Abstract
CD27 is an important co-stimulatory receptor of T cells that can potentially be exploited for immunotherapy. We developed a human IgG1 antibody that targets human CD27, and demonstrated its immunostimulatory and antineoplastic activity in various preclinical models. Currently, the antibody (1F5, CDX-1127) is being tested in patients affected by advanced malignancies.
Keywords: CD27, co-stimulation, Fc receptors, immunotherapy, monoclonal antibody
CD27 belongs to the tumor necrosis factor α receptor superfamily (TNFRSF) and has overlapping activity with other TNFRSF members including CD40, TNFRSF9 (best known as 4–1BB), and TNFRSF4 (best known as OX-40). CD27 plays a key role in diverse immunological processes, including the survival, activation and effector functions of T cells, as well as the proliferation and cytotoxic activity of natural killer (NK) cells.1 A unique feature of CD27 is its constitutive expression at significant levels on the majority of T cells, including naïve T cells. Thus, the CD27 signaling pathway is regulated, for the most part, by the expression of the CD27 ligand, CD70, and CD27 is generally available at the cell surface for targeting by specific antibodies. This was originally exploited by Glennie and colleagues, who described a monoclonal antibody specific for mouse CD27 that exerted therapeutic activity in murine tumor models.2
To translate these finding into potential clinical applications, we created a panel of fully human antibodies specific for human CD27 by immunizing mice, genetically modified to express human immunoglobulins with the extracellular domain of human CD27. This panel of monoclonal antibodies was screened by a variety of binding and functional assays, resulting in the selection of a lead IgG1 antibody, referred to as clone 1F5. This antibody exhibits a high affinity for both the human and macaque variants of CD27, binds to this TNFRSF member expressed on the cell surface, and blocks CD70 binding, suggesting that 1F5 may bind to (or near to) the ligand-binding site of CD27.3
To perform in vivo studies based on the 1F5 antibody, we generated transgenic mice expressing human CD27 (hCD27-Tg) by means of a bacterial artificial chromosome (BAC) containing the entire CD27-coding gene and presumed upstream promoter. hCD27-Tg mice were backcrossed to several strains and transgene expression was extensively characterized. Globally, the expression pattern of human CD27 in these transgenic mice, both in steady-state conditions and in the course of immune responses, suggests that the transgene of hCD27-Tg mice is expressed and regulated in a manner consistent with the biology of CD27. Importantly, the affinity of mouse CD70 for human CD27 is similar to that of human CD70, implying that natural CD70-CD27 interactions can occur in these hCD27-Tg mice.4
The agonistic activity of the 1F5 antibody was demonstrated in vitro using either human or hCD27-Tg mouse-derived T cells. In particular, 1F5 was found to significantly enhance the proliferation and cytokine expression of CD4+ and CD8+ cells exposed to sub-optimal amounts of anti-CD3 antibodies, but only when 1F5 was cross-linked with anti-human IgGs or bound to the microtiter plate.3,4 Providing 1F5 in solution or cross-linking 1F5 in the absence of T-cell receptor (TCR) stimulation resulted in no T-cell activation. Thus, 1F5 appears to be incapable of triggering a potentially dangerous polyclonal T-cell activation as co-stimulatory superagonists like some CD28-specific antibodies do.5,6
In vivo, 1F5 boosted the CD8+ T cell response elicited by an ovalbumin-targeting vaccine in hCD27-Tg mice, but not in wild-type mice. For these studies, wild-type or hCD27-Tg mice were immunized with ovalbumin and separately injected with 1F5 or an antibody specific for murine CD27 (AT-124), at the time of immunization and on the following day. One week later, splenocytes were stained with the ovalbumin-specific tetramer H-2Kb-SIINFEKL and tested by ELISPOT for interferon γ (IFNγ) secretion upon stimulation based on the same antigenic specificity. We observed a significant expansion of tetramer-specific T cells in hCD27-Tg mice receiving AT-124 or 1F5, as compared with either hCD27-Tg mice treated with isotype-matched control IgG or wild-type mice receiving 1F5. Similarly, the ELISPOT analysis showed a robust and specific IFNγ response consistent with the results of tetramer staining.4
We demonstrated the activity of 1F5 in several syngeneic murine tumor models, including the BCL1 lymphoma, CT26 colon carcinoma and E.G7 thymoma models. The administration of anti-CD27 antibodies led to complete tumor regression in the majority of hCD27-Tg mice inoculated with CT26 cells, when the treatment was started 3 d following the implantation of cancer cells. Conversely, 1F5 was less effective when treatment was delayed to start on day 15 post cancer cell implantation. The animals that survived the initial tumor challenge were shown to exhibit protective immunological memory against CT26 cells, as evidenced by the lack of tumor growth when these animals were re-challenged with tumor cells of the same type approximately 100 d later. T-cell depletion studies were also performed, and the loss of either CD4+ or CD8+ T cells diminished the efficacy of 1F5, suggesting a role for both of these T-cell subsets in the therapeutic responses following CD27 stimulation.
We sought to elucidate if 1F5 was being cross-linked in vivo, as was suggested by in vitro findings. Since human IgG1s are known to react with mouse Fc receptors (FcRs), we considered FcR-mediated cross-linking as a likely mechanism. To test this hypothesis, we introduced a point mutation into the Fc fragment of 1F5 that abolished FcR binding. Mutant 1F5 retained complete CD27-binding activity, but was unable to bind mouse FcRs as demonstrated by Biacore analyses. The mutation completely abrogated the ability of 1F5 to enhance ovalbumin-specific T-cell responses and to exert anticancer effects against the E.G7 thymoma. These data suggest that 1F5 exerts co-stimulatory effects upon the engagement of FcRs on antigen-presenting cells in the course of MHC-TCR interactions (Fig. 1).
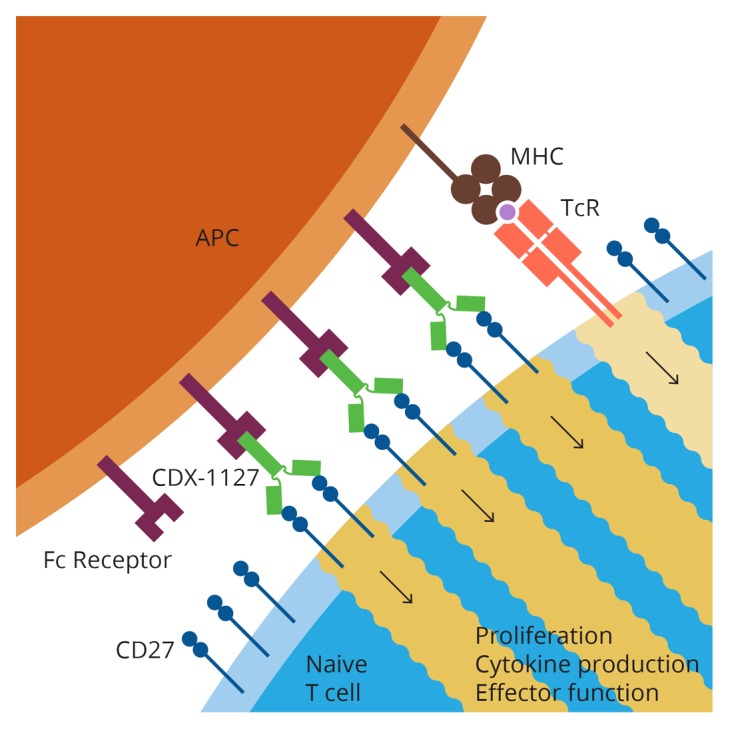
Figure 1. T-cell activation by anti-CD27 antibodies requires antibody cross-linking and TCR stimulation. 1F5 recognizes CD27 on the surface of T cells while binding Fc receptors (FcRs) on antigen-presenting cells. This favors 1F5 cross-linking and results in the co-stimulation of T-cell receptor (TCR)-transduced signals, overall stimulating proliferative, secretory and effector T-cell responses that can mediate robust antineoplastic effects.
In summary, we have developed a monoclonal antibody operating as a CD27 agonist, 1F5, which has been shown to activate human and hCD27-Tg mouse T cells in the context of TCR stimulation and to exert antitumor activity in murine tumor models. Thus, 1F5 may be particularly effective as part of combinatorial immuno(chemo)therapeutic regimens. Data from CD27-deficient mice suggest that CD27 stimulation might enhance the immunosuppressive activity of regulatory T cells and hence promote tumor growth.7 However, the immunostimulatory effects of CD27 signaling appear to override such an effect,8 and all of the studies performed to date with agonist anti-CD27 antibodies are consistent with their ability to drive therapeutically relevant antitumor immune responses.2,4,9,10 1F5 is currently being evaluated (under the name CDX-1127) in a Phase I clinical trial enrolling patients with advanced malignancies.
Disclosure of Potential Conflicts of Interest
Authors are employees of Celldex Therapeutics, Inc. and hold stock options in the company.
Glossary
Abbreviations:
- FcR
Fc receptor
- hCD27-Tg
human CD27 transgenic
- IFN
interferon
- TCR
T-cell receptor
Citation: Thomas LJ, He L, Marsh H, Keler T. Targeting human CD27 with an agonist antibody stimulates T-cell activation and anti-tumor immunity. OncoImmunology 2013; 2:e27255; 10.4161/onci.27255
Footnotes
Previously published online: www.landesbioscience.com/journals/oncoimmunology/article/27255
References
- 1.Denoeud J, Moser M. Role of CD27/CD70 pathway of activation in immunity and tolerance. J Leukoc Biol. 2011;89:195–203. doi: 10.1189/jlb.0610351. [DOI] [PubMed] [Google Scholar]
- 2.French RR, Taraban VY, Crowther GR, Rowley TF, Gray JC, Johnson PW, Tutt AL, Al-Shamkhani A, Glennie MJ. Eradication of lymphoma by CD8 T cells following anti-CD40 monoclonal antibody therapy is critically dependent on CD27 costimulation. Blood. 2007;109:4810–5. doi: 10.1182/blood-2006-11-057216. [DOI] [PubMed] [Google Scholar]
- 3.Vitale LA, He L-Z, Thomas LJ, Widger J, Weidlick J, Crocker A, O’Neill T, Storey J, Glennie MJ, Grote DM, et al. Development of a human monoclonal antibody for potential therapy of CD27-expressing lymphoma and leukemia. Clin Cancer Res. 2012;18:3812–21. doi: 10.1158/1078-0432.CCR-11-3308. [DOI] [PubMed] [Google Scholar]
- 4.He L-Z, Prostak N, Thomas LJ, Vitale L, Weidlick J, Crocker A, Pilsmaker CD, Round SM, Tutt A, Glennie MJ, et al. Agonist anti-human CD27 monoclonal antibody induces T cell activation and tumor immunity in human CD27-transgenic mice. J Immunol. 2013;191:4174–83. doi: 10.4049/jimmunol.1300409. [DOI] [PubMed] [Google Scholar]
- 5.Duff GW, et al. Expert Scientific Group on Phase One Clinical Trials (a.k.a. “The Duff Report”) 2006 www.tsoshop.co.uk
- 6.Waibler Z, Sender LY, Merten C, Hartig R, Kliche S, Gunzer M, Reichardt P, Kalinke U, Schraven B. Signaling signatures and functional properties of anti-human CD28 superagonistic antibodies. PLoS One. 2008;3:e1708. doi: 10.1371/journal.pone.0001708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Claus C, Riether C, Schürch C, Matter MS, Hilmenyuk T, Ochsenbein AF. CD27 signaling increases the frequency of regulatory T cells and promotes tumor growth. Cancer Res. 2012;72:3664–76. doi: 10.1158/0008-5472.CAN-11-2791. [DOI] [PubMed] [Google Scholar]
- 8.Aulwurm S, Wischhusen J, Friese M, Borst J, Weller M. Immune stimulatory effects of CD70 override CD70-mediated immune cell apoptosis in rodent glioma models and confer long-lasting antiglioma immunity in vivo. Int J Cancer. 2006;118:1728–35. doi: 10.1002/ijc.21544. [DOI] [PubMed] [Google Scholar]
- 9.Sakanishi T, Yagita H. Anti-tumor effects of depleting and non-depleting anti-CD27 monoclonal antibodies in immune-competent mice. Biochem Biophys Res Commun. 2010;393:829–35. doi: 10.1016/j.bbrc.2010.02.092. [DOI] [PubMed] [Google Scholar]
- 10.Roberts DJ, Franklin NA, Kingeter LM, Yagita H, Tutt AL, Glennie MJ, Bullock TNJ. Control of established melanoma by CD27 stimulation is associated with enhanced effector function and persistence, and reduced PD-1 expression of tumor infiltrating CD8(+) T cells. J Immunother. 2010;33:769–79. doi: 10.1097/CJI.0b013e3181ee238f. [DOI] [PMC free article] [PubMed] [Google Scholar]


