;287(5784):734–736. doi: 10.1038/287734a0. [DOI] [PubMed] [Google Scholar]
- Liebman P. A., Pugh E. N., Jr The control of phosphodiesterase in rod disk membranes: kinetics, possible mechanisms and significance for vision. Vision Res. 1979;19(4):375–380. doi: 10.1016/0042-6989(79)90097-x. [DOI] [PubMed] [Google Scholar]
- Miller W. H. Physiological evidence that light-mediated decrease in cyclic GMP is an intermediary process in retinal rod transduction. J Gen Physiol. 1982 Jul;80(1):103–123. doi: 10.1085/jgp.80.1.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicol G. D., Miller W. H. Cyclic GMP injected into retinal rod outer segments increases latency and amplitude of response to illumination. Proc Natl Acad Sci U S A. 1978 Oct;75(10):5217–5220. doi: 10.1073/pnas.75.10.5217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannbacker R. G. Control of guanylate cyclase activity in the rod outer segment. Science. 1973 Dec 14;182(4117):1138–1140. doi: 10.1126/science.182.4117.1138. [DOI] [PubMed] [Google Scholar]
- Racker E., Miyamoto H., Mogerman J., Simons J., O'Neal S. Cation transport in reconstituted systems. Ann N Y Acad Sci. 1980;358:64–72. doi: 10.1111/j.1749-6632.1980.tb15386.x. [DOI] [PubMed] [Google Scholar]
- Robinson W. E., Hagins W. A. GTP hydrolysis in intact rod outer segments and the transmitter cycle in visual excitation. Nature. 1979 Aug 2;280(5721):398–400. doi: 10.1038/280398a0. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Calcium translocation and storage of isolated intact cattle rod outer segments in darkness. Biochim Biophys Acta. 1979 Jul 5;554(2):441–459. doi: 10.1016/0005-2736(79)90383-3. [DOI] [PubMed] [Google Scholar]
- Schnetkamp P. P. Ion selectivity of the cation transport system of isolated intact cattle rod outer segments: evidence for a direct communication between the rod plasma membrane and the rod disk membranes. Biochim Biophys Acta. 1980 May 8;598(1):66–90. doi: 10.1016/0005-2736(80)90266-7. [DOI] [PubMed] [Google Scholar]
- Szuts E. Z., Cone R. A. Calcium content of frog rod outer segments and discs. Biochim Biophys Acta. 1977 Jul 14;468(2):194–208. doi: 10.1016/0005-2736(77)90114-6. [DOI] [PubMed] [Google Scholar]
- Woodruff M. L., Bownds M. D. Amplitude, kinetics, and reversibility of a light-induced decrease in guanosine 3',5'-cyclic monophosphate in frog photoreceptor membranes. J Gen Physiol. 1979 May;73(5):629–653. doi: 10.1085/jgp.73.5.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wormington C. M., Cone R. A. Ionic blockage of the light-regulated sodium channels in isolated rod outer segments. J Gen Physiol. 1978 Jun;71(6):657–681. doi: 10.1085/jgp.71.6.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yau K. W., Lamb T. D., Baylor D. A. Light-induced fluctuations in membrane current of single toad rod outer segments. Nature. 1977 Sep 1;269(5623):78–80. doi: 10.1038/269078a0. [DOI] [PubMed] [Google Scholar]
- Yau K. W., McNaughton P. A., Hodgkin A. L. Effect of ions on the light-sensitive current in retinal rods. Nature. 1981 Aug 6;292(5823):502–505. doi: 10.1038/292502a0. [DOI] [PubMed] [Google Scholar]
- Yee R., Liebman P. A. Light-activated phosphodiesterase of the rod outer segment. Kinetics and parameters of activation and deactivation. J Biol Chem. 1978 Dec 25;253(24):8902–8909. [PubMed] [Google Scholar]
- Yoshikami S., George J. S., Hagins W. A. Light-induced calcium fluxes from outer segment layer of vertebrate retinas. Nature. 1980 Jul 24;286(5771):395–398. doi: 10.1038/286395a0. [DOI] [PubMed] [Google Scholar]
- Yoshikami S., Robinson W. E., Hagins W. A. Topology of the outer segment membranes of retinal rods and cones revealed by a fluorescent probe. Science. 1974 Sep 27;185(4157):1176–1179. doi: 10.1126/science.185.4157.1176. [DOI] [PubMed] [Google Scholar]


