Abstract
With the remarkable increase in the fields of biomedical engineering and regenerative medicine, biomaterial design has become an indispensable approach for developing the biocompatible carriers for drug or gene cargo and extracellular matrix (ECM) for cell survival, proliferation and differentiation. Native ECM materials derived from animal tissues were believed to be the best choices for tissue engineering. However, possible pathogen contamination by cellular remnants from foreign animal tissues is an unavoidable issue that has limited the use of native ECM for human benefit. Some synthetic polymers have been used as alternative materials for manufacturing native ECM because of the biodegradability and ease of large-scale production of the polymers. However, the inherent polydispersity of the polymers causes batch-to-batch variation in polymer composition and possible cytotoxic interactions between chemical matrices and neighboring cells or tissues have not yet been fully resolved. Elastin-like proteins (ELPs) are genetically engineered biopolymers modeled after the naturally occurring tropoelastin and have emerged as promising materials for biomedical applications because they are biocompatible, non-immunogenic and biodegradable, and their composition, mechanical stiffness and even fate within the cell can be controlled at the gene level. This commentary highlights the recent progresses in the development of the ELP-based recombinant proteins that are being increasingly used for the delivery of chemotherapeutics and to provide a cell-friendly ECM environment.
Keywords: elastin-like proteins, extracellular matrix, surface-coatings, hydrogels, drug and gene delivery, regenerative medicine
Advantages of Recombinant ELP Engineering
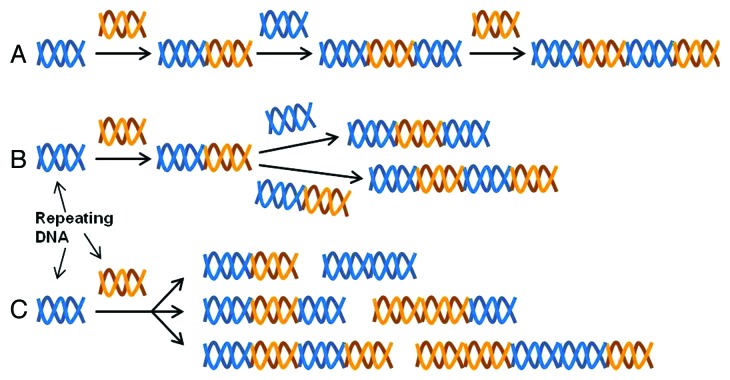
ELPs belong to a family of recombinant proteins consist of the VPGXG pentapeptide, where the guest position X accepts any amino acid except for proline. One favorable feature of the ELPs is responsiveness to temperature change; solubilized ELPs associate with each other above a certain transition temperature and form self-assembled coacervates comprising β-spiral structures.1 The thermal sensitivity of ELP has been exploited in various biomedical engineering fields that benefit from coacervation at the target site where they form an aggregating drug repository or a sold matrix, thus providing a mechanism for sustained release of the co-localized drugs or acting as a mechanically robust scaffold. Currently, three DNA manipulation methods, iterative ligation, recursive directional ligation and random concatemerization,2 are being used to oligomerize the monomer gene of repeating peptide into the gene encoding the protein of interest (Fig. 1). Implementation of such molecular biology techniques in ELP production is highly advantageous; the genetically encoded design of ELPs permits precise control over their molecular composition and chain length to meet the end users’ applications. Therefore, through the recombinant ELP engineering, bioengineers can modulate the architectural complexity as well as the diverse biochemical features, such as temperature responsiveness, mechanical stiffness, biocompatibility and cell-adhesive functions that are essential factors to be considered in developing new ELPs for the application in drug and gene delivery, cell therapy and tissue repair.
Figure 1. Schematics of iterative ligation (A), recursive directional ligation (B) and random concatemerization (C).
Drug and Gene Delivery
Positioning of amino acids such as cysteine, lysine, glutamic acid and aspartic acids at the guest residues allows covalent cross-linking of the chemotherapeutics to the backbone of ELP molecules. Therefore, to enhance the accumulation of chemotherapeutics at the tumor region that is heated by local hyperthermia, thermally responsive ELPs as the drug carrier have ever been widely used in targeted drug delivery and controlled release of radionuclides, chemical drugs and peptide therapeutics to solid tumor (Fig. 2A).3 In particular, the anticancer efficacy of ELP-doxorubicin4 and ELP-geldanamycin5 conjugates emphasizes that the ELP-drug conjugation is a useful mean not only to take advantage of thermally-induced localization to tumor site but also to overcome the drug resistance in the treatment of multidrug-resistant cancers by surpassing cellular efflux process.4

Figure 2. Schematic drawings of multifunctional ELP (A), ELP micelle (B) and ELP polyplex (C).
The recombination of ELP gene with the genes for cell-penetrating domains, cytokines or cell growth inhibitory peptides leads to a fusion form of ELPs to overcome susceptibility to degradation and poor tumor penetration in vivo (Fig. 2B). Noticeably, Raucher’s group engineered three genes of cell-penetrating Bac, ELP1 and helix 1 (H1) of c-Myc (cellular homolog of the v-myc avian myelocytomatosis viral oncogene) into a single DNA sequence.6 The fusion form of ELP, Bac-ELP1-H1, thus produced significantly reduced tumor growth in an orthotopic mouse model of breast cancer by blocking the activity of the oncogenic protein c-Myc. In a similar way, Setton’s group synthesized a recombinant protein of ELP-sTNFRII by fusion of the genes for ELP and soluble tumor necrosis factor α (TNFα) receptor II (sTNFRII).7 In in vitro bioactivity using murine L929 fibrosarcoma cells, the fusion protein shows antagonistic effects on TNFα-mediated cytotoxicity. The results indicate that the fusion protein of ELP and sTNFRII retains functionality of both domains, supporting the potential use as an immunomodulator therapeutic. Chilkoti’s8 and MacKay’s9 groups, respectively, fused the CD13 ligand NGR tripeptide and Knob domain of adenovirus serotype 5 fiber protein to the N-terminal of diblock copolymers composed of hydrophilic block and hydrophobic domains. The resultant proteins, NGR-ELP[V1A8G7]64/ELP[V]90 and Knob-G(VPGSG)48(VPGIG)48Y, self-assemble into monodisperse spherical micelles presenting NGR or Knob sequence on their coronas. The NGR-ELP[V1A8G7]64/ELP[V]90 selectively localizes to the tumor vasculature with an increased vascular retention and extravascular accumulation and the Knob-S48I48 shows more intracellular vesicular uptake into lysosomal compartments. As such, the multivalent presentation of specific ligands by micelle self-assembly on the ELP nanoparticles is a potentially useful strategy to develop new class of drug-loaded architectures that target a unique uptake mechanism.
When positively-charged ELPs interact with negatively charged molecules such as plasmid DNA, they condense into nano-sized polyplexes.10 Therefore, cationic ELPs act as a promising carrier for DNA therapeutics delivery through ionic complexation with plasmids or siRNA. In analogy with fusion forms of ELPs, combinatory use of fusogenic peptides with the cationic ELPs would improve the siRNA-mediated silencing of oncogenes by enhancing endosomal escape of polyplex particles (Fig. 2C).11
Biosurface Modification for Cellular Engineering
Spontaneous physical adsorption of ELPs onto cell culture surfaces at temperatures below their transition temperature has been successfully used to enhance cellular behaviors such as attachment, migration and differentiation. For instance, isothermal adsorption of RGD-containing ELPs (referred to as REPs) onto glass slides or polystyrene surfaces has been shown to increase neuronal cell motility and differentiation, along with neurite extension in N2a (Fig. 3A) and PC-12 cells.12-14 Similarly, multiblock ELPs composed of alternating (A)6K(A)3K(A)2QFGLVPGV and GVAPGV peptides have been shown to enhance both differentiation of skeletal muscles and development of myotubes in H9c2 myoblasts.15 Interestingly, it has been reported that the wettability of REP-treated culture surfaces can be modulated by changing the temperature and pH to promote growth of Saos-2 osteoblasts.16 However, a drawback of this technique is that the amounts of proteins adsorbed are beyond the sensitivity limits of the Lowry and the Bradford protein assays; therefore, either radioisotope-labeled ELP samples or an enzyme-linked immunosorbent assay is necessary to obtain ELP adsorption profiles, along with the quantitative analysis of cell-matrix interactions such as determination of the affinity constant and estimation of migration speed. Since protein adsorption can be easily achieved by immersing target substrates into ELP solutions for a certain period, isothermal adsorption is the most convenient way for the biofunctionalization of substrates with complex geometries, including grafts, implants and scaffolds.
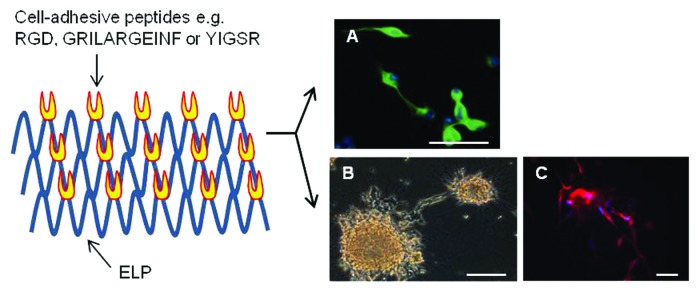
Figure 3. Differentiation of neuronal cells on the REP coatings prepared via isothermal adsorption at 4°C (A) or thermally induced coacervation at 37°C (B and C). (A) and (C) show fluorescent images of the expression of neuronal class III β-tubulin in N2a neuroblastoma cells (green color) and neural progenitor cells (red color). Blue color corresponds to nucleus. (B) is the phase contrast microscopic picture of neurite extension from human SH-SY5Y neurospheroids. Cells were cultured in the absence (A) and presence (B and C) of 10 μM all-trans retinoic acid. Scale bars = 50 μm.
Thermally induced sol-to-gel transition and chemical cross-linking leading to the formation of coacervate layers or thin films have been exploited to immobilize ELPs onto bioinert materials. The ELP aggregates or films thus formed can modulate the morphological features and functions of many different types of cells from the liver, brain, pancreatic islets, heart and eyes. Coacervate layers of an ELP-polyethyleneimine (PEI) conjugate have been reported to produce multicellular hepatocyte spheroids, which can be useful as an in vitro liver tissue model for studying liver steatosis.17,18 When SH-SY5Y neuroblasts and pancreatic β-TC6 cells were cultured on hydrophobically collapsed REP coatings, these cells clustered into multicellular neurospheroids (Fig. 3B) and pseudoislets, respectively.19,20 With regard to the high content of cell adhesion molecules (CAMs) and ECM proteins, 3-dimensional (3D) architectures such as those of neurospheroids and pseudoislets are more physiologically relevant to in vivo tissues than conventional monolayer cell cultures and were therefore recognized as in vitro models recapitulating in vivo tissues. Differentiation of embryonic stem cells into cardiomyocytes was found to be effectively promoted on surfaces coated with an ELP fusion of insulin-like growth factor-binding protein 4.21 The shape of adipose-derived stem cells cultured on photoreactive diazirine cross-linked REP films was found to change from stationary to one having spreading morphology, along with the development of F-actin cytoskeletons.22 Similarly, the phenotypes of retinal pigment epithelial cells have been found to be well maintained on chemically cross-linked REP films.23 Recently, the results obtained by this author highlighted the usability of ELPs as a biomimetic matrix for creating a cell-friendly environment on the surfaces of synthetic polymer-based scaffolds. Poly(lactide-co-glycolide) (PLGA) is an FDA-approved polymer for human use and is therefore one of the most extensively used biopolymers for casting 3D scaffolds for tissue engineering. However, the biopolymer’s hydrophobic nature makes it less effective for initial cell loading. PLGA scaffolds have been coated with cell-adhesive REP matrix via thermally driven inverse transition. On the matrix-coated PLGA scaffolds, both the adhesion and proliferation of neural progenitor cells increase significantly in a matrix dose-dependent manner. More importantly, in combination with all-trans retinoic acid, differentiation of progenitor cells into neuronal cells and astrocytes increases greatly in the cells cultured on REP-coated scaffolds than in untreated controls (Fig. 3C). The ability of REP coatings to stimulate cellular functions and differentiation could be correlated with the activation of RGD-mediated signaling cascades, which eventually upregulate the expression of CAMs and ECM proteins or promote F-actin assembly. However, signaling molecules that are activated upon REP-cell interactions remain to be identified.
To assemble multilayered films or structures, researchers have exploited the electrostatic attraction between opposite charges as the driving force for layer-by-layer (LbL) deposition. Rajagopalan’s group constructed nano-scale multilayers through sequential deposition of cationic (GVGVP)40-PEI and anionic (GVGVP)40-polyacrylic acid conjugates.24 When tested with 3T3 fibroblasts, both cell proliferation and cytoskeletal organization were observed to have increased with a rise in the number of polyelectrolyte bilayers, indicating the importance of the thickness and integrity of the multilayer in controlling cellular functions. Mano’s group fabricated biomimetic LbL coatings utilizing the electrostatic interaction between the positively charged amine groups of chitosan and the deprotonated aspartic acids of REP.25,26 The films having a terminal REP layer showed an acute and independent cyclic wettability shift in response to a change in temperature, pH and ionic strength due to the aggregation of ELP chains on the top layer. For biological evaluation, Saos-2 osteoblasts showed significantly greater adhesion and proliferation on REP-ending coatings than those ending with a chitosan layer. Herrmann’s group produced cationic and anionic ELPs by incorporating lysine and glutamic acid residues, respectively, within the repetitive pentapeptide units and constructed a hollow protein capsule through the sequential assembly of 2 oppositely supercharged ELPs onto spherical CaCO3 particles, followed by dissolution of the template core.27 Studies using LbL deposition have emphasized that polyelectrolyte ELPs with precisely defined electric charges would be attractive building blocks for the construction of electrostatically assembled architectures.
Hydrogel for Regenerative Medicine
Given the fact that mammalian cells exist in 3D environments in native tissues, it is understandable that 3D matrix systems, regardless of their biomaterial identity, have consistently been found to enhance cell growth and differentiation. With the dimensionality of native tissue, ELP-based hydrogels could serve as models for understanding the effects of matrix stiffness on cell functions (Fig. 4). Several lysine-containing ELP block copolymers have been produced by systemically incorporating lysine residues into the guest position of elastin modules. The reactivity of γ-amino groups toward chemical linkers such as glutaraldehydes,28 phosphonium compounds,29-31 genipin,32 and tris-succinimidyl aminotriacetate33 leads to the formation of interconnected hydrogel networks. Chemical derivatization of γ-amino groups into cross-linkable functionalities to formulate hydrazone-linked hydrogels has been achieved.34 Transglutaminase-catalyzed cross-linking allows cell encapsulation to occur under mild reaction conditions.35-37 Substituting the guest amino acid with cysteine in the repeating pentapeptide facilitates hydrogelation through the formation of H2O2-mediated disulphide bonds.38 Hydrogels of ELPs exhibit typical characteristics of covalently cross-linked networks as the elastic module is larger than the viscous module and is independent of the frequency in dynamic oscillatory sweep tests. The swelling and mechanical behaviors of chemically cross-linked ELP hydrogels are dependent on protein concentration, molecular weight and lysine or cysteine content of the monomeric sequence. Tailored ELP hydrogels with modular bioactive peptides and matrix stiffness regions have been utilized to understand the systemic or independent effects of each module on various types of cells, including dorsal root ganglia,31 human umbilical vein endothelial cells (HUVECs),37 and embryonic39 or adult stem cells.40 Considering the successful cartilage matrix synthesis, cardiomyocyte development and increased HUVEC proliferation, this author expects that hydrogel engineering would be more extensively used to formulate 3D microenvironments for musculoskeletal and vascular tissues.
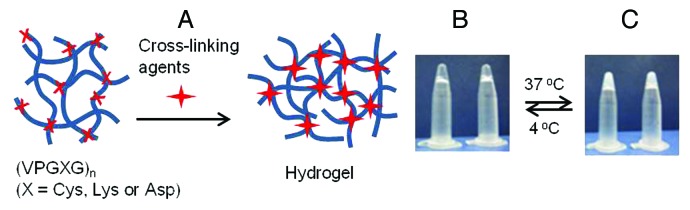
Figure 4. Schematic of hydrogel formation via intermolecular cross-linking (A) and photographs of thermoresponsive hydrogels at 4°C (B) and 37°C (C).
Conclusions and Future Prospects
Over the past 30 y, many cell culture experiments have included first-generation ELPs that are composed only of VPGXG repeats or functionalized with cell penetration sequences, apoptotic peptides or simply with cell adhesive integrin ligands. The results of the tests clearly proved the potential of ELPs as smart biomaterials that are suitable for targeted drug delivery via locally-induced hyperthermia and modifying 2D and 3D in vitro systems. However, in comparison with the study of chemotherapeutics delivery, the number of cases reporting the efficacy of ELP matrix on the repair of the injured tissues within ex vivo or in vivo models is very low.41 As described above, the effects of ELP-based scaffolds have been tested mainly for the replacement of load-bearing tissues and organs such as cartilage and intervertebral disc and are being evaluated in vascular37 or cardiac21 tissue engineering. More biological investigations with animal models are required before using ELPs in clinical practice. Along with the efforts to recover the functionality of damaged tissue, creation of new bioactive ELPs will be continued to help resolve the critical obstacles in tissue repair. In particular, stem cell transplantation has emerged as an important cell-based therapy in regenerative medicine, specially, for the treatment of neurodegenerative diseases,42,43 and thus, the use of first-generation ELPs in neural engineering is expected to increase. Therefore, researchers would likely focus their attention on designing new fusion forms of ELPs that are tailored to have biofunctional modules similar to those found in signaling proteins or growth factors that are known to influence the survival and differentiation of neuronal stem and progenitor cells. In such cases, the next-generation ELPs can provide more versatile biocompatible candidates applicable to create permissive microenvironments for stem cell transplantation into the brain tissues.
Acknowledgments
This study was supported by the Biodefense Program Fund (Project No. 13-NB-04) to WBJ from the Ministry of Education, Science and Technology of the Republic of Korea.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/24158
References
- 1.Serrano V, Liu W, Franzen S. An infrared spectroscopic study of the conformational transition of elastin-like polypeptides. Biophys J. 2007;93:2429–35. doi: 10.1529/biophysj.106.100594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rodríguez-Cabello JC, Pierna M, Fernández-Colino A, García-Arévalo C, Arias FJ. Recombinamers: combining molecular complexity with diverse bioactivities for advanced biomedical and biotechnological applications. Adv Biochem Eng Biotechnol. 2011;125:145–79. doi: 10.1007/10_2010_94. [DOI] [PubMed] [Google Scholar]
- 3.Liu W, MacKay JA, Dreher MR, Chen M, McDaniel JR, Simnick AJ, et al. Injectable intratumoral depot of thermally responsive polypeptide-radionuclide conjugates delays tumor progression in a mouse model. J Control Release. 2010;144:2–9. doi: 10.1016/j.jconrel.2010.01.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bidwell GL, 3rd, Davis AN, Fokt I, Priebe W, Raucher D. A thermally targeted elastin-like polypeptide-doxorubicin conjugate overcomes drug resistance. Invest New Drugs. 2007;25:313–26. doi: 10.1007/s10637-007-9053-8. [DOI] [PubMed] [Google Scholar]
- 5.Chen Y, Youn P, Furgeson DY. Thermo-targeted drug delivery of geldanamycin to hyperthermic tumor margins with diblock elastin-based biopolymers. J Control Release. 2011;155:175–83. doi: 10.1016/j.jconrel.2011.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bidwell GL, 3rd, Perkins E, Raucher D. A thermally targeted c-Myc inhibitory polypeptide inhibits breast tumor growth. Cancer Lett. 2012;319:136–43. doi: 10.1016/j.canlet.2011.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shamji MF, Chen J, Friedman AH, Richardson WJ, Chilkoti A, Setton LA. Synthesis and characterization of a thermally-responsive tumor necrosis factor antagonist. J Control Release. 2008;129:179–86. doi: 10.1016/j.jconrel.2008.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Simnick AJ, Amiram M, Liu W, Hanna G, Dewhirst MW, Kontos CD, et al. In vivo tumor targeting by a NGR-decorated micelle of a recombinant diblock copolypeptide. J Control Release. 2011;155:144–51. doi: 10.1016/j.jconrel.2011.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun G, Hsueh PY, Janib SM, Hamm-Alvarez S, Andrew MacKay J. Design and cellular internalization of genetically engineered polypeptide nanoparticles displaying adenovirus knob domain. J Control Release. 2011;155:218–26. doi: 10.1016/j.jconrel.2011.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen TH, Bae Y, Furgeson DY. Intelligent biosynthetic nanobiomaterials (IBNs) for hyperthermic gene delivery. Pharm Res. 2008;25:683–91. doi: 10.1007/s11095-007-9382-5. [DOI] [PubMed] [Google Scholar]
- 11.Liu Y, Jia Z, Li L, Chen F. A genetically synthetic protein-based cationic polymer for siRNA delivery. Med Hypotheses. 2011;76:239–40. doi: 10.1016/j.mehy.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 12.Jeon WB, Park BH, Wei J, Park RW. Stimulation of fibroblasts and neuroblasts on a biomimetic extracellular matrix consisting of tandem repeats of the elastic VGVPG domain and RGD motif. J Biomed Mater Res A. 2011;97:152–7. doi: 10.1002/jbm.a.33041. [DOI] [PubMed] [Google Scholar]
- 13.Jeon WB, Park BH, Choi SK, Lee KM, Park JK. Functional enhancement of neuronal cell behaviors and differentiation by elastin-mimetic recombinant protein presenting Arg-Gly-Asp peptides. BMC Biotechnol. 2012;12:61. doi: 10.1186/1472-6750-12-61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Straley KS, Heilshorn SC. Design and adsorption of modular engineered proteins to prepare customized, neuron-compatible coatings. Front Neuroeng. 2009;2 doi: 10.3389/neuro.16.009.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ciofani G, Genchi GG, Liakos I, Athanassiou A, Mattoli V, Bandiera A. Human recombinant elastin-like protein coatings for muscle cell proliferation and differentiation. Acta Biomater. 2012;9:5111–21. doi: 10.1016/j.actbio.2012.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Costa RR, Custódio CA, Arias FJ, Rodríguez-Cabello JC, Mano JF. Layer-by-layer assembly of chitosan and recombinant biopolymers into biomimetic coatings with multiple stimuli-responsive properties. Small. 2011;7:2640–9. doi: 10.1002/smll.201100875. [DOI] [PubMed] [Google Scholar]
- 17.Janorkar AV, Rajagopalan P, Yarmush ML, Megeed Z. The use of elastin-like polypeptide-polyelectrolyte complexes to control hepatocyte morphology and function in vitro. Biomaterials. 2008;29:625–32. doi: 10.1016/j.biomaterials.2007.10.022. [DOI] [PubMed] [Google Scholar]
- 18.Janorkar AV, Harris LM, Murphey BS, Sowell BL. Use of three-dimensional spheroids of hepatocyte-derived reporter cells to study the effects of intracellular fat accumulation and subsequent cytokine exposure. Biotechnol Bioeng. 2011;108:1171–80. doi: 10.1002/bit.23025. [DOI] [PubMed] [Google Scholar]
- 19.Jung GS, Lee KM, Park JK, Choi SK, Jeon WB. Morphogenetic and neuronal characterization of human neuroblastoma multicellular spheroids cultured under undifferentiated and all-trans-retinoic acid-differentiated conditions. BMB Reports. 2013 doi: 10.5483/BMBRep.2013.46.5.196. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee KM, Jung GS, Park JK, Choi SK, Jeon WB. Effects of Arg-Gly-Asp-modified elastin-like polypeptide on pseudoislet formation via up-regulation of cell adhesion molecules and extracellular matrix proteins. Acta Biomater. 2013;9:5600–8. doi: 10.1016/j.actbio.2012.10.036. [DOI] [PubMed] [Google Scholar]
- 21.Minato A, Ise H, Goto M, Akaike T. Cardiac differentiation of embryonic stem cells by substrate immobilization of insulin-like growth factor binding protein 4 with elastin-like polypeptides. Biomaterials. 2012;33:515–23. doi: 10.1016/j.biomaterials.2011.09.070. [DOI] [PubMed] [Google Scholar]
- 22.Raphel J, Parisi-Amon A, Heilshorn S. Photoreactive elastin-like proteins for use as versatile bioactive materials and surface coatings. J Mater Chem. 2012;22:19429–37. doi: 10.1039/c2jm31768k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Srivastava GK, Martín L, Singh AK, Fernandez-Bueno I, Gayoso MJ, Garcia-Gutierrez MT, et al. Elastin-like recombinamers as substrates for retinal pigment epithelial cell growth. J Biomed Mater Res A. 2011;97:243–50. doi: 10.1002/jbm.a.33050. [DOI] [PubMed] [Google Scholar]
- 24.Swierczewska M, Hajicharalambous CS, Janorkar AV, Megeed Z, Yarmush ML, Rajagopalan P. Cellular response to nanoscale elastin-like polypeptide polyelectrolyte multilayers. Acta Biomater. 2008;4:827–37. doi: 10.1016/j.actbio.2007.11.004. [DOI] [PubMed] [Google Scholar]
- 25.Barbosa JS, Costa RR, Testera AM, Alonso M, Rodríguez-Cabello JC, Mano JF. Multi-Layered Films Containing a Biomimetic Stimuli-Responsive Recombinant Protein. Nanoscale Res Lett. 2009;4:1247–53. doi: 10.1007/s11671-009-9388-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Costa RR, Custódio CA, Arias FJ, Rodríguez-Cabello JC, Mano JF. Layer-by-layer assembly of chitosan and recombinant biopolymers into biomimetic coatings with multiple stimuli-responsive properties. Small. 2011;7:2640–9. doi: 10.1002/smll.201100875. [DOI] [PubMed] [Google Scholar]
- 27.Kolbe A, del Mercato LL, Abbasi AZ, Rivera Gil P, Gorzini SJ, Huibers WH, et al. De novo design of supercharged, unfolded protein polymers, and their assembly into supramolecular aggregates. Macromol Rapid Commun. 2011;32:186–90. doi: 10.1002/marc.201000491. [DOI] [PubMed] [Google Scholar]
- 28.Girotti A, Reguera J, Rodríguez-Cabello JC, Arias FJ, Alonso M, Matestera A. Design and bioproduction of a recombinant multi(bio)functional elastin-like protein polymer containing cell adhesion sequences for tissue engineering purposes. J Mater Sci Mater Med. 2004;15:479–84. doi: 10.1023/B:JMSM.0000021124.58688.7a. [DOI] [PubMed] [Google Scholar]
- 29.Lim DW, Nettles DL, Setton LA, Chilkoti A. In situ cross-linking of elastin-like polypeptide block copolymers for tissue repair. Biomacromolecules. 2008;9:222–30. doi: 10.1021/bm7007982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nettles DL, Kitaoka K, Hanson NA, Flahiff CM, Mata BA, Hsu EW, et al. In situ crosslinking elastin-like polypeptide gels for application to articular cartilage repair in a goat osteochondral defect model. Tissue Eng Part A. 2008;14:1133–40. doi: 10.1089/ten.tea.2007.0245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lampe KJ, Antaris AL, Heilshorn SC. Design of three-dimensional engineered protein hydrogels for tailored control of neurite growth. Acta Biomater. 2013;9:5590–9. doi: 10.1016/j.actbio.2012.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hrabchak C, Rouleau J, Moss I, Woodhouse K, Akens M, Bellingham C, et al. Assessment of biocompatibility and initial evaluation of genipin cross-linked elastin-like polypeptides in the treatment of an osteochondral knee defect in rabbits. Acta Biomater. 2010;6:2108–15. doi: 10.1016/j.actbio.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 33.Trabbic-Carlson K, Setton LA, Chilkoti A. Swelling and mechanical behaviors of chemically cross-linked hydrogels of elastin-like polypeptides. Biomacromolecules. 2003;4:572–80. doi: 10.1021/bm025671z. [DOI] [PubMed] [Google Scholar]
- 34.Krishna UM, Martinez AW, Caves JM, Chaikof EL. Hydrazone self-crosslinking of multiphase elastin-like block copolymer networks. Acta Biomater. 2012;8:988–97. doi: 10.1016/j.actbio.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McHale MK, Setton LA, Chilkoti A. Synthesis and in vitro evaluation of enzymatically cross-linked elastin-like polypeptide gels for cartilaginous tissue repair. Tissue Eng. 2005;11:1768–79. doi: 10.1089/ten.2005.11.1768. [DOI] [PubMed] [Google Scholar]
- 36.Bandiera A. Transglutaminase-catalyzed preparation of human elastin-like polypeptide-based three-dimensional matrices for cell encapsulation. Enzyme Microb Technol. 2011;49:347–52. doi: 10.1016/j.enzmictec.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 37.Bozzini S, Giuliano L, Altomare L, Petrini P, Bandiera A, Conconi MT, et al. Enzymatic cross-linking of human recombinant elastin (HELP) as biomimetic approach in vascular tissue engineering. J Mater Sci Mater Med. 2011;22:2641–50. doi: 10.1007/s10856-011-4451-z. [DOI] [PubMed] [Google Scholar]
- 38.Xu D, Asai D, Chilkoti A, Craig SL, et al. Rheological properties of cysteine-containing elastin-like polypeptide solutions and hydrogels. Biomacromolecules. 2012;13:2315–21. doi: 10.1021/bm300760s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chung C, Anderson E, Pera RR, Pruitt BL, Heilshorn SC. Hydrogel crosslinking density regulates temporal contractility of human embryonic stem cell-derived cardiomyocytes in 3D cultures. Soft Matter. 2012;8:10141–8. doi: 10.1039/c2sm26082d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Betre H, Ong SR, Guilak F, Chilkoti A, Fermor B, Setton LA. Chondrocytic differentiation of human adipose-derived adult stem cells in elastin-like polypeptide. Biomaterials. 2006;27:91–9. doi: 10.1016/j.biomaterials.2005.05.071. [DOI] [PubMed] [Google Scholar]
- 41.Nettles DL, Chilkoti A, Setton LA. Applications of elastin-like polypeptides in tissue engineering. Adv Drug Deliv Rev. 2010;62:1479–85. doi: 10.1016/j.addr.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Goldman S. Stem and progenitor cell-based therapy of the human central nervous system. Nat Biotechnol. 2005;23:862–71. doi: 10.1038/nbt1119. [DOI] [PubMed] [Google Scholar]
- 43.Bell JH, Haycock JW. Next generation nerve guides: materials, fabrication, growth factors, and cell delivery. Tissue Eng Part B Rev. 2012;18:116–28. doi: 10.1089/ten.teb.2011.0498. [DOI] [PubMed] [Google Scholar]