Abstract
In this study the growth of genetically modified Lactobacillus casei LAB6, overexpressing proline iminopeptidase PepI and its capacity to increase free proline was investigated during ripening of Edam cheese. The strain successfully survived 12 weeks of ripening period in cheese. The food-grade plasmid pLEB604, carrying the pepI gene, was stable, and PepI enzyme was active in LAB6 cells isolated at different stages of the ripening process. However, HPLC analyses indicated that Lb. casei LAB6 could not increase the amount of free proline in ripened cheese.
Keywords: Edam cheese, bitterness, PepI, proline
Introduction
Bitterness is a common taste defect in semi-hard cheeses, affecting their acceptance and marketing. One reason for bitter taste in cheese is an incomplete hydrolysis of hydrophobic peptides produced during cheese ripening. The major milk protein, casein, and in particular its subunits, α- and β-casein, are an abundant source of proline.1-3 Primary degradation of casein by rennet, plasmin and starter proteinases results in the production of hydrophobic oligopeptides rich in proline.4-7 Proline as a free amino acid may have a sweet taste, but the accumulation of proline-rich oligopeptides can cause bitterness in ripened cheese.8,9 The proline-rich oligopeptides are resistant to hydrolysis by general peptidases. Therefore, the release of proline from peptides requires proline-specific peptidases.10,11 Proline-specific peptidases, such as PepI, PepP, PepQ, PepR and PepX have been characterized from several species of lactic acid bacteria, e.g., Lactococcus lactis, Lactobacillus bulgaricus, Lactobacillus helveticus, Lactobacillus delbrueckii and Lactobacillus casei.3,8,12-16 Each enzyme cleaves proline at a certain position (Table 1).
Table 1. Substrate specificities of proline-specific peptidases characterized from lactic acid bacteria. Arrows indicate the location where the enzyme cleaves.
| Peptidase | Substrate specificity | References |
|---|---|---|
| PepI | NH2–Pro↓Xn–COOH | 13, 14 |
| PepQ | NH2–X↓Pro–COOH | 15 |
| PepP | NH2–X↓Pro–Xn–COOH | 30 |
| PepR | NH2–Pro↓X–COOH | 16 |
| PepX | NH2–X–Pro↓Xn–COOH | 12, 16 |
A potential approach to reduce bitterness in cheese is the utilization of non-starter lactic acid bacteria (NSLAB) as adjunct cultures along with the starters.5,10,17 In cheese ripening, NSLAB increase from a low number in curd to the dominant microflora in the ripened cheese, thus contributing to the flavour of aged cheeses.18-20
The genome sequences of lactic acid bacteria reveal differences between proteolytic systems of LAB, which determine the various environments in which these bacteria can grow. Compared with lactococci, lactobacilli are largely deficient in amino acid biosynthesis. Lactobacilli compensate for this deficiency by expressing a range of peptidases, amino acid permeases and oligopeptide transport systems.3 Lactobacilli degrade large oligopeptides to free amino acids, which are used for metabolic activity and growth of bacteria. They can thrive in the hostile environment of cheese, reaching numbers approximating 108g-1–109g-1.18,20 Therefore, lactobacilli are more effective than lactococci on hydrolyzing bitter peptides in cheese. Several studies demonstrate the potential of lactobacilli as adjunct starters to reduce bitter taste in cheeses. For instance, cheeses made with adjunct cultures of Lb. casei, Lb. paracasei, Lb. plantarum or Lb. helveticus were less bitter than those made without adjuncts.21,22
The efficiency of lactobacilli to reduce bitterness in cheese may be further increased by the overexpression of heterologous peptidase. So far, various peptidases have been overexpressed in Lc. lactis and studied in cheese models. Courtin et al.23 cloned genes encoding six different peptidases from Lb. helveticus into Lc. lactis. The authors found increased levels of PepX, PepQ and PepW, which led to a 3-fold increase in free amino acids in the model cheese. Similarly, Guldfeldt et al.24 overexpressed PepC, PepN, PepO and PepV from different strains of Lactococcus in Lc. lactis subsp cremoris. In other studies, proline-specific peptidases have been overexpressed in Lc. lactis.23,25,26, 27 However, there are no studies regarding the overexpression of peptidases in Lactobacillus. Lactobacilli show higher potential as hosts for the expression of peptidases since they have a better protease producing potential and they survive and increase in numbers in ripened cheese.20
In the previous study, we cloned the proline iminopeptidase gene pepI from Lb. helveticus into a food-grade expression vector in NSLAB Lb. casei strain, isolated from a high-quality Edam cheese.28 The new strain, referred as Lb. casei strain LAB6, overexpresses constructed PepI. In this study, the LAB6 strain was used as an adjunct starter for Edam cheese production. The objectives were to examine: (1) the growth trend of Lb. casei strain LAB6, (2) the stability of the plasmid pLEB604, carrying the pepI gene in the strain LAB6 during ripening period and finally (3) free proline content in the final ripened Edam cheese.
Results and Discussion
Identification of Lb. casei LAB5 and LAB6
The growth of Lb. casei LAB5 and Lb. casei LAB6 in cheese and their plasmid stability were analyzed during the ripening process. Strains were isolated from cheese at different steps of ripening and identified by comparing the RAPD profiles of LAB5 and LAB6 to other casei-type lactobacilli in cheeses using primer 6789 (Fig. 1). The RAPD profiles of LAB5, LAB6 and their host Lb. casei E were identical to each other, but were different from other Lb. rhamnosus and Lb. casei strains. Therefore, it was possible to identify and track the plasmid stability of Lb. casei LAB 5 and LAB 6 strains during the ripening process.
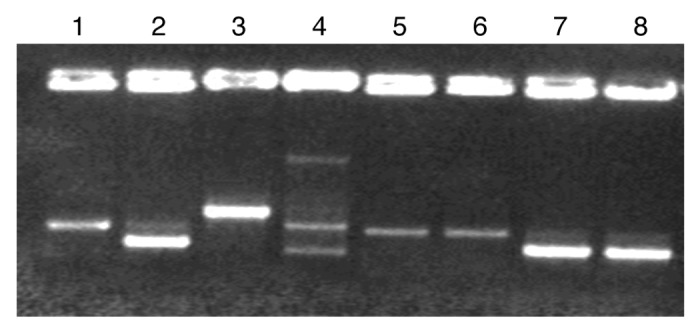
Figure 1. RAPD profiles of different Lb. casei and Lb. rhamnosus strains. (1) Lb. rhamnosus 1/6, (2) Lb. casei E, (3) Lb. casei 163, (4) Lb. casei defensis, (5) Lb. rhamnosus LC-705, (6) Lb. rhamnosus LAB11, (7) Lb. casei LAB5, (8) Lb. casei LAB6.
Survival of starters and Lb. casei LAB5 and LAB6 adjuncts in cheese
During the whole ripening period, according to RAPD profile of isolated colonies, only Lb. casei LAB5 or LAB6 were found on LBS plates. All the isolated LAB5 and LAB6 colonies showed lactose positive phenotype on lactose indicator plates, verifying the presence of the lactose-selectable food-grade plasmids in the strains. If the strains had lost the plasmids, they would have also lost the ability to metabolize lactose. All colonies from LAB6, but not from LAB5, showed PepI activity (data not shown). These tests confirmed that during the whole ripening period, both pLEB600 and pLEB604 plasmids were stable and functional in LAB5 and LAB6, respectively, during the ripening period.
The growth of starters LAB5 and LAB6 during ripening period is shown in Figure 2. Both strains LAB5 and LAB6 survived the whole 12 weeks ripening period and their growth trend was similar in both batches. Strains started to grow from approximately 106 cfu g−1 and by three weeks the bacterial counts stabilized to approximately 108–109 cfu g−1. The starters Lactococcus and Leuconostoc number remained constant throughout the ripening period in the first batch, whereas in the second batch, their number started to decrease after 28 d and they were not detectable after 10 and 12 weeks in cheeses made using LAB6 or LAB5 strains, respectively. In the research by Courtin et al.,23 in some cases more than 99% of the peptidase overexpressing lactococci were not detectable after 28 d of ripening. This decrease is in parallel with the fact that long ripening time results in lactose depletion in cheese. The lack of energy source for lactococci and starter cultures cause cells to lyse. However, in this study, for the first batch, the starter counts, for an unknown reason, did not decrease even after 84 d. Cheese batches were made on two different days. The variation in quality of milk used for the cheese making process such as milk composition, and its lactose content as well as the original microflora can affect the starter number in final product.
Figure 2. The growth of the Lactococcus and Leuconostoc starters, and Lb. casei strains LAB5 and LAB6 in Edam cheese. (A) The first cheese batch; (B) The second cheese batch. ■, Lb. casei LAB5 (vector control); ●, starters in cheese with LAB5; □, Lb. casei LAB6 (PepI-expression); ○, starters in cheese with LAB6. Data are the mean of 2 replications.
The iminopeptidase activity was higher in cheeses made with PepI producing strain LAB6 than those of cheeses containing the vector strain LAB5 (Fig. 3). This indicates that LAB6 increased PepI activity in cheese, thus the strain may promote proline release from peptides.
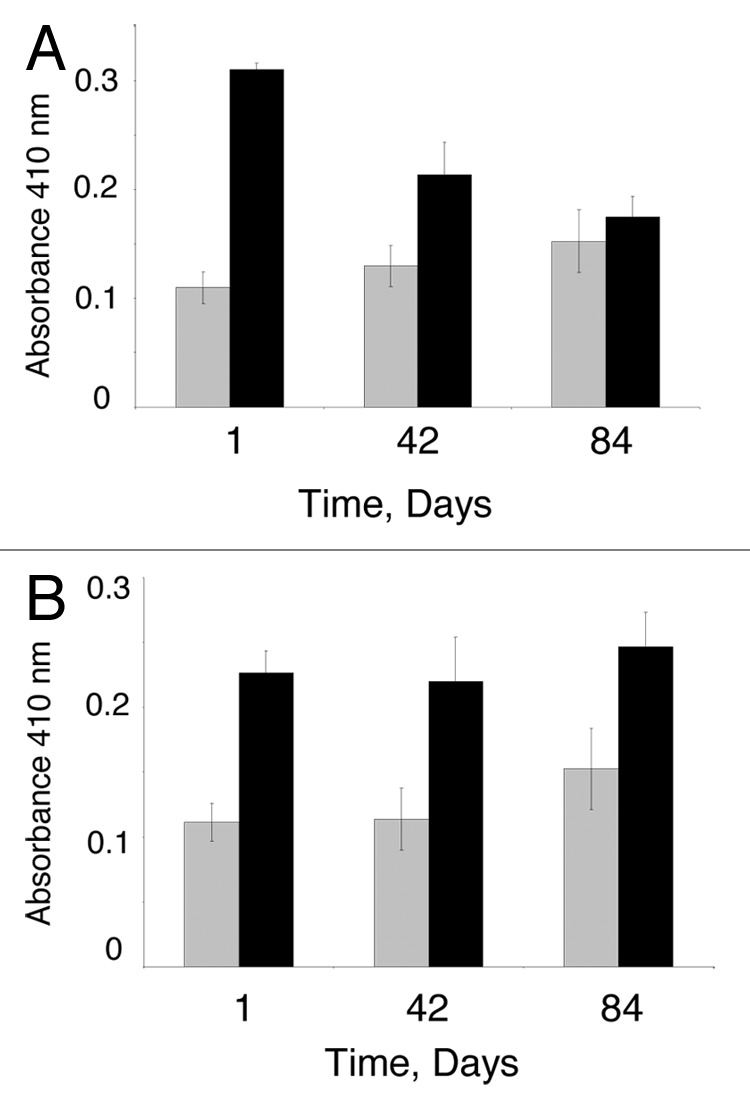
Figure 3. Proline iminopeptidase activity in cheeses made with Lb. casei LAB5 (vector control) and Lb. casei LAB6 (PepI-overexpression). (A) The first batch; (B) The second batch. Grey, cheese containing LAB5 (vector control); black, cheese containing LAB6 (PepI-expression). Data are the mean of 3 replications.
Amino acid profile of Edam cheeses made with Lb. casei LAB5 and LAB6 adjuncts
The amino acid content of cheeses was determined qualitatively and quantitatively using HPLC. The amounts of free amino acid in both cheeses containing LAB5 or LAB6 were approximately at the same level (Fig. 4), meaning LAB6 could not increase the free proline content in the cheese. The PepI is an intracellular enzyme, and its release to cheese matrix requires the lysis of LAB6 cells. If the cells are not lysed extensively, then PepI cannot be effectively released to hydrolyze the substrates. A longer ripening period of the cheese may promote the lysis of LAB6 cells and increase the peptide hydrolysis. Low temperature may also decrease enzyme activity. Edam cheeses in this study were ripened at temperatures of about 12°C. Considering the fact that proline iminopeptidase has an optimum activity at 37°C, lower temperature during ripening can significantly slow down the enzyme activity.26,27 Furthermore, the low amounts of substrate could contribute to the lack of change in the proline profile of the cheese. Proline specific enzymes i.e., PepQ, PepI, PepR, PepX and PepP enzymes isolated and identified from different lactic acid bacteria act very specifically and cleave proline only from a certain position in a polypeptide chain which is specific for that individual enzyme (Table 1). Therefore, in order to release proline from cheese substrate in an efficient way, a combination of different proline-specific enzymes would be required. In future experiments, the LAB6 strain, which showed good potential to grow and produce peptidase activity in cheese, could be engineered to produce several proline-specific enzymes in combination with a mutation weakening the cell wall for increased lysis during ripening of the cheese.
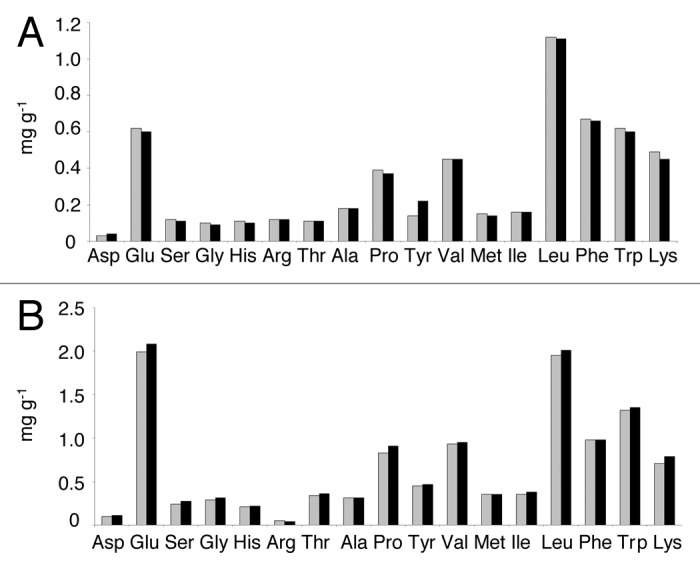
Figure 4. Liberation of amino acids in Edam cheeses after 12 weeks of ripening. (A) The first batch; (B) The second batch. Grey, cheese containing LAB5 (vector control); black, cheese containing LAB6 (PepI-expression).
Materials and Methods
Bacterial strains and growth conditions
LAB5 and LAB6—variants of Lb. casei E, a NSLAB strain isolated from a high quality Edam cheese—were used as adjunct starter cultures.28 LAB5 carries the plasmid pLEB600, a lactose-selectable food-grade vector. LAB6 is a PepI expressing strain carrying the plasmid pLEB604, in which a gene encoding the PepI enzyme is cloned under the control of a constitutive promoter in pLEB600.28 For cheese making, starter cultures Leuconostoc mesenteroides and Lc. lactis (Chr. Hansen) were added as freeze-dried cultures. For colony counting, the starters were grown in M17 agar plates (Oxoid) containing 0.5% glucose. Strains Lb. casei 163 (Danisco), Lb. casei defensis (Danisco), Lb. rhamnosus LC-705 (Valio Ltd), Lb. rhamnosus LAB1129 and Lb. rhamnosus 1/6 (Valio Ltd) were used for RAPD (random amplification of polymorphic DNA) profile comparisons. Lactobacilli other than LAB5 and LAB6 strains were grown in MRS (Oxoid). LAB5 and LAB6 were grown in modified MRS supplemented with 1% lactose at 37°C. Modified MRS lacks glucose and beef extract. In order to follow the plasmid stability during the cheese ripening, lactose utilization was determined from the Lb. casei strains. Modified MRS plates containing 1% lactose and 0.05 mg ml−1 bromocresol purple were used to determine the lactose utilization of LAB5 and LAB6 colonies isolated from cheese. The lactose indicator plates were incubated for 48 h at 37°C. A color change from purple to yellow around lactose-positive colonies was an indication for acid production by the strains.30
Cheese manufacture and ripening
Edam cheese was prepared in two batches. For each batch, cheeses were made using two different strains as adjunct starter: (1) control cheese containing Lb. casei LAB5 with no PepI expressing enzyme, (2) the cheese prepared with Lb. casei LAB6, containing PepI expressing enzyme. About 120 L of milk was used to produce 9.6 kg of cheese. The cheeses were processed essentially according to the method used to produce Edam cheese by Valio Ltd. In brief, milk was pasteurized for 15 sec at 75°C, then it was cooled down to 32°C and starters Lc. lactis and Leuc. mesenteroides (Chr. Hansen Ltd), as well as adjunct Lb. casei LAB5 or LAB6 were added to milk. Adjuncts were added approximately 3 × 105 mL −1 milk, representing about 10% of the amount of starters. After 30 min, rennet was added and coagulation took place for 35 min. Then, the curd was cut, and stirred for 11 min. After that, 30 L of whey was discarded and stirring continued for 16 min at 31°C. Next, 10 L water was added to curd and temperature was raised up to 36°C and stirring continued for 60 min. Thereafter, curd was ready for pressing. Molding process was performed for 3 h at room temperature. After that, curds were salted in 16% brine at 12°C for 8–10 h and then vacuum packaged and ripened at 12°C for 84 d. Final packages contained about 800 g cheese which were stored at 12°C . To monitor the ripening, samples were taken every second week.
Identification of Lactobacillus casei LAB5 and LAB6 in cheese samples
For isolation and identification of Lb. casei strains LAB5 and LAB6, 10 g of cheese was homogenized in a Stomacher (Lab Blender 400, Seward Medical) with 90 ml of 0.9% NaCl. Serial dilutions of homogenates were plated on LBS agar (BBL; Becton Dickinson), and the plates were incubated anaerobically (Anaerocult® A, Merck) in jars at 37°C for 48 h, after which the colonies were counted. After isolation, LAB5 and LAB6 were identified by RAPD technique. Three different RAPD primers (kindly provided by Valio Ltd.) were tested for identification of LAB5 and LAB6 from other closely related species and strains. The most suitable primer, named 6789, was chosen for RAPD-PCR identifications. The chosen RAPD-PCR primer sequence was: 5′-GCT CGT ATG TTG TGT GG-3′. To identify the lactobacilli, 100 random LBS colonies per cheese sample were chosen for colony RAPD-PCR. PCR amplification was directly performed on fresh isolated cells grown on LBS agar plates. RAPD analyses were performed in a 50 μL reaction volume consisting of 5 μL 10 × PCR reaction buffer containing 15 mM MgCl2 (Finnzymes), 1 μL dNTP (10 mM; Finnzymes), 1 μL of the primer (20 μM), 0.5 μL DNA polymerase (DyNAzyme II DNA polymerase 2 U μL−1, Finnzymes) and 42.5 μL of deionized sterile water. RAPD-PCR amplification was performed in an Eppendorf Master Cycler gradient apparatus (Eppendorf AG). The temperature profile in thermocycler was as follows: initial denaturation at 94°C for 2 min, then 35 cycles of 95°C 20 sec, 37°C 30 sec, 72°C 2 min, and final extension in 72°C for 10 min. Amplification products were analyzed electrophoretically in 1% (w/v) agarose gels containing ethidium bromide (0.5 μg mL−1) and visualized under UV light.
PepI enzyme activity in Lb. casei LAB5 and LAB6 isolated from cheese
Fifty colonies of LAB5 and LAB6 isolated from cheese samples were tested by a rapid method for the detection of PepI activity. Colonies from LBS plates were randomly picked and incubated overnight in MRS broth. Next day, the cultures were washed with 50 mM potassium phosphate buffer (pH 7). The pellets were suspended in 100 µL of the same buffer. Then, 25 µL of 20 mM l-proline-ρ-nitroanilide substrate (Sigma-Aldrich) was added, and the tubes were incubated at 37°C for 30 min. PepI producing strains degrade the substrate and release yellow-colored nitroaniline, which was measured spectrophotometrically at 410 nm.
Detection of PepI activity from cheese
Fifty mg of grated cheeses were mixed with 1.5 mL 50 mM potassium phosphate in 2 mL eppendorf tubes and mixed well. Then, 50 μL of 20 mM substrate l-proline-ρ-nitroanilide was added to the mixtures and incubated at 37°C for 24 h. After incubation, the tubes were centrifuged for 10 min at 16,000 g. The supernatants were filtered and measured spectrophotometrically at 410 nm.
Qualitative and quantitative determination of free amino acids in the ripened cheeses
Amino acid analyses were performed from full ripened cheeses (84 d). The free amino acid composition was determined as phenylthiocarbamate derivatives using the Waters Pico Tag method and the preparation of the derivatives and the HPLC analysis were performed according to the instructions of Millipore Corporation by external service (MTT Agrifood Research Center).
Acknowledgments
We want to thank Mr Jyri Rekonen at Viikki dairy pilot plant for the manufacture of the test cheeses.
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/25543
References
- 1.Lee KD, Lo CG, Warthesen JJ. Removal of bitterness from the bitter peptides extracted from cheddar cheese with peptidases from Lactococcus lactis ssp cremoris SK11. J Dairy Sci. 1996;79:1521–8. doi: 10.3168/jds.S0022-0302(96)76512-8. [DOI] [Google Scholar]
- 2.Fernández-Esplá MD, Martín-Hernández MC, Fox PF. Purification and characterization of a prolidase from Lactobacillus casei subsp. casei IFPL 731. Appl Environ Microbiol. 1997;63:314–6. doi: 10.1128/aem.63.1.314-316.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Savijoki K, Ingmer H, Varmanen P. Proteolytic systems of lactic acid bacteria. Appl Microbiol Biotechnol. 2006;71:394–406. doi: 10.1007/s00253-006-0427-1. [DOI] [PubMed] [Google Scholar]
- 4.Habibinajafi MB, Lee BH. Purification and characterization of proline iminopeptidase from Lactobacillus casei ssp. casei LLG. J Dairy Sci. 1995;78:251–9. doi: 10.3168/jds.S0022-0302(95)76632-2. [DOI] [Google Scholar]
- 5.El Soda M, Madkor SA, Tong PS. Adjunct cultures: recent developments and potential significance to the cheese industry. J Dairy Sci. 2000;83:609–19. doi: 10.3168/jds.S0022-0302(00)74920-4. [DOI] [PubMed] [Google Scholar]
- 6.Fallico V, McSweeney PLH, Horne J, Pediliggieri C, Hannon JA, Carpino S, et al. Evaluation of bitterness in Ragusano cheese. J Dairy Sci. 2005;88:1288–300. doi: 10.3168/jds.S0022-0302(05)72795-8. [DOI] [PubMed] [Google Scholar]
- 7.Azarnia S, Robert N, Lee B. Biotechnological methods to accelerate cheddar cheese ripening. Crit Rev Biotechnol. 2006;26:121–43. doi: 10.1080/07388550600840525. [DOI] [PubMed] [Google Scholar]
- 8.Soeryapranata E, Powers JR, Unlu G. Cloning and characterization of debittering peptidases, PepE, PepO, PepO2, PepO3, and PepN of Lactobacillus helveticus WSU19. Int Dairy J. 2007;17:1096–106. doi: 10.1016/j.idairyj.2007.02.002. [DOI] [Google Scholar]
- 9.Ishibashi N, Kubo T, Chino M, Fukui H, Shinoda I, Kikuchi F. Studies on flavored peptides. 4. taste of proline-containing peptides. Agric Biol Chem. 1988;52:95–8. doi: 10.1271/bbb1961.52.95. [DOI] [Google Scholar]
- 10.Christensen JE, Dudley EG, Pederson JA, Steele JL. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek. 1999;76:217–46. doi: 10.1023/A:1002001919720. [DOI] [PubMed] [Google Scholar]
- 11.Tchorbanov B, Marinova M, Grozeva L. Debittering of protein hydrolysates by Lactobacillus LBL-4 aminopeptidase. Enz Res 2011; 1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nardi M, Chopin MC, Chopin A, Cals MM, Gripon JC. Cloning and DNA sequence analysis of an X-prolyl dipeptidyl aminopeptidase gene from Lactococcus lactis subsp. lactis NCDO 763. Appl Environ Microbiol. 1991;57:45–50. doi: 10.1128/aem.57.1.45-50.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gilbert C, Atlan D, Blanc B, Portalier R. Proline iminopeptidase from Lactobacillus delbrueckii subsp. bulgaricus CNRZ 397: purification and characterization. Microbiology. 1994;140:537–42. doi: 10.1099/00221287-140-3-537. [DOI] [PubMed] [Google Scholar]
- 14.Klein JR, Schmidt U, Plapp R. Cloning, heterologous expression, and sequencing of a novel proline iminopeptidase gene, pepI, from Lactobacillus delbrueckii subsp. lactis DSM 7290. Microbiology. 1994;140:1133–9. doi: 10.1099/13500872-140-5-1133. [DOI] [PubMed] [Google Scholar]
- 15.Stucky K, Klein JR, Schüller A, Matern H, Henrich B, Plapp R. Cloning and DNA sequence analysis of pepQ, a prolidase gene from Lactobacillus delbrueckii subsp. lactis DSM7290 and partial characterization of its product. Mol Gen Genet. 1995;247:494–500. doi: 10.1007/BF00293152. [DOI] [PubMed] [Google Scholar]
- 16.Varmanen P, Savijoki K, Åvall S, Palva A, Tynkkynen S. X-prolyl dipeptidyl aminopeptidase gene (pepX) is part of the glnRA operon in Lactobacillus rhamnosus. J Bacteriol. 2000;182:146–54. doi: 10.1128/JB.182.1.146-154.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.El Abboudi M, El Soda M, Pandian S, Barreau M, Trepanier G, Simard RE. Peptidase activities in debittering and nondebittering strains of Lactobacilli. Int Dairy J. 1992;2:55–64. doi: 10.1016/0958-6946(92)90044-M. [DOI] [Google Scholar]
- 18.Beresford TP, Fitzsimons NA, Brennan NL, Cogan TM. Recent advances in cheese microbiology. Int Dairy J. 2001;11:259–74. doi: 10.1016/S0958-6946(01)00056-5. [DOI] [Google Scholar]
- 19.Marilley L, Casey MG. Flavours of cheese products: metabolic pathways, analytical tools and identification of producing strains. Int J Food Microbiol. 2004;90:139–59. doi: 10.1016/S0168-1605(03)00304-0. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson MG, Kilcawley KN. Mechanisms of incorporation and release of enzymes into cheese during ripening. Int Dairy J. 2005;15:817–30. doi: 10.1016/j.idairyj.2004.08.021. [DOI] [Google Scholar]
- 21.Gomez MJ, Gaya P, Nunez M, Medina M. Effect of Lactobacillus plantarum as adjunct starter on the flavour and texture of a semi-hard cheese made from pasteurised cows' milk. Lait. 1996;76:461–72. doi: 10.1051/lait:1996535. [DOI] [Google Scholar]
- 22.Benech RO, Kheadr EE, Lacroix C, Fliss I. Impact of nisin producing culture and liposome-encapsulated nisin on ripening of Lactobacillus added-Cheddar cheese. J Dairy Sci. 2003;86:1895–909. doi: 10.3168/jds.S0022-0302(03)73776-X. [DOI] [PubMed] [Google Scholar]
- 23.Courtin P, Nardi M, Wegmann U, Joutsjoki V, Ogier JC, Gripon JC, et al. Accelerating cheese proteolysis by enriching Lactococcus lactis proteolytic system with lactobacilli peptidases. Int Dairy J. 2002;12:447–54. doi: 10.1016/S0958-6946(02)00022-5. [DOI] [Google Scholar]
- 24.Guldfeldt LU, Sørensen KI, Stroman P, Behrndt H, Williams D, Johansen E. Effect of starter cultures with a genetically modified peptidolytic or lytic system on Cheddar cheese ripening. Int Dairy J. 2001;11:373–82. doi: 10.1016/S0958-6946(01)00066-8. [DOI] [Google Scholar]
- 25.Anastasiou R, Papadelli M, Georgalaki MD, Kalantzopoulos G, Tsakalidou E. Cloning and sequencing of the gene encoding X-prolyl-dipeptidyl aminopeptidase (PepX) from Streptococcus thermophilus strain ACA-DC 4. J Appl Microbiol. 2002;93:52–9. doi: 10.1046/j.1365-2672.2002.01659.x. [DOI] [PubMed] [Google Scholar]
- 26.Wegmann U, Klein JR, Drumm I, Kuipers OP, Henrich B. Introduction of peptidase genes from Lactobacillus delbrueckii subsp. lactis into Lactococcus lactis and controlled expression. Appl Environ Microbiol. 1999;65:4729–33. doi: 10.1128/aem.65.11.4729-4733.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luoma S, Peltoniemi K, Joutsjoki V, Rantanen T, Tamminen M, Heikkinen I, et al. Expression of six peptidases from Lactobacillus helveticus in Lactococcus lactis. Appl Environ Microbiol. 2001;67:1232–8. doi: 10.1128/AEM.67.3.1232-1238.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takala TM, Saris PE, Tynkkynen SS. Food-grade host/vector expression system for Lactobacillus casei based on complementation of plasmid-associated phospho-β-galactosidase gene lacG. Appl Microbiol Biotechnol. 2003;60:564–70. doi: 10.1007/s00253-002-1153-y. [DOI] [PubMed] [Google Scholar]
- 29.Beasley SS, Manninen TJK, Saris PEJ. Lactic acid bacteria isolated from canine faeces. J Appl Microbiol. 2006;101:131–8. doi: 10.1111/j.1365-2672.2006.02884.x. [DOI] [PubMed] [Google Scholar]
- 30.Matos J, Nardi M, Kumura H, Monnet V. Genetic characterization of pepP, which encodes an aminopeptidase P whose deficiency does not affect Lactococcus lactis growth in milk, unlike deficiency of the X-prolyl dipeptidyl aminopeptidase. Appl Environ Microbiol. 1998;64:4591–5. doi: 10.1128/aem.64.11.4591-4595.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]



