Abstract
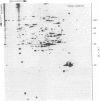
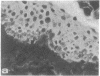
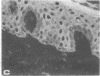
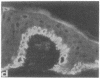
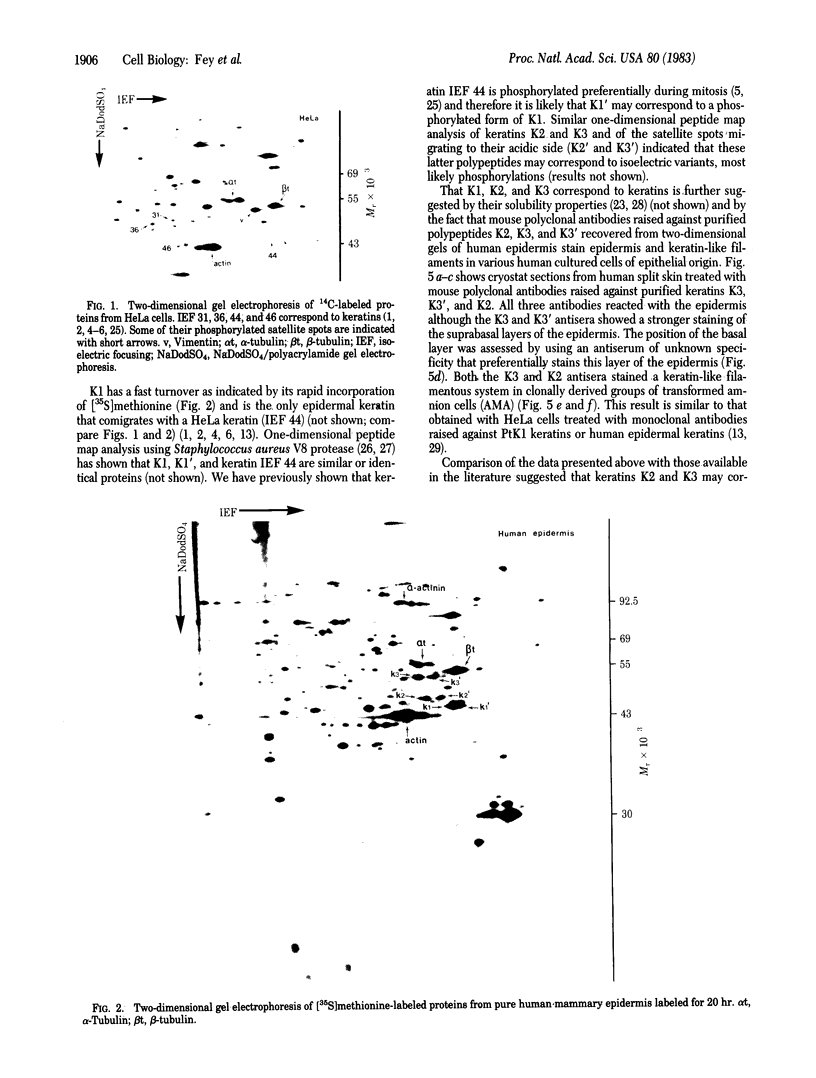
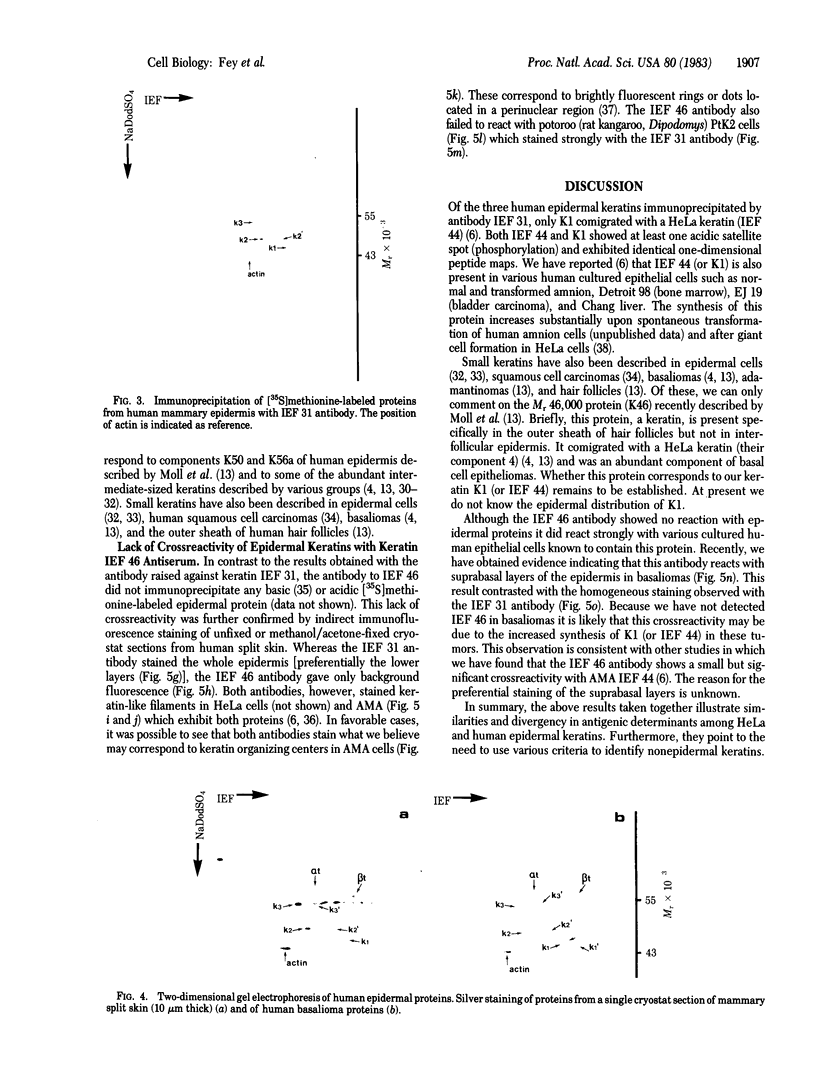
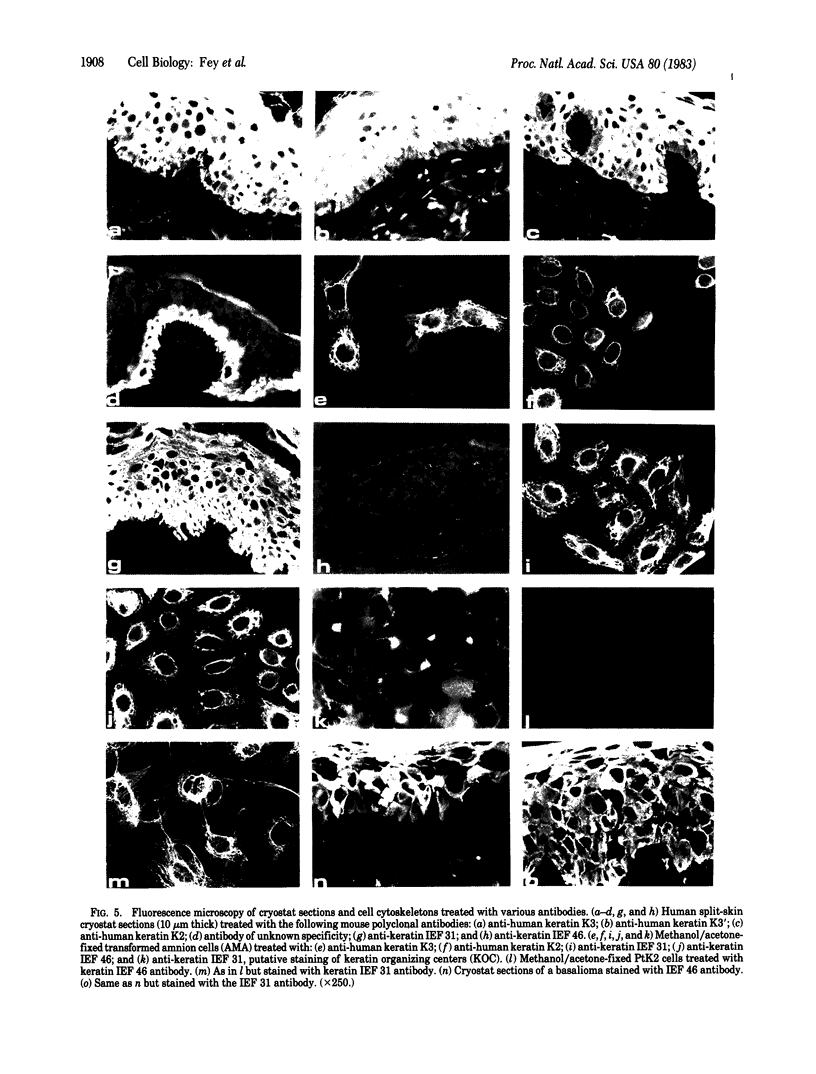
HeLa cells contain four keratin-like proteins having molecular weights of 50,000 (IEF 31), 48,500 (IEF 36), 44,000 (IEF 44), and 43,500 (IEF 46), respectively. Mouse polyclonal antibodies prepared against two of these keratins (IEF 31 and 46) have been used in this study to identify human epidermal keratins with common antigenic determinants. Using a sensitive immunoprecipitation procedure we show that the IEF 31 antibody crossreacts with three human acidic epidermal keratins, termed K1, K2, and K3, having molecular weights of 44,000, 47,500, and 54,000, respectively. One of these keratins (K1) comigrated with HeLa keratin IEF 44 and exhibited an identical one-dimensional peptide map. This protein is also abundant in basaliomas. In contrast to these results, the IEF 46 antibody showed no crossreactivity with any of the human acidic or basic [35S]methionine-labeled epidermal proteins. The lack of crossreactivity of this antibody was further confirmed by indirect immunofluorescence staining of cryostat sections from human split skin. These results emphasize both the similarity and diversity of antigenic determinants among HeLa and epidermal keratins.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banks-Schlegel S. P. Keratin alterations during embryonic epidermal differentiation: a presage of adult epidermal maturation. J Cell Biol. 1982 Jun;93(3):551–559. doi: 10.1083/jcb.93.3.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellatin J., Bravo R., Celis J. E. Changes in the relative proportion of transformation-sensitive polypeptides in giant HeLa cells produced by irradiation with lethal doses of x-rays. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4367–4370. doi: 10.1073/pnas.79.14.4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Bellatin J., Celis J. E. [35S]-methionine labelled polypeptides from HELA cells. Coordinates and percentage of some major polypeptides. Cell Biol Int Rep. 1981 Jan;5(1):93–96. doi: 10.1016/0309-1651(81)90162-4. [DOI] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. A search for differential polypeptide synthesis throughout the cell cycle of HeLa cells. J Cell Biol. 1980 Mar;84(3):795–802. doi: 10.1083/jcb.84.3.795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Human proteins sensitive to neoplastic transformation in cultured epithelial and fibroblast cells. Clin Chem. 1982 Apr;28(4 Pt 2):949–954. [PubMed] [Google Scholar]
- Bravo R., Celis J. E. Up-dated catalogue of HeLa cell proteins: percentages and characteristics of the major cell polypeptides labeled with a mixture of 16 14C-labeled amino acids. Clin Chem. 1982 Apr;28(4 Pt 2):766–781. [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Larsen P. M., Coppard N., Celis J. E. Proteins IEF (isoelectric focusing) 31 and IEF 46 are keratin-type components of the intermediate-sized filaments: keratins of various human cultured epithelial cells. J Cell Biol. 1983 Feb;96(2):416–423. doi: 10.1083/jcb.96.2.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R., Fey S. J., Small J. V., Larsen P. M., Celis J. E. Coexistence of three major isoactins in a single sarcoma 180 cell. Cell. 1981 Jul;25(1):195–202. doi: 10.1016/0092-8674(81)90244-0. [DOI] [PubMed] [Google Scholar]
- Bravo R., Small J. V., Fey S. J., Larsen P. M., Celis J. E. Architecture and polypeptide composition of HeLa cytoskeletons. Modification of cytoarchitectural polypeptides during mitosis. J Mol Biol. 1982 Jan 5;154(1):121–143. doi: 10.1016/0022-2836(82)90421-1. [DOI] [PubMed] [Google Scholar]
- Celis J. E., Small J. V., Andersen P., Celis A. Microfilament bundles in cultured cells. Correlation with anchorage independence and tumorigenicity in nude mice. Exp Cell Res. 1978 Jul;114(2):335–348. doi: 10.1016/0014-4827(78)90491-3. [DOI] [PubMed] [Google Scholar]
- Cleveland D. W., Fischer S. G., Kirschner M. W., Laemmli U. K. Peptide mapping by limited proteolysis in sodium dodecyl sulfate and analysis by gel electrophoresis. J Biol Chem. 1977 Feb 10;252(3):1102–1106. [PubMed] [Google Scholar]
- Eckert B. S., Daley R. A., Parysek L. M. In vivo disruption of the cytokeratin cytoskeleton in cultured epithelial cells by microinjection of antikeratin: evidence for the presence of an intermediate-filament-organizing center. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):403–412. doi: 10.1101/sqb.1982.046.01.039. [DOI] [PubMed] [Google Scholar]
- Fey S. J., Bravo R., Larsen P. M., Bellatin J., Celis J. E. [35S]-methionine labelled polypeptides from secondary mouse kidney fibroblasts: coordinates and one dimensional peptide maps of some major polypeptides. Cell Biol Int Rep. 1981 May;5(5):491–500. doi: 10.1016/0309-1651(81)90176-4. [DOI] [PubMed] [Google Scholar]
- Fey S. J., Larsen M., Celis J. E. Modification of vimentin polypeptides during mitosis. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):379–385. doi: 10.1101/sqb.1982.046.01.037. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schiller D. L., Moll R., Winter S., Schmid E., Engelbrecht I., Denk H., Krepler R., Platzer B. Diversity of cytokeratins. Differentiation specific expression of cytokeratin polypeptides in epithelial cells and tissues. J Mol Biol. 1981 Dec 25;153(4):933–959. doi: 10.1016/0022-2836(81)90460-5. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Weber K., Osborn M. HeLa cells contain intermediate-sized filaments of the prekeratin type. Exp Cell Res. 1979 Jan;118(1):95–109. doi: 10.1016/0014-4827(79)90587-1. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Schmid E., Winter S., Osborn M., Weber K. Widespread occurrence of intermediate-sized filaments of the vimentin-type in cultured cells from diverse vertebrates. Exp Cell Res. 1979 Oct 1;123(1):25–46. doi: 10.1016/0014-4827(79)90418-x. [DOI] [PubMed] [Google Scholar]
- Franke W. W., Weber K., Osborn M., Schmid E., Freudenstein C. Antibody to prekeratin. Decoration of tonofilament like arrays in various cells of epithelial character. Exp Cell Res. 1978 Oct 15;116(2):429–445. doi: 10.1016/0014-4827(78)90466-4. [DOI] [PubMed] [Google Scholar]
- Freudenstein C., Franke W. W., Osborn M., Weber K. Reaction of tonofilament-like intermediate-sized filaments with antibodies raised against isolated defined polypeptides of bovine hoof prekeratin. Cell Biol Int Rep. 1978 Nov;2(6):591–600. doi: 10.1016/0309-1651(78)90068-1. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. Regulation of terminal differentiation of cultured human keratinocytes by vitamin A. Cell. 1981 Sep;25(3):617–625. doi: 10.1016/0092-8674(81)90169-0. [DOI] [PubMed] [Google Scholar]
- Fuchs E., Green H. The expression of keratin genes in epidermis and cultured epidermal cells. Cell. 1978 Nov;15(3):887–897. doi: 10.1016/0092-8674(78)90273-8. [DOI] [PubMed] [Google Scholar]
- Green H., Fuchs E., Watt F. Differentiated structural components of the keratinocyte. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):293–301. doi: 10.1101/sqb.1982.046.01.031. [DOI] [PubMed] [Google Scholar]
- Lane E. B., Klymkowsky M. W. Epithelial tonofilaments: investigating their form and function using monoclonal antibodies. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 1):387–402. doi: 10.1101/sqb.1982.046.01.038. [DOI] [PubMed] [Google Scholar]
- Lee L. D., Baden H. P. Organisation of the polypeptide chains in mammalian keratin. Nature. 1976 Nov 25;264(5584):377–379. doi: 10.1038/264377a0. [DOI] [PubMed] [Google Scholar]
- Moll R., Franke W. W., Volc-Platzer B., Krepler R. Different keratin polypeptides in epidermis and other epithelia of human skin: a specific cytokeratin of molecular weight 46,000 in epithelia of the pilosebaceous tract and basal cell epitheliomas. J Cell Biol. 1982 Oct;95(1):285–295. doi: 10.1083/jcb.95.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mose-Larsen P., Bravo R., Fey S. J., Small J. V., Celis J. E. Putative association of mitochondria with a subpopulation of intermediate-sized filaments in cultured human skin fibroblasts. Cell. 1982 Dec;31(3 Pt 2):681–692. doi: 10.1016/0092-8674(82)90323-3. [DOI] [PubMed] [Google Scholar]
- O'Farrell P. H. High resolution two-dimensional electrophoresis of proteins. J Biol Chem. 1975 May 25;250(10):4007–4021. [PMC free article] [PubMed] [Google Scholar]
- O'Farrell P. Z., Goodman H. M., O'Farrell P. H. High resolution two-dimensional electrophoresis of basic as well as acidic proteins. Cell. 1977 Dec;12(4):1133–1141. doi: 10.1016/0092-8674(77)90176-3. [DOI] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Direct visualization of the 10-nm (100-A)-filament network in whole and enucleated cultured cells. J Cell Sci. 1978 Jun;31:393–409. doi: 10.1242/jcs.31.1.393. [DOI] [PubMed] [Google Scholar]
- Small J. V., Celis J. E. Filament arrangements in negatively stained cultured cells: the organization of actin. Cytobiologie. 1978 Feb;16(2):308–325. [PubMed] [Google Scholar]
- Sun T. T., Green H. Immunofluorescent staining of keratin fibers in cultured cells. Cell. 1978 Jul;14(3):469–476. doi: 10.1016/0092-8674(78)90233-7. [DOI] [PubMed] [Google Scholar]
- Sun T. T., Shih C., Green H. Keratin cytoskeletons in epithelial cells of internal organs. Proc Natl Acad Sci U S A. 1979 Jun;76(6):2813–2817. doi: 10.1073/pnas.76.6.2813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng S. C., Jarvinen M. J., Nelson W. G., Huang J. W., Woodcock-Mitchell J., Sun T. T. Correlation of specific keratins with different types of epithelial differentiation: monoclonal antibody studies. Cell. 1982 Sep;30(2):361–372. doi: 10.1016/0092-8674(82)90234-3. [DOI] [PubMed] [Google Scholar]
- Wu Y. J., Rheinwald J. G. A new small (40 kd) keratin filament protein made by some cultured human squamous cell carcinomas. Cell. 1981 Sep;25(3):627–635. doi: 10.1016/0092-8674(81)90170-7. [DOI] [PubMed] [Google Scholar]