Abstract
Enteric infections account for high morbidity and mortality and are considered to be the fifth leading cause of death at all ages worldwide. Seventy percent of all enteric infections are foodborne. Thus significant efforts have been directed toward the detection, control and prevention of foodborne diseases. Many antimicrobials including antibiotics have been used for their control and prevention. However, probiotics offer a potential alternative intervention strategy owing to their general health beneficial properties and inhibitory effects against foodborne pathogens. Often, antimicrobial probiotic action is non-specific and non-discriminatory or may be ineffective. In such cases, bioengineered probiotics expressing foreign gene products to achieve specific function is highly desirable. In this review we summarize the strategic development of recombinant bioengineered probiotics to control enteric infections, and to examine how scientific advancements in the human microbiome and their immunomodulatory effects help develop such novel and safe bioengineered probiotics.
Keywords: enteric infections, foodborne diseases, probiotics, bioengineered probiotics, recombinant probiotics, lactic acid bacteria
Introduction
Enteric infections account for about 1.5 billion episodes of diarrheal diseases with 2.2 million deaths (mostly children) annually and are the fifth leading cause of death at all ages worldwide.1 Children under 5 y of age are most susceptible and the disease burden is the greatest in developing countries.2 Consequences of childhood enteric infections are impaired physical growth and cognitive development.3 Enteric infections may be caused by bacterial, viral, parasitic or fungal agents, which disrupt intestinal function with or without causing dehydrating diarrhea. Seventy percent of all microbial diarrheal diseases are foodborne4 and foodborne illnesses are a serious public health concern (Table 1). The global burden of foodborne illness is currently unknown; however, the World Health Organization (WHO) reported that 1.8 million people died from diarrheal diseases in 2005, largely due to contaminated food and water.5,6 In the US, the Centers for Diseases Control and Prevention (CDC) estimates that each year there are about 48 million cases of foodborne infections with 128,000 hospitalizations and 3000 deaths.7 There are over 200 known microbial, chemical or physical agents that can cause foodborne illness.6 CDC estimates that of all the foodborne infections, 44% of the hospitalizations and deaths are attributed to 31 known pathogens.7 In light of this serious public health crisis, efforts have been directed toward the detection, control and prevention of food-borne pathogens and diseases. It is estimated that a reduction in foodborne illness by 10% would keep about 5 million Americans from getting sick each year.7 With increasing trend in consumer preference for safe and wholesome food, probiotics offer an effective and alternative intervention strategy to control foodborne illnesses. Among the microbial etiologies responsible for enteric infections, WHO has prioritized around 22 infectious agents for surveillance based on their higher prevalence, morbidity and mortality. These include Brucella spp, Campylobacter spp., Clostridium botulinum, Enteroaggregative E. coli (EAggEC), Enteropathogenic E. coli (EPEC), Enterotoxigenic E. coli (ETEC), Shiga-toxin producing E. coli (STEC), Helicobacter pylori, Hepatitis A virus, Hepatitis E virus, Listeria monocytogenes, Mycobacterium bovis, Vibrio cholerae O1/O139, Non-cholera Vibrio spp, Norovirus, Rotavirus, Prions, Salmonella spp. (non-typhoidal), Salmonella enterica serovar Typhi, Shigella spp., and Yersinia spp, and toxins from Staphylococcus aureus, Clostridium perfringens and Bacillus cereus.
Table 1. Diseases caused by foodborne pathogens.
| Disease or clinical symptoms | Pathogens/toxins involved |
|---|---|
| Vomiting, diarrhea, dysentery | Staphylococcus, Bacillus, Cronobacter, Salmonella, Shigella, Vibrio, Norovirus, rotavirus, Entamoeba; Cryptosporidium; Cyclospora; Giardia; Isospora; Taenia |
| Arthritis (reactive arthritis, Reiter syndrome, rheumatoid arthritis) | Campylobacter, Salmonella, Shigella, Yersinia |
| Hemorrhagic uremic syndrome (HUS) Kidney disease | Shiga-toxin producing E. coli (STEC); Shigella spa. |
| Hepatitis and jaundice | Hepatitis A virus (HAV), hepatitis E virus (HEV) |
| Guillain Barre syndrome (GBS) | Campylobacter |
| CNS/meningitis/encephalitis | Listeria, Bovine spongiform encephalopathy (BSE) |
| Miscarriage, stillbirth, neonatal infection | Listeria, Toxoplasma |
| Paralysis | Clostridium botulinum, seafood toxin, Campylobacter |
| Malignancies and auto-immune diseases | Mycotoxin |
| Allergic response | Seafood toxin |
Various strategies have been employed to control enteric pathogens in foods, food producing animals and humans. Antibiotics are used in meat animal production as prophylactic to control disease and improve growth rate and efficiency.8 However, increasing concerns about antibiotic resistance has led to research efforts to use naturally occurring antimicrobials as alternatives. Antimicrobials may include organic acids, essential oils and plant extracts, bacteriocins,9 probiotics10 and bacteriophages.11-13 Organic acids (acetic, lactic and citric acids) are commonly used to rinse animal carcasses, fruits and vegetables.14 To enhance antimicrobial efficacies, acids are also used in combination with oxidizing agents such as hydrogen peroxide. In addition, thermal (ionizing radiations and heating) and non-thermal treatments such as high hydrostatic pressure, high-intensity pulsed electric fields, oscillating magnetic fields, intense light pulse, photosensitization or a combination of above (hurdle approach) are also effective.15
Probiotics
The word “probiotic” is derived from the Greek word meaning “for life.” Probiotics are live nonpathogenic microorganisms that are administered to maintain and improve intestinal microbial balance and protect host from infective agents. Physiologically, these microbes are endowed with certain characteristics that enable them to survive in the gut environment and colonize mucosal surfaces. The rationale for the use of probiotics in the prevention of enteric infections and treatment of diarrhea are associated with three major factors: (1) maintenance of the epithelial gut barrier, (2) modulation of innate and acquired immunity, and (3) inhibition of pathogen growth by producing bacteriocins, hydrogen peroxide and other antimicrobials.16 Besides, probiotics also help prevent chronic enteric infection associated with stunted growth, abnormal low body mass indices and impairment of cognitive function in children.16
The use of probiotics, prebiotics and synbiotics (combination of prebiotics and probiotics) has also gained increased interest in recent years. The use of microflora to reduce pathogen load in the gut is termed as a probiotic strategy.17 Probiotic techniques involve the introduction of a normal microbial population into the gut to provide a nutrient (prebiotic) that is limiting and allows the growth of a specific subset of the gut microflora. The goal of this approach is to fill all the niches available in the gut so as to exclude the establishment of pathogenic microbes.18,19 Due to increased concern about the emergence of antibiotic resistance, use of probiotics provides an effective alternative to combat foodborne illnesses.20
Beneficial attributes of probiotics are broad and well documented (Table 2). These include lactose metabolism, improved digestion, increased nutritional value, production of antimicrobial factors, antimycotic effects, anti-carcinogenic properties, immunologic enhancement, production of short-chain fatty acids, anti-atherogenic and cholesterol-lowering attributes, regulatory role in allergy, protection against vaginal or urinary tract infections, maintenance of epithelial integrity and barrier, stimulation of repair mechanism in cells, and maintenance and reestablishment of a well-balanced indigenous intestinal, respiratory and urogenital microbial communities.10,21-24
Table 2. Health benefits of probiotic bacteria and their proposed mechanisms.
| Health benefits | Proposed mechanism |
|---|---|
| Resistance to enteric pathogens | Antagonism |
| Increased antibody production | |
| Colonization resistance | |
| Limiting access of enteric pathogens (pH, bacteriocins, antimicrobial peptides, lactic acid production) | |
| Aid in lactose metabolism | Bacterial lactase hydrolyzes lactose in the small intestine |
| Small bowel bacterial overgrowth | Decrease toxic metabolite production |
| Normalize small bowel flora | |
| Antibacterial characteristics | |
| Immune system modulation | Strengthening of non-specific and antigen-specific defense |
| Regulate/influence Th1/Th2 cell activation | |
| Production of anti-inflammatory cytokines | |
| Anticolon cancer effect | Antimutagenic and anticarcinogenic activity |
| Detoxification of carcinogenic metabolites | |
| Stimulation of immune function | |
| Decreased detoxification/excretion of toxic microbial metabolites | Increased bifidobacterial cell counts and shift from a preferable protein-to carbohydrate-metabolizing microbial community |
| Anti-Allergic activity (eczema or atopic dermatitis, asthma) | Prevention of antigen translocation into blood stream |
| Prevent excessive immunologic responses to increased amount of antigen | |
| Blood lipids, heart disease | Assimilation of cholestrol by bacterial cell |
| Alteration in the activity of bile salt hydrolase (BSH) | |
| Urogenital infections | Adhesion to urinary and vaginal tract cells |
| Competitive exclusion | |
| Necrotizing enterocolitis | Decrease in TLRs and signaling molecules and increase in negative regulations |
| Reduction in IL-8 response | |
| Rotavirus gastroenteritis | Increased IgA response to the virus |
| Inflammatory bowel disease | Enhancement of mucosal barrier function |
| Crohn disease | Reduction in proinflammatory cytokines production |
Adapted from Nagpal et al.23
Prevention and Control of Enteric Infections Using Wild Type Probiotics
Enteric viral infections
Probiotics have been used to control viral infections. Rotavirus is responsible for 20–25% of the diarrheal diseases worldwide. Gnotobiotic pigs fed with Lactobacillus acidophilus and L. reuteri enhanced IFNγ and IL-4 levels in serum and decreased rotavirus infection.25 Probiotics are also effective against Norovirus, which is responsible for 58% of foodborne illnesses.26,27 Probiotic fermented milk containing L. casei Shirota strain was effective in controlling norovirus gastroenteritis in a health service facility.28 A controlled double-blind study using a probiotic formulation (VSL#3) was shown to significantly reduce stool frequency and requirement for oral rehydration in children.29
Bacterial enteric infection
Among enteric pathogens that cause diarrhea, Campylobacter jejuni is responsible for about 400 million cases every year in both industrialized and developing countries.30 Several probiotic strains have been evaluated for their efficacy in controlling Campylobacter infection. Lactobacilli and Bifidobacteria were shown to enhance colonization resistance in mice that were infected by C. jejuni or Salmonella. Probiotics also increased proliferation of lymphocytes against Salmonella antigens and reversed pathogen-induced immunosuppressive activity.31 Synbiotics consisting of prebiotic galacto-oligosaccharide and probiotic Bifidobacterium longum significantly reduced C. jejuni load in poultry feces.32 Vibrio cholera causes acute dehydrating watery diarrhea with 1.8 million cases and 27,000 deaths annually.2 Experimental administration of L. acidophilus BKM B-2020 orally in mice and suckling rabbits prior to infection prevented cholera. Probiotic L. plantarum AS1 attached efficiently to cultured cell lines (HT-29) and reduced V. parahemolyticus attachment by competitive exclusion and displacement.33 Probiotics are also found to be effective against diarrhea causing E. coli including STEC and ETEC. L. acidophilus, L. casei, L. fermentum, L. plantarum and Enterococcus faecium significantly reduced E. coli O157:H7 shedding by sheep.34 Bifidobacteria caused reduced Shiga toxin production by STEC in mice and protected against E. coli O157:H7 infection.35 Nonpathogenic probiotic E. coli strains 1307 and Nissle also inhibited STEC growth and Shiga toxin production.36 Furthermore, pre-exposure to L. paracasei resulted in an upregulation of dendritic cells, activation of helper T cells and antibody production, and downregulation of proinflammatory cytokines resulting in enhanced intestinal integrity and protection against enteric infection.37 Probiotics have been widely tested to control S. enterica colonization and infection. Administration of one or several probiotic strains in broiler chicks inhibited Salmonella contamination.38 A commercial probiotic cocktail significantly reduced Salmonella counts in the tonsils and ceca of chickens and poults.39 Furthermore, administration of reuterin producing L. reutri strain significantly reduced Salmonella populations and increased the survival rate in chicks.40 In vivo study using a mouse model demonstrated that continued administration of L. casei CRL diminished Salmonella counts in the intestine and extraintestinal dissemination.41 L. casei Shirota strain also protected mice against lethal infection with multi-drug resistant S. Typhimurium DT104.42 Besides the antimicrobial effects, probiotics also increased the performance and feed conversion in chickens and turkey poults. Probiotics were also effective against other enteric pathogens such as Shigella sonnei, Staphylococcus aureus, Enterococcus faecalis, Proteus mirabilis and Pseudomonas aeruginosa.43 A bacteriocin (Microcin S) producing probiotic Escherichia coli G3/10 also suppressed EPEC adherence and pathogenesis.44
Recombinant Bioengineered Probiotics
As discussed above probiotics can be effective in the prevention and treatment of intestinal diseases. However, probiotic action is non-specific and non-discriminatory or ineffective in certain hosts.45 This is in part due to broad mode of action and strain variability (Table 2). Probiotics differ from one another, therefore, the beneficial attributes of one strain or a cocktail of strains may not be reproducible and may vary from person to person.46 Additionally, the probiotic strain, dose, route of administration, and the formulation of probiotic preparation can also affect the efficacy of a probiotic.47 Furthermore, the manufacturing process and probiotic delivery system have been shown to modify exopolysaccharide production by the probiotics and thereby modify their efficacy.48,49 Recent studies on the gut microbiome diversity have revealed that the variability in the indigenous flora among different populations may also affect probiotic efficacy.50 These limitations reinforce the need for novel and innovative approaches to design and create genetically modified probiotic strains to exclusively target a specific pathogen or toxin to be used either as a vaccine or for drug delivery.51,52
Over the last decade recombinant probiotics have been generated for mucosal delivery of therapeutic and prophylactic molecules including DNA, peptides, single-chain variable fragments, cytokines, enzymes and allergens.53,54 The major advantages of probiotic bacteria as delivery system are their (1) ability to colonize mucosal surface, (2) tolerance to gastric acid and bile salts enabling survival and transit through the gastrointestinal tract (GIT) and (3) sustained colonization and prolonged protection against pathogen.53,55 Furthermore, oral recombinant probiotics offer several advantages: direct delivery of active molecule to the mucosal surface without the need for bio-separation of the active molecules, increased shelf-life and stability, low delivery costs, and ease of technology transfer following prototype development. This led to the concept of ‘biodrug’ that is based on the oral administration of live recombinant microorganisms for the prevention and treatment of various diseases.56
In order to create therapeutically effective bioengineered recombinant probiotics, certain physiologic attributes are essential: (1) tolerance to stressors encountered during product manufacturing and storage, and during oral delivery, (2) strong mucosal colonization, (3) expression of target antigen under the gastrointestinal environment and (4) potent antipathogenic action.
Bioengineering of Probiotics to Improve Stress Tolerance
Probiotics encounter stress during manufacturing, storage and passage through the host GIT, namely temperature, acidity, salts and water activity.57 Physiologically, accumulation of compatible solutes helps stabilize protein function at low temperatures and prevent plasmolysis under low water activity. To improve stress tolerance in probiotic strains, the betaine transporter gene (betL) from Listeria monocytogenes was cloned into Lactobacillus salivarius under the control of the nisin inducible promoter.58 Thus accumulation of betaine in recombinant L. salivarius enabled it to be osmotolerant (7% NaCl), and cryo- and baro-tolerant. Similarly, cloning of the trehalose synthesis gene (ostAB) from E. coli into Lactococcus lactis protected recombinant bacteria from freeze-drying, bile toxicity and resistance to gastric acid.59 Furthermore, cloning of betL into Bifidobacterium breve UCC2003 significantly improved its survival in gastric juice thus improving its therapeutic attributes.60
Antimicrobial Action of Bioengineered Probiotics
Receptor mimicry system and toxin neutralization
To achieve pathogen/toxin-specific activity, several strategies were employed to create bioengineered probiotics. Paton and colleagues61 cloned and expressed toxin-specific host cell receptor on probiotic E. coli thus creating a competitive environment for toxin binding to host cells. They cloned glycosyltransferase genes from either Nisseria mennigitidis or C. jejuni on the surface of nonpathogenic probiotic E. coli to express chimeric lipopolysaccharide that mimics host cell receptor (ganglioside) for cholera toxin or ETEC heat labile toxin, LT. During infection enterotoxins are sequestered by the probiotic E. coli thus protecting host against diarrheal infection. In another study, L. reuteri was engineered to express ETEC heat stable (ST) and heat labile (LT) enterotoxins under the nisin inducible promoter. This recombinant probiotic successfully bound to the enterotoxins and prevented enterotoxicity in a mouse model. Furthermore, orally immunized mice with the toxin secreting recombinant L. reuteri increased serum IgG and mucosal IgA levels and protected animals from ETEC infection.62
Prevention of colonization
Cloning and expression of adhesins, toxins or secretory systems of pathogens may serve as potential targets for the development of therapeutics to prevent infection.63 Several strategies were employed to enhance probiotic adhesion to mucosal surface using gene products of target pathogen to create a competitive environment for pathogen colonization. Probiotics expressing adhesion factor, LAP (Listeria adhesion protein) from L. monocytogenes was able to exclude pathogen colonization and prevented pathogen induced cell damage.64 LAP is an adhesion factor in L. monocytogenes that interacts with the host cell receptor, heat shock protein 60 (Hsp60)65-67 and promotes listerial adhesion and transepithelial translocation during intestinal phase of infection.68,69 Pre-exposure of intestinal monolayers to the recombinant probiotic Lactobacillus paracasei expressing LAP followed by L. monocytogenes infection led to a reduction in adhesion, invasion and transepithelial translocation by 44, 45 and 46%, respectively64 (Fig. 1). The recombinant probiotic also protected the epithelial monolayers from L. monocytogenes mediated cytotoxicity and tight junction compromise.
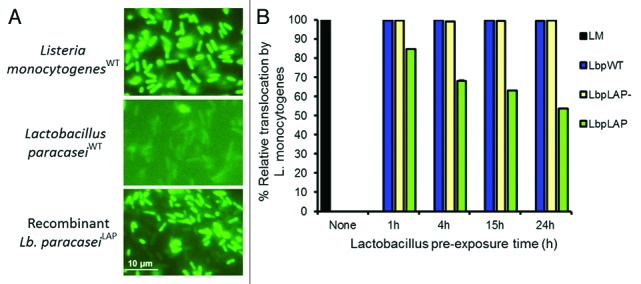
Figure 1. Inhibition of Listeria monocytogenes transepithelial translocation through epithelial barrier by bioengineered probiotic, Lactobacillus paracasei expressing Listeria adhesion protein (LAP). (A) Immunofluorescence staining of LAP expression by Listeria monocytogenesWT and recombinant Lb. paracaseiLAP. (B) Recombinant Lb. paracaseiLAP (LbpLAP) showing about 46% reduction in L. monocytogenes translocation through epithelial barrier while, Lb. paracaseiWT (LbpWT) or Lb. paracasei containing empty vector (LbpLAP-) had no effect. Figures adapted from Koo et al.64
Similarly, S. enterica attachment was inhibited by using recombinant probiotic bacteria. Lactococcus lactis expressing flagellin of a probiotic strain of Bacillus cereus CH, adhered strongly to mucin-coated polystyrene plates in an in vitro experiment and competitively inhibited the adhesion of pathogenic E. coli and S. enterica to the same molecule.70
A recombinant L. acidophilus strain carrying the K99 fimbriae from ETEC was able to reduce the attachment of ETEC to porcine intestinal brush border in a dose dependent manner.71 Similarly, L. casei was bioengineered to express ETEC adhesins K99 or K8872 and the efficacy of the recombinant probiotic to protect host from ETEC infection was verified in a mouse model. Oral vaccination of mice with the recombinant strain resulted in high levels of mucosal IgA in bronchioalveolar lavage and intestinal fluids and systemic IgG response. The recombinant probiotic protected more than 80% of the vaccinated mice after challenge with a lethal dose of ETEC.72 Likewise, L. casei expressing adhesion protein (intimin of EPEC) induced systemic and mucosal antibodies in mice and the antibodies inhibited the adhesion of EPEC in an in vitro epithelial cell culture model.73 Employing a similar approach, a recombinant L. casei strain expressing S. Enteritidis flagellar antigen FliC induced antigen specific protective immune response against S. Enteritidis in a mouse model.74
Similar strategies have also been adopted for viral pathogens. L. jensenii was engineered to secrete simian immunodeficiency virus (SIV) specific cyanovirin-N and the recombinant probiotic strain reduced the SIV infection by 62.9% in the Chinese macaque model.75,76
Regulation of virulence gene expression
Pathogenic bacteria have the ability to control the expression of virulence genes by sensing signals (termed quorum sensing) from their own species, other bacteria or their environment. Since the quorum sensing system senses population density, mediates colony-wide coordinated behavior, and controls virulence pathways,77 interruption of quorum sensing pathway may serve as a viable option for disease prevention. V. cholera release cholera autoinducer-1 (CAI-1) and autoinducer-2 (AI-2) that accumulate when the population density increases at which point bacteria produce virulence factors. An AI-2 producing E. coli Nissle strain was engineered to co-express CAI-1, which suppressed virulence gene expression in V. cholera leading to its reduced lethality on infant mouse.78
Production of antimicrobial factors
Some probiotics produce several antimicrobial compounds and peptides as a defense mechanism against pathogens. Engineering of probiotics to detect pathogen signals for timed production of antimicrobials would be a novel approach. Saeidi et al.79 engineered a commensal E. coli to detect signals from the pathogen for the production of bacteriocin.80 Pseudomonas aeruginosa quorum sensing system (LasI/LasR) controls virulence gene expression. LasI produces homoserine lactone that activates LasR and leads to virulence gene expression. A bacteriocin producing probiotic E. coli strain was engineered to express LasR (to detect homoserine lactone) under the control of the luxR promoter and E7 lysis protein to aid in release of the bacteriocin. Co-culture of P. aeruginosa and recombinant E. coli led to a decrease in P. aeruginosa growth and biofilm formation by 99% and 90%, respectively.
L. casei engineered to express human lactoferrin exhibited antimicrobial activity in the gastrointestinal tract against enteric pathogens both in vitro and in vivo.81 This recombinant strain also protected from pathogen-induced tissue injury. Bioengineered probiotics were also able to control pathogen transmission by insects. Insect synbiotics residing in the midgut of insect (reduviid bug) was engineered to express antimicrobial peptide, cercopin A and reduced the carriage of Trypanosoma cruzi, a causative agent for Chagas disease.82
Immunomodulation and cytoprotection
The most effective strategies to prevent enteric pathogen colonization in a host are to develop strains that can provide protection on the mucosal surface. It would also be easier to combat an enteric infection by blocking the infection rather than trying to eliminate the organism after the infection has already been established. Live mucosal vaccines are viable options in the prophylaxis of enteric infections since they target mucosal surfaces and elicit strong localized immune response. Recombinant probiotic bacteria would serve as ideal vectors because of their inherent ability to bind to mucosal surfaces thereby promoting effective contact between the antigen and the immune system. Additionally, colonization of the gut by live probiotic cells would enable continued production of the immunogenic molecule to stimulate humoral and cellular immune responses.55
Several studies have reported the use of attenuated pathogens as vaccines; however, a risk of virulence reversion in the attenuated strains especially in immunocompromised individuals exists. This can be overcome by the use of recombinant probiotics strains that can efficiently deliver the immunogenic molecule to the target mucosal surface.83 Such recombinant probiotics have been engineered as vaccine delivery vehicles against Yersinia pseudotuberculosis,84 S. typhimurium39,85 and Streptococcus pneumoniae infection.86 Similar recombinant vaccine was developed using L. acidophilus engineered to express protective antigen (PA) of Bacillus anthracis to activate dendritic cell to protect host against anthrax.87 Likewise, expression of PA in L. gasseri also provided 100% protection against anthrax in a mouse model.88
Probiotics were also engineered to deliver vaccines to the mucosal surfaces. The first recombinant probiotic oral vaccine was developed by expressing the tetanus toxin fragment C in Lactococcus lactis.89 Recombinant Lc. lactis strain expressing Internalin A protein of L. monocytogenes enabled this non-invasive probiotic to invade the small intestine and to deliver the immunostimulatory molecule inside the epithelial cells.90
To control rotavirus infection, several live attenuated vaccines using the human and/or bovine rotavirus strain have been developed; however, these were ineffective due to lack of robust mucosal immune response. To help elicit strong mucosal immune response, recombinant L. paracasei expressing the variable domain of llama heavy-chain antibody was developed against rotavirus. This antibody expressing probiotic was able to markedly reduce disease length, severity and viral load in a mouse model.91 In another study, recombinant Lc. lactis expressing rotavirus spike-protein VP8 induced mucosal IgA and anti-VP8 antibodies at both intestinal and systemic levels in a mouse model92 and provided 100% protection against rotavirus challenge.
Besides the use of heterologous antigens to stimulate immune responses, expression of cytokines can also help in immunostimulation. Several probiotic strains have been engineered to express cytokines and other anti-inflammatory molecules to help suppress intestinal inflammation and provide cytoprotection. Murine IL-10, an immunosuppressive and anti-inflammatory cytokine was cloned and expressed in Lc. lactis strain and the recombinant strain reduced inflammation and colitis in 40% of the mice.93 Oral administration of IL-10 secreting probiotic in a colitis murine model resulted in a reduction in inflammatory symptoms. Human interferon-β (huIFN-β) is immunomodulatory and increases IL-10 expression. Lc. lactis secreting huIFN-β was shown to significantly reduce microbial colitis and inflamation.94 In addition to huIFN-β, heme oxygenase-I (HO-1) has also been shown to modulate the anti-inflammatory effect of IL-10. Lc. lactis secreting HO-1, when administered in rats, prevented mucosal injury by LPS, reduced LPS-induced endotoxemia and significantly increased survival rate in rats.95 Oral immunization of mice with L. casei expressing IL-1β and heat-killed S. Enteritidis (SE) enhanced anti-SE antibodies demonstrating adjuvant properties of recombinant probiotics.96 Recombinant L. plantarum surface displaying invasin protein of Y. pseudotuberculosis served as a potent activator of NF-κB and was demonstrated to be a promising mucosal delivery vehicle for vaccine antigen.97 L. acidophilus was engineered to express hemagglutinin of the avian influenza virus H5N1 and induced strong mucosal and serum antibody response to H5N1.98
Safety of Probiotic Therapy and Biocontainment
The ultimate goal of developing a recombinant probiotic is its use in humans and animals. Prior to the approval of a recombinant probiotic for human use, it is essential that the bacteria be screened for potential pathogenicity and virulence traits.10,21,99 Providing evidence for the absence of virulence properties is relatively straightforward in elucidating the pathogenic potential. Besides phenotypic characterization, it is also essential to genetically screen potential candidates for use as probiotics. Another critical consideration is the scope for antimicrobial resistance. In addition to being sensitive to antibiotics, it is also essential that the probiotic bacteria do not carry any transferrable antibiotic resistance genes, which can serve as genetic reservoirs for other potentially pathogenic bacteria. Besides acquisition of antibiotic resistant genes, there is also the risk for uptake of virulence genes from pathogens that co-inhabit the intestinal tract at the same time. However, there is no evidence in the literature for such event taking place in the gut. This could partly be due to the transient colonization of the gut by probiotics. Considering all the factors that are essential in assessment of safety of probiotic therapy, it is paramount that the general conclusion “probiotics are safe” cannot be broadly made. Prior to the use of a probiotic or probiotic cocktail in foods or dietary supplement, they need to be determined to be safe for the general population. Therefore, when intended for use as drugs, the safety assessment must balance risk with benefit.99
Another important consideration for genetically modified probiotic is preventing its accumulation in the environment and preventing lateral dissemination of the genetic material to other bacteria. The best approach to address this concern is to use a biological system that is propagated along with the probiotic termed as biological containment systems.100 Biocontainment systems can be active or passive. Active containment involves the conditional production of a bacterial toxin through tightly regulated gene expression that is controlled by an environmental cue. Passive containment results in growth dependence on the complementation of an auxotrophy or gene defect, by supplementing another gene or essential metabolite.101 Hillman102 used the passive approach to contain recombinant Streptococcus mutans. They deleted the alr gene necessary for d-alanine synthesis that is essential for biosynthesis of cell wall. Similarly, Fu and Xu103 developed a containment system for recombinant L. acidophilus using the thymidilmate synthase gene (thyA) from L. casei as a marker for plasmid maintenance.
Conclusions and Future Perspectives
Although probiotics have been used in food to enhance flavor or to provide health benefits, currently there is an increasing trend for their use in medicine. They provide a viable alternative especially in the treatment and prevention of enteric diseases. Over the years, several probiotics have been demonstrated to be effective against enteropathogens and their mode of action has been elucidated. A better understanding of the host pathogen interaction has also enabled the development of bioengineered probiotics that can be used for the targeted elimination of pathogens. The use of engineered probiotics helps overcome the short-half life and stability of other therapeutic alternatives and also provides access to a cost-effective alternative. Recombinant probiotics can be used in a variety of applications. However, there is a need to contain the modified organism to prevent its uninhibited spread. Also, it is essential to consider their biosafety and their ability to cause allergy due to prolonged consumption. Although there are several hurdles in the development of safe and effective bioengineered probiotics, advancements in technologies and further refinements in techniques will continue to provide novel bio-therapeutics for the treatment and prevention of enteric infections both in rich and economically challenged countries.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/23574
References
- 1.Mandeville KL, Krabshuis J, Ladep NG, Mulder CJJ, Quigley EMM, Khan SA. Gastroenterology in developing countries: issues and advances. World J Gastroenterol. 2009;15:2839–54. doi: 10.3748/wjg.15.2839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Girard MP, Steele D, Chaignat C-L, Kieny MP. A review of vaccine research and development: human enteric infections. Vaccine. 2006;24:2732–50. doi: 10.1016/j.vaccine.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 3.Guerrant RL, Kosek M, Moore S, Lorntz B, Brantley R, Lima AAM. Magnitude and impact of diarrheal diseases. Arch Med Res. 2002;33:351–5. doi: 10.1016/S0188-4409(02)00379-X. [DOI] [PubMed] [Google Scholar]
- 4.Buzby JC, Roberts T. The economics of enteric infections: human foodborne disease costs. Gastroenterology. 2009;136:1851–62. doi: 10.1053/j.gastro.2009.01.074. [DOI] [PubMed] [Google Scholar]
- 5.Greig JD, Ravel A. Analysis of foodborne outbreak data reported internationally for source attribution. 2009;130:77–87. doi: 10.1016/j.ijfoodmicro.2008.12.031. [DOI] [PubMed] [Google Scholar]
- 6.Newell DG, Koopmans M, Verhoef L, Duizer E, Aidara-Kane A, Sprong H, et al. Food-borne diseases - the challenges of 20 years ago still persist while new ones continue to emerge. Int J Food Microbiol. 2010;139(Suppl 1):S3–15. doi: 10.1016/j.ijfoodmicro.2010.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Scallan E, Hoekstra RM, Angulo FJ, Tauxe RV, Widdowson MA, Roy SL, et al. Foodborne illness acquired in the United States--major pathogens. Emerg Infect Dis. 2011;17:7–15. doi: 10.3201/eid1701.P11101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaway TR, Edrington TS, Rychlik JL, Genovese KJ, Poole TL, Jung YS, et al. Ionophores: their use as ruminant growth promotants and impact on food safety. Curr Issues Intest Microbiol. 2003;4:43–51. [PubMed] [Google Scholar]
- 9.Juneja VK, Dwivedi HP, Yan X. Novel natural food antimicrobials. Annu Rev Food Sci Technol. 2012;3:381–403. doi: 10.1146/annurev-food-022811-101241. [DOI] [PubMed] [Google Scholar]
- 10.Amalaradjou MAR, Bhunia AK. Modern approaches in probiotics research to control foodborne pathogens. Adv Food Nutr Res. 2012;67:185–239. doi: 10.1016/B978-0-12-394598-3.00005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hagens S, Loessner MJ. Bacteriophage for biocontrol of foodborne pathogens: calculations and considerations. Curr Pharm Biotechnol. 2010;11:58–68. doi: 10.2174/138920110790725429. [DOI] [PubMed] [Google Scholar]
- 12.Fenton M, Ross P, McAuliffe O, O’Mahony J, Coffey A. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs. 2010;1:9–16. doi: 10.4161/bbug.1.1.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brovko LY, Anany H, Griffiths MW. Bacteriophages for detection and control of bacterial pathogens in food and food-processing environment. Adv Food Nutr Res. 2012;67:241–88. doi: 10.1016/B978-0-12-394598-3.00006-X. [DOI] [PubMed] [Google Scholar]
- 14.Sirsat SA, Muthaiyan A, Ricke SC. Antimicrobials for foodborne pathogen reduction in organic and natural poultry production. J Appl Poult Res. 2009;18:379–88. doi: 10.3382/japr.2008-00140. [DOI] [Google Scholar]
- 15.Luksienė Z, Zukauskas A. Prospects of photosensitization in control of pathogenic and harmful micro-organisms. J Appl Microbiol. 2009;107:1415–24. doi: 10.1111/j.1365-2672.2009.04341.x. [DOI] [PubMed] [Google Scholar]
- 16.Monachese M, Cunningham-Rundles S, Diaz MA, Guerrant R, Hummelen R, Kemperman R, et al. Probiotics and prebiotics to combat enteric infections and HIV in the developing world: a consensus report. Gut Microbes. 2011;2:198–207. doi: 10.4161/gmic.2.3.16106. [DOI] [PubMed] [Google Scholar]
- 17.Callaway TR, Anderson RC, Edrington TS, Elder RO, Genovese KJ, Bischoff KM, et al. Preslaughter intervention strategies to reduce food-borne pathogens in food animals. J Anim Sci. 2003;81:E17–23. doi: 10.2527/2003.812553x. [DOI] [PubMed] [Google Scholar]
- 18.Doyle M, Erickson M. Reducing the carriage of foodborne pathogens in livestock and poultry. 2006;85:960–973. doi: 10.1093/ps/85.6.960. [DOI] [PubMed] [Google Scholar]
- 19.Gaggìa F, Mattarelli P, Biavati B. Probiotics and prebiotics in animal feeding for safe food production. Int J Food Microbiol. 2010;141(Suppl 1):S15–28. doi: 10.1016/j.ijfoodmicro.2010.02.031. [DOI] [PubMed] [Google Scholar]
- 20.Preidis GA, Versalovic J. Targeting the human microbiome with antibiotics, probiotics, and prebiotics: gastroenterology enters the metagenomics era. Gastroenterology. 2009;136:2015–31. doi: 10.1053/j.gastro.2009.01.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dicks LMT, Botes M. Probiotic lactic acid bacteria in the gastro-intestinal tract: health benefits, safety and mode of action. Benef Microbes. 2010;1:11–29. doi: 10.3920/BM2009.0012. [DOI] [PubMed] [Google Scholar]
- 22.Thomas LV, Ockhuizen T. New insights into the impact of the intestinal microbiota on health and disease: a symposium report. Br J Nutr. 2012;107(Suppl 1):S1–13. doi: 10.1017/S0007114511006970. [DOI] [PubMed] [Google Scholar]
- 23.Nagpal R, Kumar A, Kumar M, Behare PV, Jain S, Yadav H. Probiotics, their health benefits and applications for developing healthier foods: a review. FEMS Microbiol Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 24.Salminen S, Nybom S, Meriluoto J, Collado MC, Vesterlund S, El-Nezami H. Interaction of probiotics and pathogens--benefits to human health? Curr Opin Biotechnol. 2010;21:157–67. doi: 10.1016/j.copbio.2010.03.016. [DOI] [PubMed] [Google Scholar]
- 25.Wen K, Azevedo MSP, Gonzalez A, Zhang W, Saif LJ, Li GH, et al. Toll-like receptor and innate cytokine responses induced by lactobacilli colonization and human rotavirus infection in gnotobiotic pigs. Vet Immunol Immunopathol. 2009;127:304–15. doi: 10.1016/j.vetimm.2008.10.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mattison K. Norovirus as a foodborne disease hazard. Adv Food Nutr Res. 2011;62:1–39. doi: 10.1016/B978-0-12-385989-1.00001-6. [DOI] [PubMed] [Google Scholar]
- 27.Marshall JA, Bruggink LD. The dynamics of norovirus outbreak epidemics: recent insights. Int J Environ Res Public Health. 2011;8:1141–9. doi: 10.3390/ijerph8041141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nagata S, Asahara T, Ohta T, Yamada T, Kondo S, Bian L, et al. Effect of the continuous intake of probiotic-fermented milk containing Lactobacillus casei strain Shirota on fever in a mass outbreak of norovirus gastroenteritis and the faecal microflora in a health service facility for the aged. Br J Nutr. 2011;106:549–56. doi: 10.1017/S000711451100064X. [DOI] [PubMed] [Google Scholar]
- 29.Dubey AP, Rajeshwari K, Chakravarty A, Famularo G. Use of VSL[sharp]3 in the treatment of rotavirus diarrhea in children: preliminary results. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 1):S126–9. doi: 10.1097/MCG.0b013e31816fc2f6. [DOI] [PubMed] [Google Scholar]
- 30.Ruiz-Palacios GM. The health burden of Campylobacter infection and the impact of antimicrobial resistance: playing chicken. Clin Infect Dis. 2007;44:701–3. doi: 10.1086/509936. [DOI] [PubMed] [Google Scholar]
- 31.Wagner RD, Johnson SJ, Kurniasih Rubin D. Probiotic bacteria are antagonistic to Salmonella enterica and Campylobacter jejuni and influence host lymphocyte responses in human microbiota-associated immunodeficient and immunocompetent mice. Mol Nutr Food Res. 2009;53:377–88. doi: 10.1002/mnfr.200800101. [DOI] [PubMed] [Google Scholar]
- 32.Baffoni L, Gaggìa F, Di Gioia D, Santini C, Mogna L, Biavati B. A Bifidobacterium-based synbiotic product to reduce the transmission of C. jejuni along the poultry food chain. Int J Food Microbiol. 2012;157:156–61. doi: 10.1016/j.ijfoodmicro.2012.04.024. [DOI] [PubMed] [Google Scholar]
- 33.Satish Kumar R, Kanmani P, Yuvaraj N, Paari KA, Pattukumar V, Arul V. Lactobacillus plantarum AS1 binds to cultured human intestinal cell line HT-29 and inhibits cell attachment by enterovirulent bacterium Vibrio parahaemolyticus. Lett Appl Microbiol. 2011;53:481–7. doi: 10.1111/j.1472-765X.2011.03136.x. [DOI] [PubMed] [Google Scholar]
- 34.Lema M, Williams L, Rao DR. Reduction of fecal shedding of enterohemorrhagic Escherichia coli O157:H7 in lambs by feeding microbial feed supplement. Small Rumin Res. 2001;39:31–9. doi: 10.1016/S0921-4488(00)00168-1. [DOI] [PubMed] [Google Scholar]
- 35.Asahara T, Shimizu K, Nomoto K, Hamabata T, Ozawa A, Takeda Y. Probiotic bifidobacteria protect mice from lethal infection with Shiga toxin-producing Escherichia coli O157:H7. Infect Immun. 2004;72:2240–7. doi: 10.1128/IAI.72.4.2240-2247.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reissbrodt R, Hammes WP, dal Bello F, Prager R, Fruth A, Hantke K, et al. Inhibition of growth of Shiga toxin-producing Escherichia coli by nonpathogenic Escherichia coli. FEMS Microbiol Lett. 2009;290:62–9. doi: 10.1111/j.1574-6968.2008.01405.x. [DOI] [PubMed] [Google Scholar]
- 37.Tsai YT, Cheng PC, Pan TM. Immunomodulating cctivity of Lactobacillus paracasei subsp. paracasei NTU 101 in Enterohemorrhagic Escherichia coli O157H7 - infected mice. J Agric Food Chem. 2010;58:11265–72. doi: 10.1021/jf103011z. [DOI] [PubMed] [Google Scholar]
- 38.Higgins SE, Higgins JP, Wolfenden AD, Henderson SN, Torres-Rodriguez A, Tellez G, et al. Evaluation of a Lactobacillus-based probiotic culture for the reduction of Salmonella enteritidis in neonatal broiler chicks. Poult Sci. 2008;87:27–31. doi: 10.3382/ps.2007-00210. [DOI] [PubMed] [Google Scholar]
- 39.Menconi A, Wolfenden AD, Shivaramaiah S, Terraes JC, Urbano T, Kuttel J, et al. Effect of lactic acid bacteria probiotic culture for the treatment of Salmonella enterica serovar Heidelberg in neonatal broiler chickens and turkey poults. Poult Sci. 2011;90:561–5. doi: 10.3382/ps.2010-01220. [DOI] [PubMed] [Google Scholar]
- 40.Zhang D, Li R, Li J. Lactobacillus reuteri ATCC 55730 and L22 display probiotic potential in vitro and protect against Salmonella-induced pullorum disease in a chick model of infection. Res Vet Sci. 2012;93:366–73. doi: 10.1016/j.rvsc.2011.06.020. [DOI] [PubMed] [Google Scholar]
- 41.de LeBlanc AdeM, Castillo NA, Perdigon G. Anti-infective mechanisms induced by a probiotic Lactobacillus strain against Salmonella enterica serovar Typhimurium infection. Int J Food Microbiol. 2010;138:223–31. doi: 10.1016/j.ijfoodmicro.2010.01.020. [DOI] [PubMed] [Google Scholar]
- 42.Asahara T, Shimizu K, Takada T, Kado S, Yuki N, Morotomi M, et al. Protective effect of Lactobacillus casei strain Shirota against lethal infection with multi-drug resistant Salmonella enterica serovar Typhimurium DT104 in mice. J Appl Microbiol. 2011;110:163–73. doi: 10.1111/j.1365-2672.2010.04884.x. [DOI] [PubMed] [Google Scholar]
- 43.Varma P, Dinesh KR, Menon KK, Biswas R. Lactobacillus fermentum isolated from human colonic mucosal biopsy inhibits the growth and adhesion of enteric and foodborne pathogens. J Food Sci. 2010;75:M546–51. doi: 10.1111/j.1750-3841.2010.01818.x. [DOI] [PubMed] [Google Scholar]
- 44.Zschüttig A, Zimmermann K, Blom J, Goesmann A, Pöhlmann C, Gunzer F. Identification and characterization of microcin S, a new antibacterial peptide produced by probiotic Escherichia coli G3/10. PLoS One. 2012;7:e33351. doi: 10.1371/journal.pone.0033351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bomba A. Nemcová Rr, Mudroňová D, Guba P. The possibilities of potentiating the efficacy of probiotics. Trends Food Sci Technol. 2002;13:121–6. doi: 10.1016/S0924-2244(02)00129-2. [DOI] [Google Scholar]
- 46.Karimi O, Peña AS. Indications and challenges of probiotics, prebiotics, and synbiotics in the management of arthralgias and spondyloarthropathies in inflammatory bowel disease. J Clin Gastroenterol. 2008;42(Suppl 3 Pt 1):S136–41. doi: 10.1097/MCG.0b013e3181662455. [DOI] [PubMed] [Google Scholar]
- 47.Morrow LE, Kollef MH. Probiotics in the intensive care unit: why controversies and confusion abound. Crit Care. 2008;12:160. doi: 10.1186/cc6927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grześkowiak L, Isolauri E, Salminen S, Gueimonde M. Manufacturing process influences properties of probiotic bacteria. Br J Nutr. 2011;105:887–94. doi: 10.1017/S0007114510004496. [DOI] [PubMed] [Google Scholar]
- 49.Salazar N, Gueimonde M, Hernández-Barranco AM, Ruas-Madiedo P, de los Reyes-Gavilán CG. Exopolysaccharides produced by intestinal Bifidobacterium strains act as fermentable substrates for human intestinal bacteria. Appl Environ Microbiol. 2008;74:4737–45. doi: 10.1128/AEM.00325-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barzegari A, Saei AA. Designing probiotics with respect to the native microbiome. Future Microbiol. 2012;7:571–5. doi: 10.2217/fmb.12.37. [DOI] [PubMed] [Google Scholar]
- 51.Culligan EP, Hill C, Sleator RD. Probiotics and gastrointestinal disease: successes, problems and future prospects. Gut Pathog. 2009;1:19. doi: 10.1186/1757-4749-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhunia AK. Bioengineered probiotics – A solution to broaden probiotics efficacy. J Nutr Food Sci. 2012;2:e105. doi: 10.4172/2155-9600.1000e105. [DOI] [Google Scholar]
- 53.Wells J. Mucosal vaccination and therapy with genetically modified lactic acid bacteria. Annu Rev Food Sci Technol. 2011;2:423–45. doi: 10.1146/annurev-food-022510-133640. [DOI] [PubMed] [Google Scholar]
- 54.Sleator RD, Hill C. Battle of the bugs. Science. 2008;321:1294–5. doi: 10.1126/science.321.5894.1294b. [DOI] [PubMed] [Google Scholar]
- 55.Wells JM, Mercenier A. Mucosal delivery of therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol. 2008;6:349–62. doi: 10.1038/nrmicro1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.D’Silva I. Recombinant technology and probiotics. Int J Eng Technol. 2011;3:288–93. [Google Scholar]
- 57.Hill C, Cotter PD, Sleator RD, Gahan CGM. Bacterial stress response in Listeria monocytogenes: jumping the hurdles imposed by minimal processing. Int Dairy J. 2002;12:273–83. doi: 10.1016/S0958-6946(01)00125-X. [DOI] [Google Scholar]
- 58.Sheehan VM, Sleator RD, Fitzgerald GF, Hill C. Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Appl Environ Microbiol. 2006;72:2170–7. doi: 10.1128/AEM.72.3.2170-2177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Termont S, Vandenbroucke K, Iserentant D, Neirynck S, Steidler L, Remaut E, et al. Intracellular accumulation of trehalose protects Lactococcus lactis from freeze-drying damage and bile toxicity and increases gastric acid resistance. Appl Environ Microbiol. 2006;72:7694–700. doi: 10.1128/AEM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheehan VM, Sleator RD, Hill C, Fitzgerald GF. Improving gastric transit, gastrointestinal persistence and therapeutic efficacy of the probiotic strain Bifidobacterium breve UCC2003. Microbiology. 2007;153:3563–71. doi: 10.1099/mic.0.2007/006510-0. [DOI] [PubMed] [Google Scholar]
- 61.Paton AW, Morona R, Paton JC. Designer probiotics for prevention of enteric infections. Nat Rev Microbiol. 2006;4:193–200. doi: 10.1038/nrmicro1349. [DOI] [PubMed] [Google Scholar]
- 62.Wu C-M, Chung T-C. Mice protected by oral immunization with Lactobacillus reuteri secreting fusion protein of Escherichia coli enterotoxin subunit protein. FEMS Immunol Med Microbiol. 2007;50:354–65. doi: 10.1111/j.1574-695X.2007.00255.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rasko DA, Sperandio V. Anti-virulence strategies to combat bacteria-mediated disease. Nat Rev Drug Discov. 2010;9:117–28. doi: 10.1038/nrd3013. [DOI] [PubMed] [Google Scholar]
- 64.Koo OK, Amalaradjou MAR, Bhunia AK. Recombinant probiotic expressing Listeria adhesion protein attenuates Listeria monocytogenes virulence in vitro. PLoS One. 2012;7:e29277. doi: 10.1371/journal.pone.0029277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jagadeesan B, Fleishman Littlejohn AE, Amalaradjou MAR, Singh AK, Mishra KK, La D, et al. N-terminal Gly(224)-Gly(411) domain in Listeria adhesion protein interacts with host receptor Hsp60. PLoS One. 2011;6:e20694. doi: 10.1371/journal.pone.0020694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jagadeesan B, Koo OK, Kim KP, Burkholder KM, Mishra KK, Aroonnual A, et al. LAP, an alcohol acetaldehyde dehydrogenase enzyme in Listeria, promotes bacterial adhesion to enterocyte-like Caco-2 cells only in pathogenic species. Microbiology. 2010;156:2782–95. doi: 10.1099/mic.0.036509-0. [DOI] [PubMed] [Google Scholar]
- 67.Wampler JL, Kim KP, Jaradat Z, Bhunia AK. Heat shock protein 60 acts as a receptor for the Listeria adhesion protein in Caco-2 cells. Infect Immun. 2004;72:931–6. doi: 10.1128/IAI.72.2.931-936.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Burkholder KM, Bhunia AK. Listeria monocytogenes uses Listeria adhesion protein (LAP) to promote bacterial transepithelial translocation and induces expression of LAP receptor Hsp60. Infect Immun. 2010;78:5062–73. doi: 10.1128/IAI.00516-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burkholder KM, Kim KP, Mishra KK, Medina S, Hahm BK, Kim H, Bhunia AK. Expression of LAP, a SecA2-dependent secretory protein, is induced under anaerobic environment. 2009;11:859–67. doi: 10.1016/j.micinf.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 70.Sánchez B, López P, González-Rodríguez I, Suárez A, Margolles A, Urdaci MC. A flagellin-producing Lactococcus strain: interactions with mucin and enteropathogens. FEMS Microbiol Lett. 2011;318:101–7. doi: 10.1111/j.1574-6968.2011.02244.x. [DOI] [PubMed] [Google Scholar]
- 71.Chu H, Kang S, Ha S, Cho K, Park SM, Han KH, et al. Lactobacillus acidophilus expressing recombinant K99 adhesive fimbriae has an inhibitory effect on adhesion of enterotoxigenic Escherichia coli. Microbiol Immunol. 2005;49:941–8. doi: 10.1111/j.1348-0421.2005.tb03687.x. [DOI] [PubMed] [Google Scholar]
- 72.Wen L-J, Hou X-L, Wang G-H, Yu L-Y, Wei X-M, Liu J-K, et al. Immunization with recombinant Lactobacillus casei strains producing K99, K88 fimbrial protein protects mice against enterotoxigenic Escherichia coli. Vaccine. 2012;30:3339–49. doi: 10.1016/j.vaccine.2011.08.036. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira PCD, Campos IB, Abe CM, Trabulsi LR, Elias WP, Ho PL, et al. Immunization of mice with Lactobacillus casei expressing intimin fragments produces antibodies able to inhibit the adhesion of enteropathogenic Escherichia coli to cultivated epithelial cells. FEMS Immunol Med Microbiol. 2008;54:245–54. doi: 10.1111/j.1574-695X.2008.00471.x. [DOI] [PubMed] [Google Scholar]
- 74.Kajikawa A, Igimi S. Innate and acquired immune responses induced by recombinant Lactobacillus casei displaying flagellin-fusion antigen on the cell-surface. Vaccine. 2010;28:3409–15. doi: 10.1016/j.vaccine.2010.02.077. [DOI] [PubMed] [Google Scholar]
- 75.Lagenaur LA, Sanders-Beer BE, Brichacek B, Pal R, Liu X, Liu Y, et al. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011;4:648–57. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fahey JV, Bodwell JE, Hickey DK, Ghosh M, Muia MN, Wira CR. New approaches to making the microenvironment of the female reproductive tract hostile to HIV. Am J Reprod Immunol. 2011;65:334–43. doi: 10.1111/j.1600-0897.2010.00949.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Waters CM, Bassler BL. Quorum sensing: cell-to-cell communication in bacteria. Annu Rev Cell Dev Biol. 2005;21:319–46. doi: 10.1146/annurev.cellbio.21.012704.131001. [DOI] [PubMed] [Google Scholar]
- 78.Duan F, March JC. Engineered bacterial communication prevents Vibrio cholerae virulence in an infant mouse model. Proc Natl Acad Sci U S A. 2010;107:11260–4. doi: 10.1073/pnas.1001294107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Saeidi N, Wong CK, Lo T-M, Nguyen HX, Ling H, Leong SSJ, et al. Engineering microbes to sense and eradicate Pseudomonas aeruginosa, a human pathogen. Mol Syst Biol. 2011;7:521. doi: 10.1038/msb.2011.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goh Y-L, He H, March JC. Engineering commensal bacteria for prophylaxis against infection. Curr Opin Biotechnol. 2012;23:924–30. doi: 10.1016/j.copbio.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chen HL, Lai YW, Chen CS, Chu TW, Lin W, Yen CC, et al. Probiotic Lactobacillus casei expressing human lactoferrin elevates antibacterial activity in the gastrointestinal tract. Biometals. 2010;23:543–54. doi: 10.1007/s10534-010-9298-0. [DOI] [PubMed] [Google Scholar]
- 82.Hurwitz I, Fieck A, Klein N, Jose C, Kang A, Durvasula R. A paratransgenic strategy for the control of Chagas disease. Psyche. 2012 doi: 10.1155/2012/178930. [DOI] [Google Scholar]
- 83.Tarahomjoo S. Development of vaccine delivery vehicles based on lactic acid bacteria. Mol Biotechnol. 2012;51:183–99. doi: 10.1007/s12033-011-9450-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Daniel C, Sebbane F, Poiret S, Goudercourt D, Dewulf J, Mullet C, et al. Protection against Yersinia pseudotuberculosis infection conferred by a Lactococcus lactis mucosal delivery vector secreting LcrV. Vaccine. 2009;27:1141–4. doi: 10.1016/j.vaccine.2008.12.022. [DOI] [PubMed] [Google Scholar]
- 85.Kajikawa A, Zhang L, Long J, Nordone S, Stoeker L, LaVoy A, et al. Construction and immunological evaluation of dual cell surface display of HIV-1 gag and Salmonella enterica serovar Typhimurium FliC in Lactobacillus acidophilus for vaccine delivery. Clin Vaccine Immunol. 2012;19:1374–81. doi: 10.1128/CVI.00049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hernani MdeL, Ferreira PCD, Ferreira DM, Miyaji EN, Ho PL, Oliveira MLS. Nasal immunization of mice with Lactobacillus casei expressing the pneumococcal surface protein C primes the immune system and decreases pneumococcal nasopharyngeal colonization in mice. FEMS Immunol Med Microbiol. 2011;62:263–72. doi: 10.1111/j.1574-695X.2011.00809.x. [DOI] [PubMed] [Google Scholar]
- 87.Mohamadzadeh M, Duong T, Sandwick SJ, Hoover T, Klaenhammer TR. Dendritic cell targeting of Bacillus anthracis protective antigen expressed by Lactobacillus acidophilus protects mice from lethal challenge. Proc Natl Acad Sci U S A. 2009;106:4331–6. doi: 10.1073/pnas.0900029106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mohamadzadeh M, Durmaz E, Zadeh M, Pakanati KC, Gramarossa M, Cohran V, et al. Targeted expression of anthrax protective antigen by Lactobacillus gasseri as an anthrax vaccine. Future Microbiol. 2010;5:1289–96. doi: 10.2217/fmb.10.78. [DOI] [PubMed] [Google Scholar]
- 89.Robinson K, Chamberlain LM, Schofield KM, Wells JM, Le Page RWF. Oral vaccination of mice against tetanus with recombinant Lactococcus lactis. Nat Biotechnol. 1997;15:653–7. doi: 10.1038/nbt0797-653. [DOI] [PubMed] [Google Scholar]
- 90.Innocentin S, Guimarães V, Miyoshi A, Azevedo V, Langella P, Chatel JM, et al. Lactococcus lactis expressing either Staphylococcus aureus fibronectin-binding protein A or Listeria monocytogenes internalin A can efficiently internalize and deliver DNA in human epithelial cells. Appl Environ Microbiol. 2009;75:4870–8. doi: 10.1128/AEM.00825-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Pant N, Hultberg A, Zhao YF, Svensson L, Pan-Hammarstrom Q, Johansen K, et al. Lactobacilli expressing variable domain of llama heavy-chain antibody fragments (lactobodies) confer protection against rotavirus-induced diarrhea. J Infect Dis. 2006;194:1580–8. doi: 10.1086/508747. [DOI] [PubMed] [Google Scholar]
- 92.Marelli B, Perez AR, Banchio C, de Mendoza D, Magni C. Oral immunization with live Lactococcus lactis expressing rotavirus VP8 subunit induces specific immune response in mice. J Virol Methods. 2011;175:28–37. doi: 10.1016/j.jviromet.2011.04.011. [DOI] [PubMed] [Google Scholar]
- 93.Steidler L, Hans W, Schotte L, Neirynck S, Obermeier F, Falk W, et al. Treatment of murine colitis by Lactococcus lactis secreting interleukin-10. Science. 2000;289:1352–5. doi: 10.1126/science.289.5483.1352. [DOI] [PubMed] [Google Scholar]
- 94.Zhuang Z, Wu ZG, Chen M, Wang PG. Secretion of human interferon-β 1b by recombinant Lactococcus lactis. Biotechnol Lett. 2008;30:1819–23. doi: 10.1007/s10529-008-9761-y. [DOI] [PubMed] [Google Scholar]
- 95.Pang Q, Ji Y, Li Y, Bermúdez-Humarán LG, Hu G, Zeng Y. Intragastric administration with recombinant Lactococcus lactis producing heme oxygenase-1 prevents lipopolysaccharide-induced endotoxemia in rats. FEMS Microbiol Lett. 2008;283:62–8. doi: 10.1111/j.1574-6968.2008.01141.x. [DOI] [PubMed] [Google Scholar]
- 96.Kajikawa A, Masuda K, Katoh M, Igimi S. Adjuvant effects for oral immunization provided by recombinant Lactobacillus casei secreting biologically active murine interleukin-1β. Clin Vaccine Immunol. 2010;17:43–8. doi: 10.1128/CVI.00337-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fredriksen L, Kleiveland CR, Hult LT, Lea T, Nygaard CS, Eijsink VGH, et al. Surface display of N-terminally anchored invasin by Lactobacillus plantarum activates NF-κB in monocytes. Appl Environ Microbiol. 2012;78:5864–71. doi: 10.1128/AEM.01227-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang Z, Yu Q, Gao J, Yang Q. Mucosal and systemic immune responses induced by recombinant Lactobacillus spp. expressing the hemagglutinin of the avian influenza virus H5N1. Clin Vaccine Immunol. 2012;19:174–9. doi: 10.1128/CVI.05618-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sanders ME, Akkermans LMA, Haller D, Hammerman C, Heimbach J, Hörmannsperger G, et al. Safety assessment of probiotics for human use. Gut Microbes. 2010;1:164–85. doi: 10.4161/gmic.1.3.12127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee P. Biocontainment strategies for live lactic acid bacteria vaccine vectors. Bioeng Bugs. 2010;1:75–7. doi: 10.4161/bbug.1.1.10594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Steidler L. Genetically engineered probiotics. Best Pract Res Clin Gastroenterol. 2003;17:861–76. doi: 10.1016/S1521-6918(03)00072-6. [DOI] [PubMed] [Google Scholar]
- 102.Hillman JD. Genetically modified Streptococcus mutans for the prevention of dental caries. Antonie Van Leeuwenhoek. 2002;82:361–6. doi: 10.1023/A:1020695902160. [DOI] [PubMed] [Google Scholar]
- 103.Fu XL, Xu JG. Development of a chromosome-plasmid balanced lethal system for Lactobacillus acidophilus with thyA gene as selective marker. Microbiol Immunol. 2000;44:551–6. doi: 10.1111/j.1348-0421.2000.tb02533.x. [DOI] [PubMed] [Google Scholar]


