Abstract
Background
Metabolic syndrome (MeS) is being recognized as a risk factor for insulin resistance and cardiovascular disease. The present study was aimed to find out the possible association between 45-bp I/D polymorphism of uncoupling protein 2 (UCP2) and MeS.
Methods
DNA was extracted from peripheral blood of 151 subjects with and 149 subjects without MeS. 45-bp I/D variant of UCP2 was detected using polymerase chain reaction (PCR).
Results
Our finding showed that 45-bp I/D polymorphism was associated with protection against MeS (OR = 0.56, 95% CI = 0.34-0.92, p = 0.020 D/I vs DD and OR = 0.54, 95% CI = 0.34-0.86, p = 0.009; D/I + I/I vs D/D). The I allele decreased the risk of MeS (OR = 0.62, 95% CI = 0.44-0.90, p = 0.011) in comparison with D allele.
Conclusion
In conclusion, our result suggests that 45-bp I/D polymorphism is associated with the risk of MeS, which remains to be cleared.
Keywords: Metabolic syndrome, UCP2, Polymorphism, Insertion/deletion
Introduction
Metabolic syndrome (MeS) is described as the combination of clinical disorders that increase the risk for obesity (central adiposity), insulin resistance, glucose intolerance, dyslipidemia, non-alcoholic fatty liver disease and cardiovascular diseases including atherosclerosis, stroke and hypertension [1,2]. The prevalence of MeS is considerably increasing globally, and is becoming an important health problem [3]. The etiology of this syndrome is complex and is thought to be the result of interaction between genetic and environmental factors [4-6]. Uncoupling proteins (UCPs) are mitochondrial membrane transporters that disturb the coupling between the mitochondrial proton gradient and ATP synthesis. In humans, the gene for UCP2 is located on chromosome 11q13 and contains 8 exons which exons 1 and 2 of UCP2 are untranslated [7]. Among the five UCP homologs (UCP1–UCP5), UCP2 is the most widely expressed, being involved in thermal regulation in various tissues, including white adipose tissue, liver, kidney, pancreatic islets, macrophages as well as retinal endothelial cells [8,9], and it is thought to play a role in the progress of obesity [10]. This protein uncouples oxidation of substrate from phosphorylation, dissipating the membrane potential energy and consequently decreasing ATP production by the mitochondrial respiratory chain [8]. It has been proposed that uncoupling leads to tissue-specific functions such as decreasing ROS formation by mitochondria, regulation of free fatty acids metabolism and inhibition of insulin secretion from beta cells [8,11]. Increased ROS production has been observed in macrophages [12] and pancreatic islets of UCP2 knockout mice [13]. It has been reported that overexpression of UCP2 attenuates ROS production and prevents oxidative damage of tissues [14,15]. α-Cells of pancreas secrete glucagon in response to low blood glucose. It has been shown that UCP2 is required for appropriate glucagon secretion and the absence of UCP2 impairs α-cell function [16].
The dysregulation of uncoupling proteins (UCPs), which translocate protons into the mitochondrial matrix resulting in heat generation without ATP synthesis [17], may contribute to the pathogenesis of obesity. It has been proposed that carriers of the exon-8 insertion allele in the UCP2 gene may have a greater risk of developing obesity [18]. Papazoglou et al. have found no association between UCP2 ins/del polymorphism and morbid obesity [19]. No association between UCP2-45 bp I/D and obesity was found in a Chinese population [20]. It has been shown that the UCP gene cluster variation may not be useful predictor for type 2 diabetes mellitus (T2DM) risk assessment [21]. In the current study, we aimed to evaluate the possible association between UCP 45-bp I/D and MeS in a sample of Iranian population.
Materials and methods
This case-control study was done on 151 patients with and 149 without MeS which we used in previous studies [4,5]. MeS was defined using the National Cholesterol Education Program Adult Treatment Panel III (NCEP ATP III) criteria [22] as described previously [23]. Ethical approvals for recruitment were obtained from local Ethics Committee of Zahedan University of Medical Sciences, and informed consent was obtained from all individuals. The data included weight, height, waist circumference, systolic and diastolic blood pressure; blood levels of glucose, triglycerides, total cholesterol, HDL cholesterol and LDL cholesterol were collected as described previously [23-25]. Blood samples were collected in EDTA-containing tubes and genomic DNA were extracted using salting out method as described previously [26].
Position of the 45-bp I/D polymorphism of UCP2 is shown schematically in Figure 1. The forward and reverse primers for detection of 45-bp ins/del polymorphism were 5′-TCTGGCTGAACTTTCCAA-3′ and 5′-TTCATGCCCTCCTTTCTC-3′, respectively. PCR was performed by using commercially available PCR premix (AccuPower PCR PreMix; BIONEER, Daejeon, Korea) according to the manufacturer’s instructions. Briefly, 1 μL template DNA (~100 ng/mL), 1 μL of each primer (10 pmol/mL), and 17 μL DNase-free water were added to AccuPower PCR PreMix. Amplification was done with an initial denaturation step at 95°C for 5 min, followed by 30 cycles at 95°C for 30 s, 60°C for 30 s, and 72°C for 23 s with a final extension at 72°C for 10 min. The amplified PCR product was resolved on 2% agarose gel. The PCR products for insertion and deletion alleles were 428-bp and 383-bp, respectively (Figure 2). Random samples were regenotyped to verify the accuracy of genotyping. We found no genotyping mistake.
Figure 1.
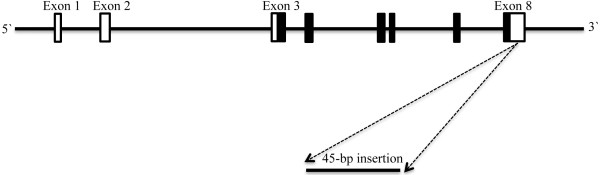
Map of the human UCP2 gene. Exons 1–8 are numbered and represented by black (coding exons) and white boxes (3′ and 5′ UTRs). Position of the 45-bp insertion/deletion in exon 8 in the 3′ UTR is designated.
Figure 2.
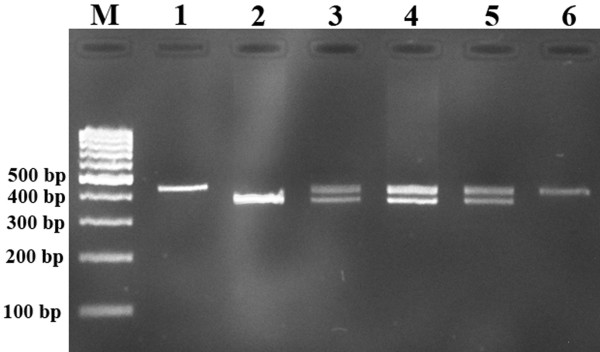
Representative PCR products resolved by agarose gel electrophoresis to detect the presence or absence of the 45 bp I/D of UCP2 gene. M, DNA marker. Lanes 1, 6: I/I; lane 2: D/D; lanes 3,4,5: I/D.
Statistical Analysis
The differences between the categorical and contentious data were assessed by chi-square and independent sample t-tests, respectively. The association between genotypes and metabolic syndrome was assessed by computing the odds ratio (OR) and 95% confidence intervals (95% CI) from logistic regression analyses. P-value less than 0.05 were considered statistically significant. The data analysis was performed using the SPSS18 software. According to our findings, sample power was calculated by using STATA 10 software.
Results
A total of 300 subjects including 151 MeS patients and 149 subjects without MeS were recruited in the study. The demographic and biochemical characteristics of the study participants are shown in Table 1. There were no significant differences between the groups regarding sex and age (p > 0.05). As shown in Table 2, our finding showed that the 45-bp I/D polymorphism of UCP2 decreased the risk of MeS in codominant (OR = 0.56, 95% CI = 0.34-0.92, p = 0.020 D/I vs D/D) and dominant (OR = 0.54, 95% CI = 0.34-0.86, p = 0.009; D/I + I/I vs D/D) inheritance model tested (Table 2). The I allele is associated with decreased risk of MeS (OR = 0.62, 95% CI = 0.44-0.90, p = 0.011) in comparison to D allele.
Table 1.
Demoraphic, clinical and biochemical characteristics of individuals with and without metabolic syndrome (MeS)
| |
Metabolic syndrome |
p |
|
|---|---|---|---|
| Yes | No | ||
| Sex (M/F) |
45/104 |
50/101 |
0.901 |
| Age (year) |
43.53 ± 11.96 |
41.98 ± 14.65 |
0.382 |
| Height (cm) |
160.21 ± 9.54 |
161.33 ± 10.24 |
0.330 |
| Weight (kg) |
71.07 ± 16.48 |
66.35 ± 14.19 |
0.009 |
| BMI (kg/m2) |
27.59 ± 5.40 |
25.49 ± 4.89 |
0.001 |
| Waist circumference (cm) |
95.36 ± 15.54 |
90.19 ± 12.87 |
0.002 |
| FBG (mg/dL) |
96.51 ± 25.99 |
94.25 ± 31.27 |
0.459 |
| Triglycerides (mg/dL) |
184.03 ± 110.81 |
141.66 ± 67.23 |
<0.001 |
| Total cholestrol (mg/dL) |
200.03 ± 45.74 |
190.91 ± 39.09 |
0.065 |
| HDL-C (mg/dL) |
43.05 ± 9.06 |
45.61 ± 7.70 |
0.019 |
| LDL-C (mg/dL) |
120.21 ± 44.76 |
116.78 ± 34.61 |
0.460 |
| SBP (mmHg) |
122.85 ± 17.90 |
117.49 ± 21.57 |
0.020 |
| DBP (mmHg) | 77.32 ± 13.51 | 73.95 ± 13.61 | 0.032 |
FBG, fasting blood glucose; SBP, systolic blood pressure; DBP, diastolic blood pressure.
Table 2.
Association of the 45-bp I/D polymorphism of UCP2 gene in individulas with and without metabolic syndrome (MeS)
| UCP2 (45-bp I/D) | MeS (yes) | MeS (No) | a OR (95% CI) | p | Study power % |
|---|---|---|---|---|---|
| Codominant |
|
|
|
|
|
| D/D |
90 (59.6) |
67 (45.0) |
1.00 |
- |
68 |
| D/I |
50 (33.1) |
65 (43.6) |
0.56 (0.34-0.92) |
0.020 |
42 |
| I/I |
11 (7.3) |
17 (11.4) |
0.47 (0.21-1.08) |
0.074 |
18 |
| Dominanat |
|
|
|
|
|
| D/D |
90 (59.6) |
67 (45.0) |
1.00 |
- |
68 |
| D/I + I/I |
61 (40.4) |
82 (55.0) |
0.54 (0.34-0.86) |
0.009 |
|
| Recessive |
|
|
|
|
|
| D/D + D/I |
140 (92.7) |
132 (88.6) |
1.00 |
- |
18 |
| I/I |
11 (7.3) |
17 (11.4) |
0.60 (0.27-1.34) |
0.213 |
18 |
| Alleles |
|
|
|
|
|
| D |
230 (76.2) |
199 (66.8) |
1.00 |
- |
70 |
| I | 72 (23.8) | 99 (33.2) | 0.62 (0.44-0.90) | 0.011 | 70 |
aAdjusted for sex and age.
The genotype distribution of 45-bp I/D polymorphism of UCP2 in subjects with and without MeS were in Hardy Weinberg equilibrium (HWE) (χ2 = 1.173, p = 0.278, χ2 = 0.042, p = 0.837, respectively).
Discussion
In the present study, we analyzed the possible association 45-bp I/D polymorphism of UCP2 gene and MeS in a sample of Iranian population. The UCP2 45-bp I/D was associated with MeS, so that the frequency distribution of I/D genotype as well as I allele were significantly lower in cases than that of controls. Metabolic syndrome is a combination of risk factors for cardiovascular disease (CVD) and T2DM. These factors include hyperglycemia, hypertension, dyslipidemia (high level of triglyceride and low HDL-cholesterol), and obesity (particularly with abdominal localization) [23,27]. Prevalence of MeS varies globally and depends in part on lifestyle, sex, age and ethnicity [23,28].
Some studies have found no associations between UCP2 45-bp I/D polymorphism and obesity, resting energy expenditure, BMI and insulin secretion [29-32]. Though, in some studies, the I-allele of UCP-2 has been found to be associated with development of obesity [18,33-35].
Oguzkan-Balci et al. [36] have found That UCP I/I genotype as well as I allele was associated with childhood obesity and related metabolic disorders.
Papazoglou et al. [19] have found no association between UCP2 45-bp I/D polymorphism and morbid obesity. They found that this polymorphism has effect on weight loss in metabolically healthy subjects so that individuals with I-allele had significantly greater reduction in body mass index (BMI) and fat-free mass as well as a slight significant improvement in the homeostatic model assessment index. No association was found between UCP2 45-bp I/D and obesity in a Chinese population [20] as well as Italian Caucasians [37].
Crispim et al. [38] investigated the -866G/A (rs659366), Ala55Val (rs660339) and 45 bp I/D polymorphisms in the UCP2 gene in diabetes mellitus. They found that the haplotype [A Val I] appears to be an important risk factor associated with proliferative diabetic retinopathy in both type 1 and 2 diabetic groups.
It has been reported that UCP2 I/D heterozygous decreased the risk of end-stage renal disease (ESRD) [39]. Mitchell et al. [40] have found no association between UCP2 I/D polymorphism and neural tube defects (NTDs). While, Wang et al. [41] proposed that this variant might be a potential genetic risk factor for NTDs.
It has been proposed that impaired adipose tissue expression of UCP2 may play a role in the pathophysiology of obesity [42]. To date, very little is known about the biological effects of the UCP2 45-bp I/D polymorphism, although its location in the 3′UTR of exon 8 suggests its potential involvement in mRNA processing or in transcript stability. It has been reported that UCP2 45-bp I/D polymorphism had no apparent effect on skeletal muscle UCP2 mRNA levels in 22 randomly chosen Pima Indians [43]. Wang et al. have reported that 3′UTR I/D variant had no impact on adipose mRNA levels [44]. Esterbauer et al. showed that the ratio of inserted to deleted mRNA expression was highly variable in the adipose tissue of subjects heterozygous for 45-bp I/D. The findings suggested an independent role for the 3′UTR I/D variant in mRNA stability [45].
One of the limitations of the present study is relatively small sample size. Consequently, analysis according to MeS components was not possible.
In conclusion, our findings showed that the 45-bp I/D polymorphism of UCP2 was associated with decreased risk of MeS. Larger studies with different ethnicity are required to validate our findings.
Competing interests
No competing financial interests.
Authors’ contributions
MH designed the study, supervised the study, analyzed the data and drafting the manuscript. HR collected the data and carried out the laboratory studies. MAK collected the data, and reviewed the manuscript. MT analyzed the data and reviewed the manuscript. All authors read and approved the final manuscript.
Contributor Information
Mohammad Hashemi, Email: mhd.hashemi@gmail.com.
Hamzeh Rezaei, Email: rezaeihamzeh@yahoo.com.
Mahmoud-Ali Kaykhaei, Email: mazyar44@yahoo.com.
Mohsen Taheri, Email: amirt112@yahoo.com.
Acknowledgements
This study was supported by a dissertation grant (M.Sc. thesis of HR) from Zahedan University of Medical Sciences. The authors thank all subjects who willingly participated in the study.
References
- Church T. Exercise in obesity, metabolic syndrome, and diabetes. Prog Cardiovasc Dis. 2011;53:412–418. doi: 10.1016/j.pcad.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Abete I, Goyenechea E, Zulet MA, Martinez JA. Obesity and metabolic syndrome: potential benefit from specific nutritional components. Nutr Metab Cardiovasc Dis. 2011;21(Suppl 2):B1–B15. doi: 10.1016/j.numecd.2011.05.001. [DOI] [PubMed] [Google Scholar]
- Azimi-Nezhad M, Herbeth B, Siest G, Dade S, Ndiaye NC, Esmaily H, Hosseini SJ, Ghayour-Mobarhan M, Visvikis-Siest S. High prevalence of metabolic syndrome in Iran in comparison with France: what are the components that explain this? Metab Syndr Relat Disord. 2012;10:181–188. doi: 10.1089/met.2011.0097. [DOI] [PubMed] [Google Scholar]
- Hahsemi M, Rezaei H, Eskandari Nasab E, Kaykhaei MA, Zakeri Z, Taheri M. Association between chemerin rs17173608 and vaspin rs2236242 gene polymorphisms and the metabolic syndrome, a preliminary report. Gene. 2012;510:113–117. doi: 10.1016/j.gene.2012.08.048. [DOI] [PubMed] [Google Scholar]
- Hashemi M, Rezaei H, Eskandari-Nasab E, Kaykhaei MA, Taheri M. Association of promoter methylation and 32-bp deletion of the PTEN gene with susceptibility to metabolic syndrome. Mol Med Rep. 2013;7:342–346. doi: 10.3892/mmr.2012.1174. [DOI] [PubMed] [Google Scholar]
- Deger O, Yandi YE, Ayvaz M, Erem C, Hacihasanoglu AB. Polymorphisms in ABC transporters (ABCA1 and ABCC8) in metabolic syndrome. Turk J Med Sci. 2013;43:214–221. [Google Scholar]
- Jia JJ, Zhang X, Ge CR, Jois M. The polymorphisms of UCP2 and UCP3 genes associated with fat metabolism, obesity and diabetes. Obes Rev. 2009;10:519–526. doi: 10.1111/j.1467-789X.2009.00569.x. [DOI] [PubMed] [Google Scholar]
- Dalgaard LT, Pedersen O. Uncoupling proteins: functional characteristics and role in the pathogenesis of obesity and type II diabetes. Diabetologia. 2001;44:946–965. doi: 10.1007/s001250100596. [DOI] [PubMed] [Google Scholar]
- Cui Y, Xu X, Bi H, Zhu Q, Wu J, Xia X, Qiushi R, Ho PC. Expression modification of uncoupling proteins and MnSOD in retinal endothelial cells and pericytes induced by high glucose: the role of reactive oxygen species in diabetic retinopathy. Exp Eye Res. 2006;83:807–816. doi: 10.1016/j.exer.2006.03.024. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Lowell BB. The mitochondrial uncoupling-protein homologues. Nat Rev Mol Cell Biol. 2005;6:248–261. doi: 10.1038/nrm1592. [DOI] [PubMed] [Google Scholar]
- Affourtit C, Brand MD. On the role of uncoupling protein-2 in pancreatic beta cells. Biochim Biophys Acta. 2008;1777:973–979. doi: 10.1016/j.bbabio.2008.03.022. [DOI] [PubMed] [Google Scholar]
- Arsenijevic D, Onuma H, Pecqueur C, Raimbault S, Manning BS, Miroux B, Couplan E, Alves-Guerra MC, Goubern M, Surwit R. et al. Disruption of the uncoupling protein-2 gene in mice reveals a role in immunity and reactive oxygen species production. Nat Genet. 2000;26:435–439. doi: 10.1038/82565. [DOI] [PubMed] [Google Scholar]
- Krauss S, Zhang CY, Scorrano L, Dalgaard LT, St-Pierre J, Grey ST, Lowell BB. Superoxide-mediated activation of uncoupling protein 2 causes pancreatic beta cell dysfunction. J Clin Invest. 2003;112:1831–1842. doi: 10.1172/JCI19774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li LX, Skorpen F, Egeberg K, Jorgensen IH, Grill V. Uncoupling protein-2 participates in cellular defense against oxidative stress in clonal beta-cells. Biochem Biophys Res Commun. 2001;282:273–277. doi: 10.1006/bbrc.2001.4577. [DOI] [PubMed] [Google Scholar]
- Teshima Y, Akao M, Jones SP, Marban E. Uncoupling protein-2 overexpression inhibits mitochondrial death pathway in cardiomyocytes. Circ Res. 2003;93:192–200. doi: 10.1161/01.RES.0000085581.60197.4D. [DOI] [PubMed] [Google Scholar]
- Allister EM, Robson-Doucette CA, Prentice KJ, Hardy AB, Sultan S, Gaisano HY, Kong D, Gilon P, Herrera PL, Lowell BB, Wheeler MB. UCP2 regulates the glucagon response to fasting and starvation. Diabetes. 2013;62:1623–1633. doi: 10.2337/db12-0981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingenberg M. Uncoupling protein–a useful energy dissipator. J Bioenerg Biomembr. 1999;31:419–430. doi: 10.1023/A:1005440221914. [DOI] [PubMed] [Google Scholar]
- Marti A, Corbalan MS, Forga L, Martinez-Gonzalez MA, Martinez JA. Higher obesity risk associated with the exon-8 insertion of the UCP2 gene in a Spanish case-control study. Nutrition. 2004;20:498–501. doi: 10.1016/j.nut.2004.03.019. [DOI] [PubMed] [Google Scholar]
- Papazoglou D, Papathanasiou P, Papanas N, Papatheodorou K, Chatziangeli E, Nikitidis I, Kotsiou S, Maltezos E. Uncoupling protein-2 45-base pair insertion/deletion polymorphism: is there an association with severe obesity and weight loss in morbidly obese subjects? Metab Syndr Relat Disord. 2012;10:307–311. doi: 10.1089/met.2012.0003. [DOI] [PubMed] [Google Scholar]
- Liu X, Zhang B, Liu X, Shen Y, Li J, Zhao N, Ma L, Du Q, Liu L, Zhao J, Wang X. A 45-bp insertion/deletion polymorphism in uncoupling protein 2 is not associated with obesity in a Chinese population. Biochem Genet. 2012;50:784–796. doi: 10.1007/s10528-012-9520-6. [DOI] [PubMed] [Google Scholar]
- Zee RY, Ridker PM, Chasman DI. Mitochondrial uncoupling protein gene cluster variation (UCP2-UCP3) and the risk of incident type 2 diabetes mellitus: the Women’s Genome Health Study. Atherosclerosis. 2011;214:107–109. doi: 10.1016/j.atherosclerosis.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Expert Panel on Detection E. Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (adult treatment panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- Kaykhaei M, Hashemi M, Narouie B, Shikhzadeh A, Jahantigh M, Shirzaei E, Rezazehi B, Hoseinian M, Yousefi S, Masoudian S. et al. Prevalence of metabolic syndrome in adult population from zahedan, southeast iran. Iran J Public Health. 2012;41:70–76. [PMC free article] [PubMed] [Google Scholar]
- Kordi-Tamandani DM, Hashemi M, Sharifi N, Kaykhaei MA, Torkamanzehi A. Association between paraoxonase-1 gene polymorphisms and risk of metabolic syndrome. Mol Biol Rep. 2012;39:937–943. doi: 10.1007/s11033-011-0819-x. [DOI] [PubMed] [Google Scholar]
- Hashemi M, Kordi-Tamandani DM, Sharifi N, Moazeni-Roodi A, Kaykhaei MA, Narouie B, Torkmanzehi A. Serum paraoxonase and arylesterase activities in metabolic syndrome in Zahedan, southeast Iran. Eur J Endocrinol. 2011;164:219–222. doi: 10.1530/EJE-10-0881. [DOI] [PubMed] [Google Scholar]
- Hashemi M, Moazeni-Roodi AK, Fazaeli A, Sandoughi M, Bardestani GR, Kordi-Tamandani DM, Ghavami S. Lack of association between paraoxonase-1 Q192R polymorphism and rheumatoid arthritis in southeast Iran. Genet Mol Res. 2010;9:333–339. doi: 10.4238/vol9-1gmr728. [DOI] [PubMed] [Google Scholar]
- Aydin S, Gemalmaz A, Nayir T, Ozkan S. Which predicts the cardiovascular risk best in elderly metabolic syndrome patients: ATP III or IDF? Turk J Med Sci. 2011;14:125–129. [Google Scholar]
- Cameron AJ, Shaw JE, Zimmet PZ. The metabolic syndrome: prevalence in worldwide populations. Endocrinol Metab Clin North Am. 2004;33:351–375. doi: 10.1016/j.ecl.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Dalgaard LT, Andersen G, Larsen LH, Sorensen TI, Andersen T, Drivsholm T, Borch-Johnsen K, Fleckner J, Hansen T, Din N, Pedersen O. Mutational analysis of the UCP2 core promoter and relationships of variants with obesity. Obes Res. 2003;11:1420–1427. doi: 10.1038/oby.2003.191. [DOI] [PubMed] [Google Scholar]
- Avesani CM, Kamimura MA, Utaka S, Pecoits-Filho R, Nordfors L, Stenvinkel P, Lindholm B, Draibe SA, Cuppari L. Is UCP2 gene polymorphism associated with decreased resting energy expenditure in nondialyzed chronic kidney disease patients? J Ren Nutr. 2008;18:489–494. doi: 10.1053/j.jrn.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Berentzen T, Dalgaard LT, Petersen L, Pedersen O, Sorensen TI. Interactions between physical activity and variants of the genes encoding uncoupling proteins -2 and -3 in relation to body weight changes during a 10-y follow-up. Int J Obes (Lond) 2005;29:93–99. doi: 10.1038/sj.ijo.0802841. [DOI] [PubMed] [Google Scholar]
- Ochoa MC, Santos JL, Azcona C, Moreno-Aliaga MJ, Martinez-Gonzalez MA, Martinez JA, Marti A. Association between obesity and insulin resistance with UCP2-UCP3 gene variants in Spanish children and adolescents. Mol Genet Metab. 2007;92:351–358. doi: 10.1016/j.ymgme.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Lee YH, Kim W, Yu BC, Park BL, Kim LH, Shin HD. Association of the ins/del polymorphisms of uncoupling protein 2 (UCP2) with BMI in a Korean population. Biochem Biophys Res Commun. 2008;371:767–771. doi: 10.1016/j.bbrc.2008.04.144. [DOI] [PubMed] [Google Scholar]
- Evans D, Minouchehr S, Hagemann G, Mann WA, Wendt D, Wolf A, Beisiegel U. Frequency of and interaction between polymorphisms in the beta3-adrenergic receptor and in uncoupling proteins 1 and 2 and obesity in Germans. Int J Obes Relat Metab Disord. 2000;24:1239–1245. doi: 10.1038/sj.ijo.0801402. [DOI] [PubMed] [Google Scholar]
- Cassell PG, Neverova M, Janmohamed S, Uwakwe N, Qureshi A, McCarthy MI, Saker PJ, Albon L, Kopelman P, Noonan K. et al. An uncoupling protein 2 gene variant is associated with a raised body mass index but not type II diabetes. Diabetologia. 1999;42:688–692. doi: 10.1007/s001250051216. [DOI] [PubMed] [Google Scholar]
- Oguzkan-Balci S, Col-Araz N, Nacak M, Araz M, Sabanci H, Balat A, Pehlivan S. Mitochondrial uncoupling protein 2 (UCP2) gene polymorphisms are associated with childhood obesity and related metabolic disorders. J Pediatr Endocrinol Metab. 2013;26:277–283. doi: 10.1515/jpem-2012-0267. [DOI] [PubMed] [Google Scholar]
- Maestrini S, Podesta F, Di Blasio AM, Savia G, Brunani A, Tagliaferri A, Mencarelli M, Chiodini I, Liuzzi A. Lack of association between UCP2 gene polymorphisms and obesity phenotype in Italian Caucasians. J Endocrinol Invest. 2003;26:985–990. doi: 10.1007/BF03348196. [DOI] [PubMed] [Google Scholar]
- Crispim D, Fagundes NJ, Dos Santos KG, Rheinheimer J, Boucas AP, De Souza BM, Macedo GS, Leiria LB, Gross JL, Canani LH. Polymorphisms of the UCP2 gene are associated with proliferative diabetic retinopathy in patients with diabetes mellitus. Clin Endocrinol (Oxf) 2010;72:612–619. doi: 10.1111/j.1365-2265.2009.03684.x. [DOI] [PubMed] [Google Scholar]
- Sharma R, Agrawal S, Saxena A, Pandey M, Sharma RK. Association of genetic variants of ghrelin, leptin and UCP2 with malnutrition inflammation syndrome and survival in end-stage renal disease patients. Genes Nutr. 2013;8:611–621. doi: 10.1007/s12263-013-0353-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell A, Pangilinan F, Van der Meer J, Molloy AM, Troendle J, Conley M, Kirke PN, Scott JM, Brody LC, Mills JL. Uncoupling protein 2 polymorphisms as risk factors for NTDs. Birth Defects Res A Clin Mol Teratol. 2009;85:156–160. doi: 10.1002/bdra.20520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Liu C, Zhao H, Wang F, Guo J, Xie H, Lu X, Bao Y, Pei L, Niu B. et al. Association between a 45-bp 3′untranslated insertion/deletion polymorphism in exon 8 of UCP2 gene and neural tube defects in a high-risk area of China. Reprod Sci. 2011;18:556–560. doi: 10.1177/1933719110393026. [DOI] [PubMed] [Google Scholar]
- Oberkofler H, Liu YM, Esterbauer H, Hell E, Krempler F, Patsch W. Uncoupling protein-2 gene: reduced mRNA expression in intraperitoneal adipose tissue of obese humans. Diabetologia. 1998;41:940–946. doi: 10.1007/s001250051011. [DOI] [PubMed] [Google Scholar]
- Walder K, Norman RA, Hanson RL, Schrauwen P, Neverova M, Jenkinson CP, Easlick J, Warden CH, Pecqueur C, Raimbault S. et al. Association between uncoupling protein polymorphisms (UCP2-UCP3) and energy metabolism/obesity in Pima indians. Hum Mol Genet. 1998;7:1431–1435. doi: 10.1093/hmg/7.9.1431. [DOI] [PubMed] [Google Scholar]
- Wang H, Chu WS, Lu T, Hasstedt SJ, Kern PA, Elbein SC. Uncoupling protein-2 polymorphisms in type 2 diabetes, obesity, and insulin secretion. Am J Physiol Endocrinol Metab. 2004;286:E1–7. doi: 10.1152/ajpendo.00231.2003. [DOI] [PubMed] [Google Scholar]
- Esterbauer H, Schneitler C, Oberkofler H, Ebenbichler C, Paulweber B, Sandhofer F, Ladurner G, Hell E, Strosberg AD, Patsch JR. et al. A common polymorphism in the promoter of UCP2 is associated with decreased risk of obesity in middle-aged humans. Nat Genet. 2001;28:178–183. doi: 10.1038/88911. [DOI] [PubMed] [Google Scholar]


