Abstract
Betaine uptake in Listeria monocytogenes is mediated by three independent transport systems, the simplest of which in genetic terms is the secondary transporter BetL. Using a random mutagenesis approach, based on the E. coli XL1 Red mutator strain, we identified a single point mutation in a putative promoter region upstream of the BetL coding region which leads to a significant increase in betL transcript levels under osmo- and chill-stress conditions and a concomitant increase in stress tolerance. Furthermore, the mutation appears to counter the heretofore unreported “twisted” cell morphology observed for L. monocytogenes grown at elevated osmolarities in tryptone soy broth.
Keywords: Listeria, osmotolerance, chill-tolerance, salt stress, twisted cells
Introduction
A characteristic feature of the intracellular foodborne pathogen Listeria monocytogenes is its ability to thrive in a variety of stressful environments.1,2 This phenotypic robustness can be attributed at least in part to the ability of the organism to accumulate a variety of protective compounds, termed compatible solutes, which help to buffer the cell from the detrimental effects of a range of environmental insults.3-5
Previous work in our labs6-8 (and others9,10) led to the identification and characterization of the principal compatible solute uptake/synthesis systems in L. monocytogenes. The first of these loci to be identified, betL, encodes a high affinity (Km 7.9 mM; Vmax 134 nmol/min/mg of protein) sodium-motive force dependent secondary betaine uptake system (BetL),6 which is a member of the BCCT family of osmolyte transport systems.3 Detailed in silico analysis revealed the presence of two putative promoter regions; a σA-like promoter and a σB-dependent promoter, respectively.11 However, while transcriptional control through alternative sigma factors is important, the final yield of BetL protein product is also likely determined by translation efficiency.4 In E. coli, and to a lesser extent Bacillus subtilis, for example, the use of non-ATG initiation codons has previously been shown to modulate expression at the translational level.12 Given that betL is initiated with an alternative TTG start codon, it is likely that the locus is also regulated to some degree at the level of translation.6,11
Furthermore, in addition to transcriptional and translational control, detailed biochemical analyses revealed that the BetL protein is itself activated in response to changes in osmolarity. Rapid activation of pre-existing BetL protein [half-life (t1/2), 2 min] in response to relatively low NaCl concentrations (1 to 2% NaCl) suggests that BetL is one of the primary responders to rapid fluxes in external osmolarity.11
We outline the use of a random mutagenesis strategy, employing the Epicurian coli® mutator strain XLI-Red, to screen for mutations in the betL gene which result in improved stress resistance. The deletion of a single thymine residue (from a string of seven thymines), within the spacer region between the -10 and -35 binding sites of the σA-like promoter, resulted in a dramatically improved osmo- and chill-tolerance phenotype when expressed against both E. coli MKH13 and L. monocytogenes LO28BCGSOE backgrounds. Furthermore, we also report for the first time an unusual “twisted-cell” morphology exhibited by L. monocytogenes when grown at elevated osmolarity (> 7% NaCl). Interestingly, this twisted cell morphology is not observed in L. monocytogenes LO28BCGSOE cells expressing the betL* gene.
Results and Discussion
Shotgun cloning of the L. monocytogenes LO28 genome, followed by heterologous complementation of the compatible solute uptake mutant Escherichia coli MKH13, led to the identification of betL—the first genetic element linked to listerial osmotolerance.6 In silico analysis revealed the presence of a consensus σB-dependent promoter-binding site downstream of a putative σA-like promoter, suggesting that in addition to being regulated at the protein level, betL is also likely regulated at the level of transcription.11 RNA slot blot and reverse transcription analysis proved that this is indeed the case, with the gene showing a 1.6-fold increase in the level of transcription following 15 min exposure to 4% NaCl.13 Furthermore, deleting betL from the listerial chromosome resulted in a dramatic reduction in the ability of the mutant to accumulate betaine (~19% that of the wild type), with an associated drop in the growth rate of the mutant at elevated osmolarities.6
In the current study, the complete betL gene (under the transcriptional control of its native promoters) was amplified from pCPL1 (using the primer pair betLFPstΙ / betLRXbaΙ) and cloned into pCI372, a shuttle vector capable of replicating in both E. coli and L. monocytogenes. As expected, the resulting construct, designated pRS1, reversed the salt sensitive phenotypes of the osmotically challenged strains E. coli MKH13 and L. monocytogenes LO28BCGSOE14 (data not shown).
In an effort to improve BetL mediated osmotolerance, a random mutagenesis strategy was employed to introduce point mutations into the cloned listerial betL gene. Plasmid pRS1 (harbouring betL) was transformed into the E. coli mutator strain XL1-Red. Mutations in three of the primary DNA repair pathways of XL1-Red result in a mutation rate which is ~5,000-fold higher than that of the wild type; hence, pRS1 replication within this strain led to the introduction of random point mutations throughout the plasmid, including the target betL gene. The randomly mutated pRS1 “bank,” designated pRS1mut, was subsequently transformed into E. coli MKH13, and transformants were selected on LB agar plates containing 7% added NaCl (a salt concentration above the growth limit for MKH13::pRS1). No colonies were obtained following a control transformation with unmutated pRS1, but transformation efficiencies of 55 CFU/μg of DNA were achieved from pRS1mut; with colonies appearing after 48 h at 37°C. Following overnight growth at elevated osmolarities, plasmids from selected osmotolerant transformants were extracted and the cloned insert sequenced. In each case, the same mutation was observed; i.e. a deletion of one of a string of seven thymines within the spacer region between the -10 and -35 binding sites of the betL σA-like promoter (Fig. 1). Proof that the observed phenotype was the result of the single point mutation in the cloned betL gene (as opposed to random mutations in the pCI372 plasmid backbone) was obtained by re-complementation studies, in which the mutated betL (designated betL*) was cloned into a pPL2 backbone, creating pRS2. Further proof that the observed “hyper-osmotolerance” phenotype was the result of the betL* mutation was obtained using the QuikChange®XL Site-Directed Mutagenesis Kit—replacing the missing thymine reversed the observed phenotype from “hyper-osmotolerance” to normal. Confirmation that the increased osmotolerance phenotype of betL* is the direct result of improved betaine mediated osmoprotection was obtained following growth in defined medium at elevated osmolarity (5% added NaCl) in the presence and absence of betaine. While no growth was observed for any of the E. coli strains in the absence of betaine (data not shown), the strain expressing betL* grew significantly better than the control strains in the presence of betaine (Fig. 2).
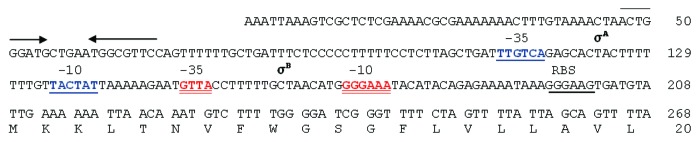
Figure 1. DNA sequence upstream of the betL coding region. Inverted repeats, indicated by pairs of arrows, delineate a likely rho-independent transcription terminal signal (ΔG -13 kcal). The predicted ribosome-binding site (RBS) and the putative σA and σB-dependent -10 and -35 sites are underlined. The first 20 amino acids of the predicted BetL protein are also presented; beginning with the alternative initiation codon TTG.
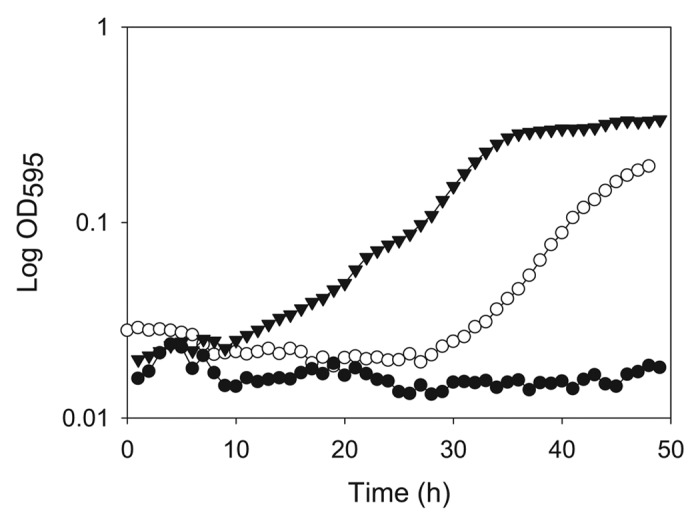
Figure 2. Growth of E. coli MKH13::pRS2 (▼; betL*), E. coli MKH13::pRS3 (○; betL) and E. coli MKH13::pPL2 (●; negative control) in M9 minimal medium containing 2 mM betaine and 5% added NaCl.
In order to assess the effect of the betL* mutation against the native listerial background, the constructs were transformed into LO28ΔBCGSOE; a strain which is devoid of betaine uptake.15 When expressed against the Listeria background, a significant advantage of pPL2 (the pRS2 and pRS3 backbone) over the multi-copy cytoplasmic pCI372 plasmid, is that pPL2 integrates as a single copy in the listerial chromosome (specifically the tRNAArg-attBB′ in both serotype 1/2 and 4b strains of Listeria16,17), thereby removing plasmid copy number as a variable in assessing the true contribution of betL* to L. monocytogenes osmotolerance. Given the location of the thymine deletion, between the -10 and -35 promoter binding sites, it seemed plausible that the increased osmotolerance (Fig. 3A) was the result of increased transcription in betL* relative to the betL wild type. RT-PCR analysis of the mutant and wild type genes suggests that this is indeed the case (Fig. 3B). While no significant difference in transcript levels were observed between betL and betL* at 0% NaCl, at both log and stationary phase growth, expression levels do appear to differ at 4% NaCl, in both growth phases. Similarly, betL* appears to exhibit slightly higher transcript levels under cold stress conditions (particularly at stationary phase Fig. 4B) and a concomitant increase in growth at refrigeration temperatures (Fig. 4A); a finding which is not altogether surprising given that BetL has previously been linked to chill tolerance in L. monocytogenes.18 Promoter variants in genes encoding compatible solute uptake is not a new phenomenon and has been reported previously in the literature for a number of microorganisms; including E. coli,19 Salmonella typhimurium20 and B. subtilis.21,22 While the mechanism of promoter activation in the current study is still unclear, it is tempting to speculate that the deleted thymine may affect DNA topology, thereby facilitating improved promoter binding and activation. In support of this hypothesis, promoters of osmotolerance genes in different organisms have previously been shown to be induced only when DNA is highly supercoiled.23,24 Interestingly, proU (encoding betaine uptake in E. coli) is controlled by changes in DNA topology25; while the osmoregulated promoter for opuA, encoding a betaine uptake system in B. subtilis, is likely subject to similar control . Indeed, both the osmoregulated proU and opuA promoters deviate from the consensus 17-bp in the length of their -10 and -35 spacer regions; with sub-optimal spacing of 16 and 18-bp respectively24,26,27—the latter being the predicted distance between the -10 and -35 binding sites of the betL σA-like promoter (Fig. 1). Given that RNA polymerase makes specific contacts with both the -10 and -35 regions; the relative orientation of these sequences is likely an important determinant for efficient transcription initiation.28 Promoters with sub-optimal spacer regions, like the betL σA-like promoter, are thus likely to respond sensitively to environmentally controlled alterations in DNA topology and as such belong to a special class of DNA twist-sensitive promoters.29 Indeed, Alice and Sanchez-Rivas30 observed a direct link between osmotolerance and DNA supercoiling in B. subtilis, while Grau et al.,31 noted similar fluctuations in DNA supercoiling during cold adaptation.
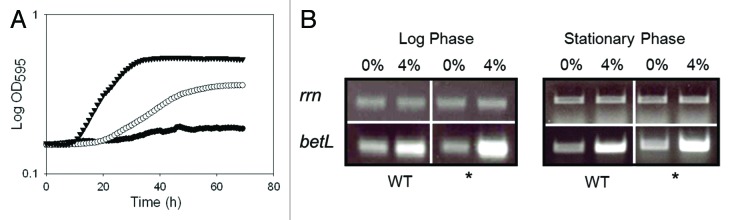
Figure 3.(A) Growth of L. monocytogenes LO28BCGSOE::pRS2 (▼; betL*), LO28BCGSOE::pRS3 (○; betL) and LO28BCGSOE::pPL2 (●; negative control) in TSB with 7% added NaCl. (B) Transcript levels of betL and betL* against the L. monocytogenes LO28BCGSOE background, following exposure to 4% NaCl for 15 min. Control PCRs were performed with the rrn primers U141 and L142. For each RT-PCR, results are presented after 22 cycles and are representative of three biological replicates.
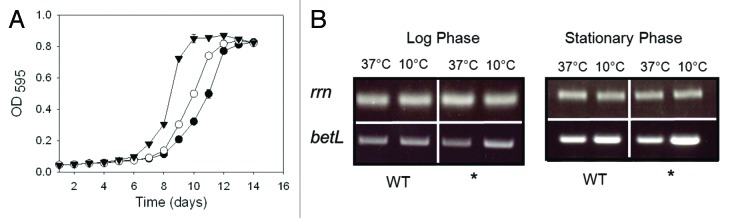
Figure 4.(A) Growth of L. monocytogenes LO28BCGSOE::pRS2 (▼; betL*), LO28BCGSOE::pRS3 (○; betL) and LO28BCGSOE::pPL2 (●; negative control) in TSB at 4°C. (B) Transcript levels of betL and betL* against the L. monocytogenes LO28BCGSOE background, following exposure to 10°C for 30 min. Control PCRs were performed with the rrn primers U141 and L142. For each RT-PCR, results are presented after 22 cycles and are representative of three biological replicates.
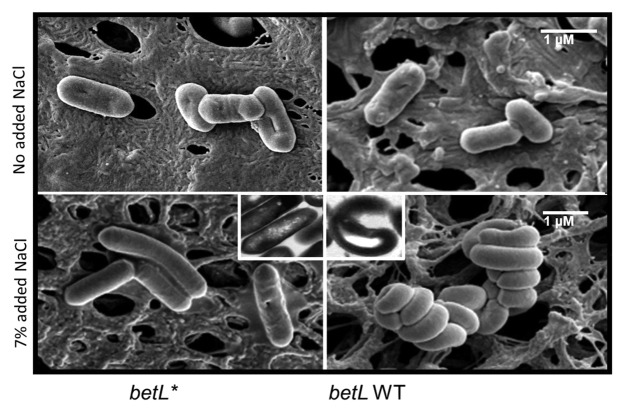
In addition to dramatically improving the growth of L. monocytogenes at elevated osmolarities, the betL* mutation reverses a previously unreported “twisted-cell” morphology for Listeria grown in complex media (BHI or TSB) at 7% NaCl (Fig. 5). While the existence of elongated listerial cells at elevated osmolarities is not a new phenomenon32,33; the “twisted-cell” phenotype has not previously been reported. While the exact role of this twisted morphology is unclear, it may function as a survival strategy reducing the cellular surface area exposed to the bathing solution, thereby reducing the severity of the stress. Indeed, bacterial “huddling”—the observed close association of individual bacterial cells—has previously been reported by Corcoran et al.,34 in probiotic lactobacilli subjected to in both spray and freeze-drying. The lack of a twisted morphology for L. monocytogenes LO28BCGSOE expressing betL* suggests that the cells are less stressed and thus better equipped for growth and survival at elevated osmolarities.
Figure 5. Scanning electron micrograph (SEM) at × 20k magnification of L. monocytogenes LO28BCGSOE::pRS2 expressing betL* (column 1) and LO28BCGSOE::pRS3 expressing betL WT (column 2), with no added NaCl (row 1) and 7% added NaCl (row 2). Inset—transmission electron microscopy (TEM) of L. monocytogenes LO28BCGSOE::pRS2 expressing betL* (column 1) and LO28BCGSOE::pRS3 expressing betL WT (column 2), with 7% added NaCl (row 2)
Finally, the betL* mutation in L. monocytogenes represents a double edged sword; from a food safety perspective, a single point mutation with the potential to induce such dramatic shifts in cell growth and survival at low aw and temperatures—making an already dangerous pathogen even more formidable—raises significant food-safety concerns which need to be addressed.1,35 However, from a synthetic biology point of view, a boosted-stress resistance locus, such as betL*, represents a useful BioBrick36 for the design of more physiologically robust pharmabiotic strains.37-42
Materials and Methods
Bacterial strains, plasmids and culture conditions
Bacterial strains and plasmids used in this study are listed in Table 1. E. coli MKH13 was grown at 37°C in either LB medium or M9 minimal medium (GIBCO/BRL, Eggenstein) containing 0.5% glucose, 0.04% arginine, 0.04% isoleucine and 0.04% valine. Glycine betaine (Sigma) was added to M9 as a filter-sterilised solution to a final concentration of 1 mM.. L. monocytogenes strains were grown in brain heart infusion (BHI) broth or in tryptone soy broth (TSB) supplemented with 0.6% yeast extract (Sigma Chemical Co.) or in chemically defined minimal medium44 (DM). Antibiotics, when needed, were made up as concentrated stocks and added to media at the required levels. Where indicated, media osmolarity was adjusted by the addition of NaCl.
Table 1. Bacterial strains and plasmids.
| Strain or plasmid | Relevant genotype or characteristicsa | Source or reference |
|---|---|---|
| Strains | ||
|
L. monocytogenes LO28 |
Serotype 1/2c | P. Cossart, Institut Pasteur |
| LO28ΔBCGSOE | LO28 ΔbetL, ΔopuC, Δgbu | Wemekamp-Kamphuis et al.,15 |
|
E. coli MKH13 |
MC4100D(putPA)101D(proP)2D(proU) | Haardt et al.,43 |
| XL1-Red | endA1 gyrA96 thi-1 hsdR17 supE44 relA1 lac mutD5 mutS mutT Tn10 (Tetr) | Stratagene |
| Plasmids | ||
| pUC18 | ColE1 ori, Apr | Vieira and Messing 43 |
| pCPL1 | pUC18 containing 2.5 kb of L. monocytogenes genomic DNA | Sleator et al.,6 |
| pCI372 | E. coli/L. lactis shuttle vector, 5.7 kb, Cmr | Sleator et al.,6 |
| pPL2 | Site-specific listerial integrative vector, 6.1 kb, Cmr | Lauer et al.,16,17 |
| pRS1 | pCI372::betL | This study |
| pRS1mut | Randomly mutated pRS1 from E. coli XL1-Red | This study |
| pRS2 | pPL2::betL* | This study |
| pRS3 | pPL2::betL | This study |
a Apr ampicillin resistance, Cmr chloramphenicol resistance, Tetr tetracycline resistance.
DNA manipulations and sequence analysis
Plasmid DNA was isolated using the Qiagen QIAprep Spin Miniprep Kit (Qiagen). E. coli was transformed by standard methods while electro-transformation of L. monocytogenes was achieved by the protocol outlined by Park and Stewart.45 Polymerase chain reaction (PCR) reagents (Taq polymerase and deoxynucleoside triphosphates dNTPs) were purchased from Boehringer GmbH and used according to the manufacturer’s instructions with a Hybaid PCR express system. Oligonucleotide primers, listed in Table 2, were synthesized on a Beckman oligo 1000M DNA synthesizer (Beckman Instruments Inc.). Nucleotide sequence determination was performed on an ABI 373 automated sequencer using the BigDye™ Terminator sequence kit (Lark Technologies, Inc.). Nucleotide sequence analysis was performed using BioMapper (nSilico Lifescience Ltd.).
Table 2. PCR primers used in this study.
| Primer | Sequence (5′-3′) |
|---|---|
| betLFPstΙ | CATCTGCAGGCTTTCTCCCCCTTTTTCCTC |
| betLRXbaΙ | CATTCTAGAGCTCTATTCCAATTACCGCCATTTC |
| XbaIKO | TAAGCGCCACTCTAGACC |
| EcoRIKO | GCACGAATTCACCAAGTA |
| U141 | TTGCTCTTCCAATGTTAG |
| L142 | GAGTGCTTAATGCGTTAG |
aNucleotides introduced to create restriction sites are underlined
Generation and screening of betL*
Random mutagenesis was performed using the strategy outlined previously.46 Plasmid pRS1 harbouring the listerial betL gene was transformed into the mutator strain Epicurian coli® XL1-Red (Stratagene) and transformants were selected on LB plates containing chloramphenicol (30 μg/ml). Transformants were then pooled and grown overnight at 37°C in LB broth. Randomly mutated plasmid DNA extracted from this culture was then used to transform the osmolyte uptake mutant E. coli MKH13. Mutations leading to enhanced osmotolerance were selected by plating transformants on LB medium containing 7% added NaCl (a salt concentration which does not permit the growth of MKH13 expressing pRS1). Plasmids isolated from the resultant osmotolerant MKH13 clones were then used to transform L. monocytogenes LO28BCGSOE (Δbet, ΔopuC, Δgbu).
Site directed mutagenesis
Single nucleotide additions and deletions within the putative betL σA-like promoter region were achieved using the QuikChange®XL Site-Directed Mutagenesis Kit (Stratagene) in accordance with the manufacturer’s instructions.
Transcriptional analysis
Reverse transcriptase (RT)-PCR analysis was performed as previously described.13 Essentially L. monocytogenes cells were grown, at 37°C with shaking, to mid-exponential phase in BHI. Ten ml of culture were centrifuged and re-suspended in 1 ml of BHI with 4% added NaCl, for analysis of salt stress, and BHI pre-chilled to 10°C for chill stress. BHI at 37°C with no added salt was used as a control. After 15 and 30 min incubation, cells were harvested by centrifugation and flash-frozen at -80°C with liquid nitrogen. Total RNA was extracted using the hot acid phenol procedure described by Ripio et al.,47 and cDNA was synthesized by adding 1 μg of total RNA to 4 μl of 5× RT buffer (Roche), 2 μl of 100 mM dithiothreitol, 0.5 μl of a deoxynucleoside triphosphate mix, 0.25 μl of RNasin, 100 ng of the random primer p(dN)6 and 1 μl of Expand reverse transcriptase (Roche). The reaction mixture was incubated at 42°C for 9 h.
In all cases control PCR reactions were used to ensure the complete removal of DNA from RNA preparations prior to reverse transcription and to ensure that levels of cDNA for samples to be compared were equal. Oligonucleotide primer pairs XbaIKO / EcoRIKO and U141 / L142 were used for PCR amplification of a 551-bp fragment from the center of betL and a 806 bp rrnA DNA fragment respectively. Each (RT)-PCR reaction was performed in triplicate from three biological replicates.
All glass and plastic-ware used in RNA analysis was first treated with 2% sodium dodecyl sulfate (SDS) for 15 min, before rinsing with 1:10 in diethyl-pyrocarbonate (DEPC) treated water.
Microscopy
Microscopy was performed essentially as described by Considine et al.48 Safranin (0.25%) stained samples of individual strains were viewed using Bright-field light microscopy at 1000 × magnification to determine cell morphology. For scanning electron microscopy (SEM), overnight cultures in TSB ± 7% added NaCl (5 mL in a 30 mL round-bottomed tube) were centrifuged at 5,000 rpm × 10 min. Pellets were immediately re-suspended in 2% glutaraldehyde, 2.5% paraformaldehyde in 0.165M phosphate buffer (pH 7.3) and left to fix overnight at 4°C. After fixation, the cells were subjected to three 10 min washes with 0.165M phosphate buffer. The cells were post-fixed with 2% osmium tetroxide for 2 h before being washed again in buffer. Cells were dehydrated by to successive 25%, 50%, 75% and 100% (100% x four times) solutions of acetone for 10 min, before being transferred to tetramethylsilane (TMS) for 15 min and air-dried in a fume hood. The samples were mounted on metal stubs using double-sided carbon tape and sputter coated with ~28 nm layer of gold using a BioRAD Polaron Sputter Coating System. The samples were viewed with a Joel JSM-5510 scanning electron microscope. For transmission electron microscopy (TEM), overnight cultures were fixed and post-fixed as for the SEM preparation. Following a buffer rinse, the cells were dehydrated in a series of solutions of ethanol: 10% for 15 min (three times), 50% for 30 min, 100% for 15 min (three times) and finally in epoxy propane for 30 min. The dehydrated cells were infiltrated with an epoxypropane:araldite mixture (3:1) overnight and then embedded in araldite and cured for 48 h in molds at 50°C. Thin (70 nm) sections were obtained from the polymerized blocks using a Reichert-Jung Ultracut E ultramicrotome and collected on formvar-coated copper grids (100 mesh). The sections were stained with 2% uranyl acetate and Reynolds lead citrate stain and examined using a Joel JEM-2000FXII electron microscope. Electron micrographs were obtained for areas of interest with a Megaview-III digital camera and analySIS software.
Acknowledgments
R.D.S. is an ESCMID Research Fellow and Coordinator of ClouDx-i, an FP7-PEOPLE-2012-IAPP project. A.F. is the recipient of an IRCSET EMBARK Postgraduate Scholarship RS/2010/2300.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/24094
References
- 1.Hill C, Cotter PD, Sleator RD, Gahan CGM. Bacterial stress response in Listeria monocytogenes: jumping the hurdles imposed by minimal processing. Int Dairy J. 2001;12:273–83. doi: 10.1016/S0958-6946(01)00125-X. [DOI] [Google Scholar]
- 2.Sleator RD, Watson D, Hill C, Gahan CG. The interaction between Listeria monocytogenes and the host gastrointestinal tract. Microbiology. 2009;155:2463–75. doi: 10.1099/mic.0.030205-0. [DOI] [PubMed] [Google Scholar]
- 3.Sleator RD, Hill C. Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol Rev. 2002;26:49–71. doi: 10.1111/j.1574-6976.2002.tb00598.x. [DOI] [PubMed] [Google Scholar]
- 4.Sleator RD, Gahan CG, Hill C. A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Appl Environ Microbiol. 2003;69:1–9. doi: 10.1128/AEM.69.1.1-9.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sleator RD, Hill C. Compatible solutes: the key to Listeria’s success as a versatile gastrointestinal pathogen? Gut Pathog. 2010;2:20. doi: 10.1186/1757-4749-2-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sleator RD, Gahan CG, Abee T, Hill C. Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl Environ Microbiol. 1999;65:2078–83. doi: 10.1128/aem.65.5.2078-2083.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sleator RD, Gahan CG, Hill C. Identification and disruption of the proBA locus in Listeria monocytogenes: role of proline biosynthesis in salt tolerance and murine infection. Appl Environ Microbiol. 2001;67:2571–7. doi: 10.1128/AEM.67.6.2571-2577.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sleator RD, Wouters J, Gahan CG, Abee T, Hill C. Analysis of the role of OpuC, an osmolyte transport system, in salt tolerance and virulence potential of Listeria monocytogenes. Appl Environ Microbiol. 2001;67:2692–8. doi: 10.1128/AEM.67.6.2692-2698.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ko R, Smith LT. Identification of an ATP-driven, osmoregulated glycine betaine transport system in Listeria monocytogenes. Appl Environ Microbiol. 1999;65:4040–8. doi: 10.1128/aem.65.9.4040-4048.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fraser KR, Harvie D, Coote PJ, O’Byrne CP. Identification and characterization of an ATP binding cassette L-carnitine transporter in Listeria monocytogenes. Appl Environ Microbiol. 2000;66:4696–704. doi: 10.1128/AEM.66.11.4696-4704.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sleator RD, Wood JM, Hill C. Transcriptional regulation and posttranslational activity of the betaine transporter BetL in Listeria monocytogenes are controlled by environmental salinity. J Bacteriol. 2003;185:7140–4. doi: 10.1128/JB.185.24.7140-7144.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Reddy P, Peterkofsky A, McKenney K. Translational efficiency of the Escherichia coli adenylate cyclase gene: mutating the UUG initiation codon to GUG or AUG results in increased gene expression. Proc Natl Acad Sci U S A. 1985;82:5656–60. doi: 10.1073/pnas.82.17.5656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sleator RD, Gahan CGM, O’Driscoll B, Hill C. Analysis of the role of betL in contributing to the growth and survival of Listeria monocytogenes LO28. Int J Food Microbiol. 2000;60:261–8. doi: 10.1016/S0168-1605(00)00316-0. [DOI] [PubMed] [Google Scholar]
- 14.Wemekamp-Kamphuis HH, Wouters JA, Sleator RD, Gahan CG, Hill C, Abee T. Multiple deletions of the osmolyte transporters BetL, Gbu, and OpuC of Listeria monocytogenes affect virulence and growth at high osmolarity. Appl Environ Microbiol. 2002;68:4710–6. doi: 10.1128/AEM.68.10.4710-4716.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wemekamp-Kamphuis HH, Sleator RD, Wouters JA, Hill C, Abee T. Molecular and physiological analysis of the role of osmolyte transporters BetL, Gbu, and OpuC in growth of Listeria monocytogenes at low temperatures. Appl Environ Microbiol. 2004;70:2912–8. doi: 10.1128/AEM.70.5.2912-2918.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lauer P, Chow MYN, Loessner MJ, Portnoy DA, Calendar R. Construction, characterization, and use of two Listeria monocytogenes site-specific phage integration vectors. J Bacteriol. 2002;184:4177–86. doi: 10.1128/JB.184.15.4177-4186.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Angelidis AS, Smith GM. Role of the glycine betaine and carnitine transporters in adaptation of Listeria monocytogenes to chill stress in defined medium. Appl Environ Microbiol. 2003;69:7492–8. doi: 10.1128/AEM.69.12.7492-7498.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mellies J, Wise A, Villarejo M. Two different Escherichia coli proP promoters respond to osmotic and growth phase signals. J Bacteriol. 1995;177:144–51. doi: 10.1128/jb.177.1.144-151.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang X, Fletcher SA, Csonka LN. Site-directed mutational analysis of the osmotically regulated proU promoter of Salmonella typhimurium. J Bacteriol. 1996;178:3377–9. doi: 10.1128/jb.178.11.3377-3379.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Spiegelhalter F, Bremer E. Osmoregulation of the opuE proline transport gene from Bacillus subtilis: contributions of the sigma A- and sigma B-dependent stress-responsive promoters. Mol Microbiol. 1998;29:285–96. doi: 10.1046/j.1365-2958.1998.00929.x. [DOI] [PubMed] [Google Scholar]
- 21.Hoffmann T, Wensing A, Brosius M, Steil L, Völker U, Bremer E. Osmotic control of opuA expression in Bacillus subtilis and its modulation in response to intracellular glycine betaine and proline pools. J Bacteriol. 2013;195:510–22. doi: 10.1128/JB.01505-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Graeme-Cook KA, May G, Bremer E, Higgins CF. Osmotic regulation of porin expression: a role for DNA supercoiling. Mol Microbiol. 1989;3:1287–94. doi: 10.1111/j.1365-2958.1989.tb00279.x. [DOI] [PubMed] [Google Scholar]
- 23.Kempf B, Bremer E. OpuA, an osmotically regulated binding protein-dependent transport system for the osmoprotectant glycine betaine in Bacillus subtilis. J Biol Chem. 1995;270:16701–13. doi: 10.1074/jbc.270.28.16701. [DOI] [PubMed] [Google Scholar]
- 24.Jordi BJAM, Owen-Hughes TA, Hulton CSJ, Higgins CF. DNA twist, flexibility and transcription of the osmoregulated proU promoter of Salmonella typhimurium. EMBO J. 1995;14:5690–700. doi: 10.1002/j.1460-2075.1995.tb00256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Higgins CF, Dorman CJ, Stirling DA, Waddell L, Booth IR, May G, et al. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988;52:569–84. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 26.Owen-Hughes TA, Pavitt GD, Santos DS, Sidebotham JM, Hulton CS, Hinton JC, et al. The chromatin-associated protein H-NS interacts with curved DNA to influence DNA topology and gene expression. Cell. 1992;71:255–65. doi: 10.1016/0092-8674(92)90354-F. [DOI] [PubMed] [Google Scholar]
- 27.Auble DT, deHaseth PL. Promoter recognition by Escherichia coli RNA polymerase. Influence of DNA structure in the spacer separating the -10 and -35 regions. J Mol Biol. 1988;202:471–82. doi: 10.1016/0022-2836(88)90279-3. [DOI] [PubMed] [Google Scholar]
- 28.Wang JY, Syvanen M. DNA twist as a transcriptional sensor for environmental changes. Mol Microbiol. 1992;6:1861–6. doi: 10.1111/j.1365-2958.1992.tb01358.x. [DOI] [PubMed] [Google Scholar]
- 29.Alice AF, Sanchez-Rivas C. DNA supercoiling and osmoresistance in Bacillus subtilis 168. Curr Microbiol. 1997;35:309–15. doi: 10.1007/s002849900260. [DOI] [PubMed] [Google Scholar]
- 30.Grau R, Gardiol D, Glikin GC, de Mendoza D. DNA supercoiling and thermal regulation of unsaturated fatty acid synthesis in Bacillus subtilis. Mol Microbiol. 1994;11:933–41. doi: 10.1111/j.1365-2958.1994.tb00372.x. [DOI] [PubMed] [Google Scholar]
- 31.Hazeleger WC, Dalvoorde M, Beumer RR. Fluorescence microscopy of NaCl-stressed, elongated Salmonella and Listeria cells reveals the presence of septa in filaments. Int J Food Microbiol. 2006;112:288–90. doi: 10.1016/j.ijfoodmicro.2006.04.026. [DOI] [PubMed] [Google Scholar]
- 32.Zaika LL, Fanelli JS. Growth kinetics and cell morphology of Listeria monocytogenes Scott A as affected by temperature, NaCl, and EDTA. J Food Prot. 2003;66:1208–15. doi: 10.4315/0362-028x-66.7.1208. [DOI] [PubMed] [Google Scholar]
- 33.Corcoran BM, Ross RP, Fitzgerald GF, Dockery P, Stanton C. Enhanced survival of GroESL-overproducing Lactobacillus paracasei NFBC 338 under stressful conditions induced by drying. Appl Environ Microbiol. 2006;72:5104–7. doi: 10.1128/AEM.02626-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sleator RD, Hill C. Food reformulations for improved health: A potential risk for microbial food safety? Med Hypotheses. 2007;69:1323–4. doi: 10.1016/j.mehy.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 35.Ho-Shing O, Lau KH, Vernon W, Eckdahl TT, Campbell AM. Assembly of standardized DNA parts using BioBrick ends in E. coli. Methods Mol Biol. 2012;852:61–76. doi: 10.1007/978-1-61779-564-0_6. [DOI] [PubMed] [Google Scholar]
- 36.Sheehan VM, Sleator RD, Fitzgerald GF, Hill C. Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Appl Environ Microbiol. 2006;72:2170–7. doi: 10.1128/AEM.72.3.2170-2177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheehan VM, Sleator RD, Hill C, Fitzgerald GF. Improving gastric transit, gastrointestinal persistence and therapeutic efficacy of the probiotic strain Bifidobacterium breve UCC2003. Microbiology. 2007;153:3563–71. doi: 10.1099/mic.0.2007/006510-0. [DOI] [PubMed] [Google Scholar]
- 38.Sleator RD, Hill C. Battle of the bugs. Science. 2008;321:1294–5. doi: 10.1126/science.321.5894.1294b. [DOI] [PubMed] [Google Scholar]
- 39.Sleator RD, Hill C. New frontiers in probiotic research. Lett Appl Microbiol. 2008;46:143–7. doi: 10.1111/j.1472-765X.2007.02293.x. [DOI] [PubMed] [Google Scholar]
- 40.Sleator RD, Hill C. Rational design of improved pharmabiotics. J Biomed Biotechnol. 2009;2009:275287. doi: 10.1155/2009/275287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sleator RD, Hill C. Patho-biotechnology: using bad bugs to do good things. Curr Opin Biotechnol. 2006;17:211–6. doi: 10.1016/j.copbio.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 42.Haardt M, Kempf B, Faatz E, Bremer E. The osmoprotectant proline betaine is a major substrate for the binding-protein-dependent transport system ProU of Escherichia coli K-12. Mol Gen Genet. 1995;246:783–6. doi: 10.1007/BF00290728. [DOI] [PubMed] [Google Scholar]
- 43.Vieira J, Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982;19:259–68. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- 44.Premaratne RJ, Lin WJ, Johnson EA. Development of an improved chemically defined minimal medium for Listeria monocytogenes. Appl Environ Microbiol. 1991;57:3046–8. doi: 10.1128/aem.57.10.3046-3048.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Park SF, Stewart GSAB. High-efficiency transformation of Listeria monocytogenes by electroporation of penicillin-treated cells. Gene. 1990;94:129–32. doi: 10.1016/0378-1119(90)90479-B. [DOI] [PubMed] [Google Scholar]
- 46.Sleator RD, Gahan CG, Hill C. Mutations in the listerial proB gene leading to proline overproduction: effects on salt tolerance and murine infection. Appl Environ Microbiol. 2001;67:4560–5. doi: 10.1128/AEM.67.10.4560-4565.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ripio MT, Vázquez-Boland JA, Vega Y, Nair S, Berche P. Evidence for expressional crosstalk between the central virulence regulator PrfA and the stress response mediator ClpC in Listeria monocytogenes. FEMS Microbiol Lett. 1998;158:45–50. doi: 10.1111/j.1574-6968.1998.tb12798.x. [DOI] [PubMed] [Google Scholar]
- 48.Considine KM, Sleator RD, Kelly AL, Fitzgerald GF, Hill C. Novel listerial genetic loci conferring enhanced barotolerance in Escherichia coli. J Appl Microbiol. 2011;110:618–30. doi: 10.1111/j.1365-2672.2010.04924.x. [DOI] [PubMed] [Google Scholar]