Abstract
The co-fermentation of glucose and xylose is one of the issues in decreasing the price of biofuel or chemicals produced from lignocellulosic materials. A glucose and xylose co-utilizing Saccharomyces cerevisiae was obtained through rational genetic manipulation. Non-rational evolution in xylose was performed, and the xylose utilization efficiency of the engineered strain was significantly enhanced. The results of transcriptome study suggested that Snf1/Mig1-mediated regulation, a part of glucose sensing and repression network, was altered in the evolved strain and might be related to the enhancement of xylose utilization.
Keywords: xylose fermentation, ethanol, Saccharomyces cerevisiae, adaptive evolution, glucose repression, Mig1, Snf1
With the worldwide interest in producing fuel ethanol from lignocellulosic feedstock, the utilization of xylose, the second most abundant component in lignocelluloses, has been widely studied. Saccharomyces cerevisiae is an ethanol-producing microorganism with high tolerance to stressful environment. This microorganism has excellent capacity to produce ethanol from hexose and can be endowed with xylose-utilizing capacity by introducing heterogeneous xylose metabolic pathways.1-3 Xylose is usually converted to xylulose by xylose reductase-xylitol dehydrogenase or xylose isomerase (XI). The produced xylulose is phosphorylated by endogenous xylulokinase (XK) and enters the endogenous pentose phosphate pathway (PPP) in recombinant S.cerevisiae. Although overexpressing the genes of XK and enzymes involved in PPP can improve the xylose metabolism of the recombinant strain, the efficiency is still low.1,4 Previous studies used the adaptive evolution strategy to obtain the mutants of engineered S.cerevisiae with significantly enhanced xylose fermentation capacity.1,2 With subsequent “omics” analysis and inverse metabolic engineering works, several facilitated factors for xylose utilization were discovered.5-7
Our previous study2 constructed a strain that expresses the Piromyces sp XI gene Pi-xylA, overexpresses the genes of XK and PPP, deletes the aldose reductase gene GRE3, and destroys the respiration by deleting the subunit IV of cytochrome c oxidase gene COX4. After the long-term evolution in xylose, the strain had improved xylose fermentation ability and obtained the capacity to simultaneously convert glucose and xylose to ethanol.2 The global transcription profile of the evolved strain BSPX013 (Pi-XI, XK, gre3::PPP, cox4,AE) was compared with that of the reference strain BSPC095 (Pi-XI, XK, gre3::PPP, cox4). Aside from the doubled xylose isomerase activity, the effects of several functional genes, such as PFK27, PDC6, and PHO13, to xylose fermentation were further confirmed. The results suggested that these genes are not the only ones endowing the evolved strain with efficient xylose metabolism capacity.
So far, the repression of glucose to xylose was mainly described at the absorption level because the native hexose transporters in charge of the xylose uptake in S.cerevisiae but has much lower affinity to xylose than to glucose.1 Nonetheless, as a novel substrate for S.cerevisiae, xylose metabolism is suggested to be sub-optimal because the xylose-grown cells fail to activate appropriate genes.3 In S.cerevisiae, the enzymes required for the utilization of alternative carbon source were absent or kept in a low level when glucose was present. This phenomenon is known as carbon catabolite repression or glucose repression. Glucose repression mainly occurs at the transcriptional level; although it is also related to the alteration of protein synthesis and degradation.8 In the present addendum, the possible mechanisms underlying xylose metabolism in the glucose repression system through transcriptional analysis were further discussed.
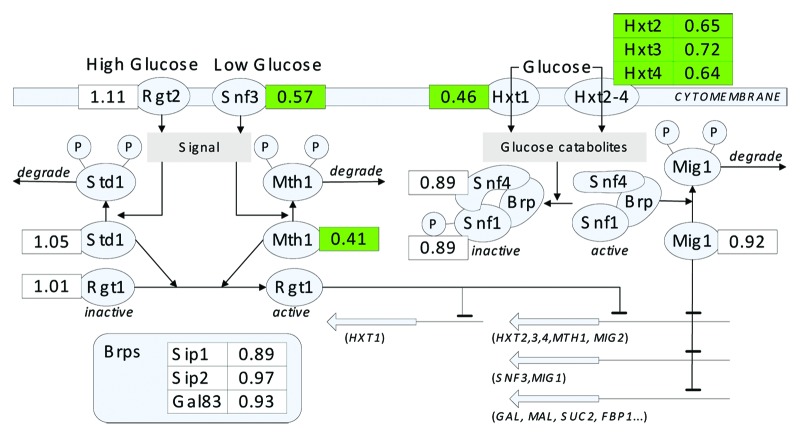
The RNAs used for transcriptional analysis were extracted from the cells cultured in a mixture of 10 g l−1 glucose and 20 g l−1 xylose and collected in the middle of exponential phase (OD600 = 0.8). The expression levels of genes encoding plasma membrane low glucose sensor (Snf3), negative regulator of the glucose-sensing signal transduction pathway (Mth1), and the glucose transporters (Hxts) in the evolved strain were notably lower than those in its parent strain (Fig. 1). Furthermore, the transcriptional levels of genes involved in the utilization of alternative carbon sources, such as the GAL1, GAL2, MAL12, MAL31and MAL32, were significantly decreased in the evolved strain; only the expression levels of SUC2 and FBP1 were increased (Table 1). Current studies found that the glucose catabolites inhibit the activity of the Snf1 protein kinase complex (including Snf1, Snf4, Sip1, Sip2, Gal83, and so on) to prevent Mig1 phosphorylation. The hypophosphorylated Mig1 moves into the nucleus and represses the expression of several genes, such as HXT2, HXT3, HXT4, MTH1, SNF3, and genes involved in the utilization of alternative carbon sources such as galactose, maltose, and sucrose.8,9 The decreased transcription of the genes mentioned above indicated that less Mig1 was phosphorylate by Snf1 complex. Aside from Mig1, other Snf1-related pathways, such as response to stress (GO: 0006950), glycogen biosynthesis (GO: 0005978), and trehalose biosynthesis (GO: 0005992),10,11 were reported in the transcriptional analysis of our evolved strain. All of the results suggested an altered glucose sensing and repression network in the evolved strain. It was reported that the gene HXT2, HXT3, SNF3, MTH1, HXK1, HXT16, SUC2, MAL11, MAL31, and MAL32, which are all repressed by glucose via the Snf1/Mig1-pathway, expressed higher level in the xylose-grown cells compared with the glucose repressed cells.12 This suggested that the xylose metabolism capacity of strain related to the regulation of Snf1/Mig1-pathway. Given that although the deletion of MIG1 gene did not improve the xylose utilization during batch cultivation but increased 25% xylose consumption rate during chemostat cultivation,13 we proposed that the altered Snf/Mig1 regulation might affect the xylose utilization in some aspects. Nonetheless, how this benefit to the xylose utilization of S.cerevisiae has remained to be elucidated.
Figure 1. Comparison of transcriptional changes in the genes involved in glucose sensing and repression network between the evolved strain BSPX013 and the reference strain BSPC095.2 The cells were cultured in minimal medium with 10 g l−1 glucose and 20 g l−1 xylose, and then collected when OD600 was 0.8. In yeast, the glucose sensing and repression network consists of two signal transduction pathways. In the first pathway, the glucose is sensed by Rgt2 and Snf3, whereas the generated signal leads to the degradation of Std1 and Mth1. Without Std1 and Mth1, Rgt1 is inactive, thus resulting in the transcriptional derepression of its target genes. In the second pathway, the glucose catabolites inhibit the activity of the Snf1 protein kinase complex to prevent Mig1 phosphorylation. The Snf1 protein kinase complex consists of Snf1, Snf4, and bridging proteins (Brp). The Brps can be Gal83, Sip1, Sip2, or some other protein as yet unidentified. The hypophosphorylated Mig1 moves into the nucleus and represses the expression of several genes, such as HXT2, HXT3, HXT4, MTH1, SNF3, and genes involved in the utilization of alternative carbon sources.8-10
Table 1. Transcriptional changes of the genes involved in the utilization of alternative carbon source.
| Genes | Description | Fold-change (BSPX013 vs. BSPC095)2 |
|---|---|---|
| HXK1 | Hexokinase isoenzyme | 0.47 |
| HXT16 | Protein of unknown function with similarity to hexose transporter family members | 0.13 |
| GAL1 | Galactokinase | 0.42 |
| GAL2 | Galactosepermease | 0.18 |
| MAL12 | Alpha-glucosidase | 0.45 |
| MAL31 | Maltose permease | 0.33 |
| MAL32 | Alpha-glucosidase | 0.36 |
| SUC2 | Invertase | 1.23 |
| FBP1 | Fructose-1,6-bisphosphatase | 1.16 |
In conclusion, the results of transcriptome analysis suggested that the alteration in glucose sensing and repression network occurred in our evolved strain. This global alteration might contribute to the enhancement of xylose utilization capacity.
Acknowledgments
This work was supported by the National Key Basic Research Program (2011CB707405), the National High-Technology Research and Development Program of China under Grant (2012AA022106), the National Natural Science Foundation of China (30970091, 31070096 and 31270151), the International S&T Cooperation Program of China (2010DFA32560), and Independent Innovation Foundation of Shandong University, IIFSDU (2012TB003).
Disclosure of Potential Conflicts of Interest
No potential conflict of interest was disclosed.
Footnotes
Previously published online: www.landesbioscience.com/journals/bioe/article/25542
References
- 1.Kim SR, Park YC, Jin YS, Seo JH. Strain engineering of Saccharomyces cerevisiae for enhanced xylose metabolism. Biotechnol Adv. 2013 doi: 10.1016/j.biotechadv.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Shen Y, Chen X, Peng B, Chen L, Hou J, Bao X. An efficient xylose-fermenting recombinant Saccharomyces cerevisiae strain obtained through adaptive evolution and its global transcription profile. Appl Microbiol Biotechnol. 2012;96:1079–91. doi: 10.1007/s00253-012-4418-0. [DOI] [PubMed] [Google Scholar]
- 3.Souto-Maior AM, Runquist D, Hahn-Hägerdal B. Crabtree-negative characteristics of recombinant xylose-utilizing Saccharomyces cerevisiae. J Biotechnol. 2009;143:119–23. doi: 10.1016/j.jbiotec.2009.06.022. [DOI] [PubMed] [Google Scholar]
- 4.Cai Z, Zhang B, Li Y. Engineering Saccharomyces cerevisiae for efficient anaerobic xylose fermentation: reflections and perspectives. Biotechnol J. 2012;7:34–46. doi: 10.1002/biot.201100053. [DOI] [PubMed] [Google Scholar]
- 5.Kuyper M, Hartog MM, Toirkens MJ, Almering MJ, Winkler AA, van Dijken JP, et al. Metabolic engineering of a xylose-isomerase-expressing Saccharomyces cerevisiae strain for rapid anaerobic xylose fermentation. FEMS Yeast Res. 2005;5:399–409. doi: 10.1016/j.femsyr.2004.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Sonderegger M, Sauer U. Evolutionary engineering of Saccharomyces cerevisiae for anaerobic growth on xylose. Appl Environ Microbiol. 2003;69:1990–8. doi: 10.1128/AEM.69.4.1990-1998.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou H, Cheng JS, Wang BL, Fink GR, Stephanopoulos G. Xylose isomerase overexpression along with engineering of the pentose phosphate pathway and evolutionary engineering enable rapid xylose utilization and ethanol production by Saccharomyces cerevisiae. Metab Eng. 2012;14:611–22. doi: 10.1016/j.ymben.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Gancedo JM. Yeast carbon catabolite repression. Microbiol Mol Biol Rev. 1998;62:334–61. doi: 10.1128/mmbr.62.2.334-361.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuttykrishnan S, Sabina J, Langton LL, Johnston M, Brent MR. A quantitative model of glucose signaling in yeast reveals an incoherent feed forward loop leading to a specific, transient pulse of transcription. Proc Natl Acad Sci U S A. 2010;107:16743–8. doi: 10.1073/pnas.0912483107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedbacker K, Carlson M. SNF1/AMPK pathways in yeast. Front Biosci. 2008;13:2408–20. doi: 10.2741/2854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Usaite R, Jewett MC, Oliveira AP, Yates JR, 3rd, Olsson L, Nielsen J. Reconstruction of the yeast Snf1 kinase regulatory network reveals its role as a global energy regulator. Mol Syst Biol. 2009;5:319. doi: 10.1038/msb.2009.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Salusjärvi L, Kankainen M, Soliymani R, Pitkänen JP, Penttilä M, Ruohonen L. Regulation of xylose metabolism in recombinant Saccharomyces cerevisiae. Microb Cell Fact. 2008;7:18. doi: 10.1186/1475-2859-7-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roca C, Haack MB, Olsson L. Engineering of carbon catabolite repression in recombinant xylose fermenting Saccharomyces cerevisiae. Appl Microbiol Biotechnol. 2004;63:578–83. doi: 10.1007/s00253-003-1408-2. [DOI] [PubMed] [Google Scholar]