Abstract
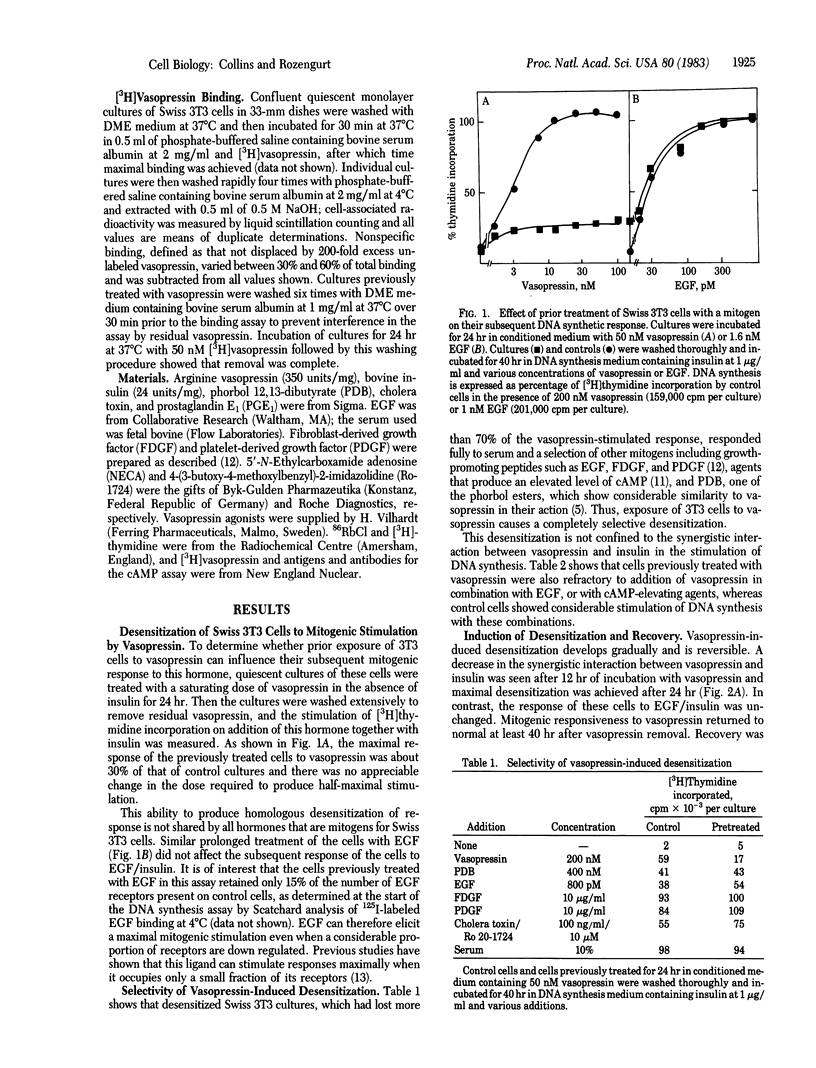
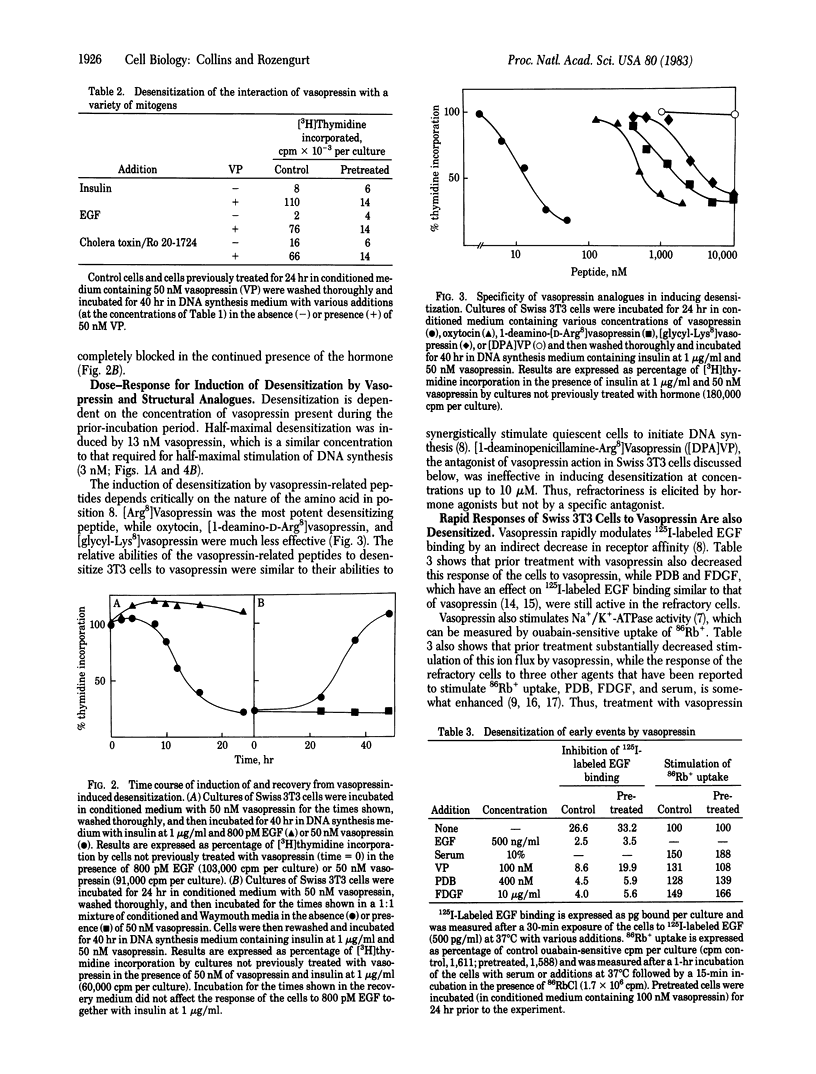
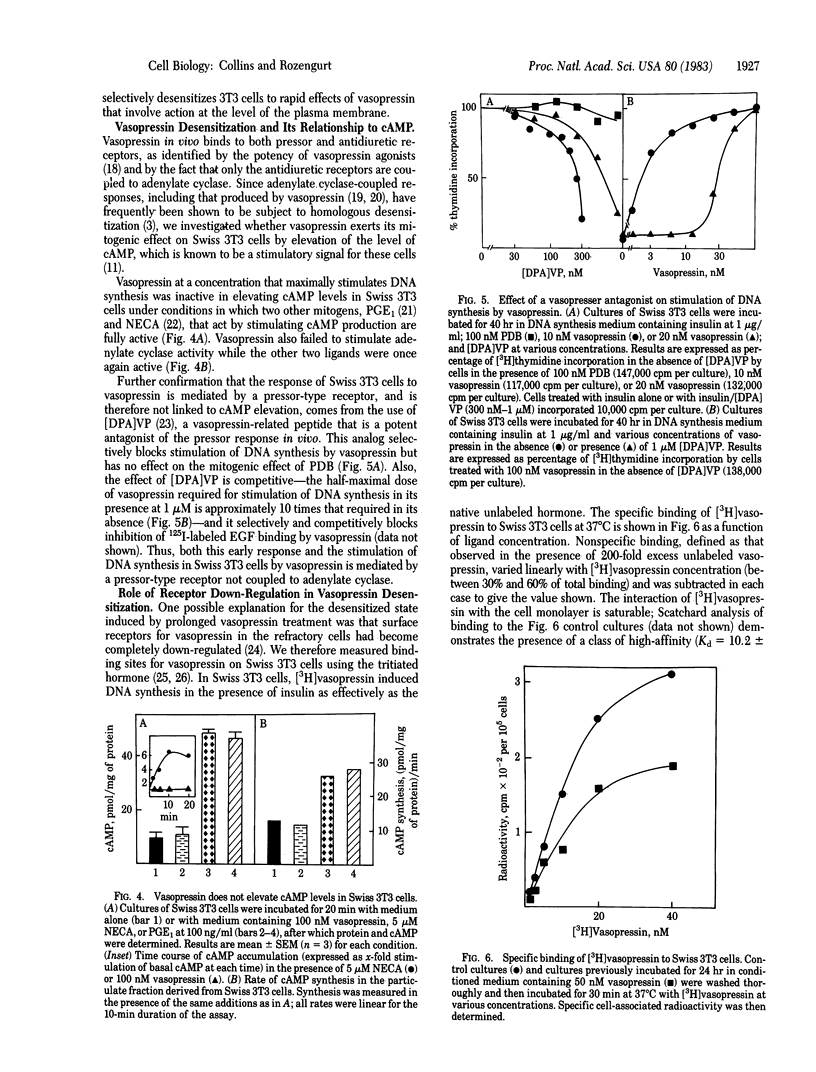
Prior incubation of quiescent cultures of Swiss 3T3 cells with vasopressin leads to loss of mitogenic stimulation on its subsequent addition in the presence of a synergistic growth factor. This desensitization is selective for vasopressin, requires prolonged incubation (half-maximal desensitization after 12 hr of treatment) for its induction, and is reversed after a 48-hr incubation in the absence of vasopressin. It is elicited by concentrations of vasopressin, and several analogues, similar to those required for stimulation of DNA synthesis. Inhibition of 125I-labeled epidermal growth factor binding and stimulation of 86Rb+ uptake by vasopressin are also selectively decreased in the refractory cells. The vasopressin receptors that mediate mitogenesis in Swiss 3T3 cells are of the pressor type, not coupled to adenylate cyclase. These cells bind [3H]vasopressin in a specific and saturable (Kd = 1 X 10(-8) M) manner. The receptors are down-regulated after prolonged vasopressin treatment; however, this cannot provide a complete explanation of desensitization because cells that are completely refractory to vasopressin retain 60% of their [3H]vasopressin binding sites. Vasopressin refractoriness must therefore occur partly at a post-receptor locus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bankowski K., Manning M., Haldar J., Sawyer W. H. Design of potent antagonists of the vasopressor response to arginine-vasopressin. J Med Chem. 1978 Sep;21(9):850–853. doi: 10.1021/jm00207a002. [DOI] [PubMed] [Google Scholar]
- Bourne H. R., Rozengurt E. An 18,000 molecular weight polypeptide induces early events and stimulates DNA synthesis in cultured cells. Proc Natl Acad Sci U S A. 1976 Dec;73(12):4555–4559. doi: 10.1073/pnas.73.12.4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown K. D., Dicker P., Rozengurt E. Inhibition of epidermal growth factor binding to surface receptors by tumor promotors. Biochem Biophys Res Commun. 1979 Feb 28;86(4):1037–1043. doi: 10.1016/0006-291x(79)90221-3. [DOI] [PubMed] [Google Scholar]
- Catt K. J., Harwood J. P., Aguilera G., Dufau M. L. Hormonal regulation of peptide receptors and target cell responses. Nature. 1979 Jul 12;280(5718):109–116. doi: 10.1038/280109a0. [DOI] [PubMed] [Google Scholar]
- Collins M. K., Rozengurt E. Binding of phorbol esters to high-affinity sites on murine fibroblastic cells elicits a mitogenic response. J Cell Physiol. 1982 Jul;112(1):42–50. doi: 10.1002/jcp.1041120108. [DOI] [PubMed] [Google Scholar]
- Collins M., Rozengurt E. Stimulation of DNA synthesis in murine fibroblasts by the tumour promoter teleocidin: relationship to phorbol esters and vasopressin. Biochem Biophys Res Commun. 1982 Feb 26;104(4):1159–1166. doi: 10.1016/0006-291x(82)91372-9. [DOI] [PubMed] [Google Scholar]
- Dicker P., Pohjanpelto P., Pettican P., Rozengurt E. Similarities between fibroblast-derived growth factor and platelet-derived growth factor. Exp Cell Res. 1981 Sep;135(1):221–227. doi: 10.1016/0014-4827(81)90314-1. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol ester stimulation of Na influx and Na-K pump activity in Swiss 3T3 cells. Biochem Biophys Res Commun. 1981 May 15;100(1):433–441. doi: 10.1016/s0006-291x(81)80115-5. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Phorbol esters and vasopressin stimulate DNA synthesis by a common mechanism. Nature. 1980 Oct 16;287(5783):607–612. doi: 10.1038/287607a0. [DOI] [PubMed] [Google Scholar]
- Dicker P., Rozengurt E. Stimulation of DNA synthesis by transient exposure of cell cultures to TPA or polypeptide mitogens: induction of competence or incomplete removal? J Cell Physiol. 1981 Oct;109(1):99–109. doi: 10.1002/jcp.1041090112. [DOI] [PubMed] [Google Scholar]
- Lefkowitz R. J., Wessels M. R., Stadel J. M. Hormones, receptors, and cyclic AMP: their role in target cell refractoriness. Curr Top Cell Regul. 1980;17:205–230. doi: 10.1016/b978-0-12-152817-1.50011-0. [DOI] [PubMed] [Google Scholar]
- Mendoza S. A., Wigglesworth N. M., Rozengurt E. Vasopressin rapidly stimulates Na entry and Na-K pump activity in quiescent cultures of mouse 3T3 cells. J Cell Physiol. 1980 Oct;105(1):153–162. doi: 10.1002/jcp.1041050117. [DOI] [PubMed] [Google Scholar]
- Michell R. H., Kirk C. J., Billah M. M. Hormonal stimulation of phosphatidylinositol breakdown with particular reference to the hepatic effects of vasopressin. Biochem Soc Trans. 1979 Oct;7(5):861–865. doi: 10.1042/bst0070861. [DOI] [PubMed] [Google Scholar]
- Roy C., Ausiello D. A. Characterization of (8-lysine) vasopressin binding sites on a pig kidney cell line (LLC-PK1). Evidence for hormone-induced receptor transition. J Biol Chem. 1981 Apr 10;256(7):3415–3422. [PubMed] [Google Scholar]
- Roy C., Guillon G., Jard S. Hormone-dependent desensitization of vasopressin-sensitive adenylate cyclase. Biochem Biophys Res Commun. 1976 Oct 18;72(4):1265–1270. doi: 10.1016/s0006-291x(76)80151-9. [DOI] [PubMed] [Google Scholar]
- Rozengurt E. Adenosine receptor activation in quiescent Swiss 3T3 cells. Enhancement of cAMP levels, DNA synthesis and cell division. Exp Cell Res. 1982 May;139(1):71–78. doi: 10.1016/0014-4827(82)90319-6. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Brown K. D., Pettican P. Vasopressin inhibition of epidermal growth factor binding to cultured mouse cells. J Biol Chem. 1981 Jan 25;256(2):716–722. [PubMed] [Google Scholar]
- Rozengurt E., Collins M., Brown K. D., Pettican P. Inhibition of epidermal growth factor binding to mouse cultured cells by fibroblast-derived growth factor. Evidence for an indirect mechanism. J Biol Chem. 1982 Apr 10;257(7):3680–3686. [PubMed] [Google Scholar]
- Rozengurt E. Cyclic AMP: a growth-promoting signal for mouse 3T3 cells. Adv Cyclic Nucleotide Res. 1981;14:429–442. [PubMed] [Google Scholar]
- Rozengurt E., Heppel L. A. Serum rapidly stimulates ouabain-sensitive 86-RB+ influx in quiescent 3T3 cells. Proc Natl Acad Sci U S A. 1975 Nov;72(11):4492–4495. doi: 10.1073/pnas.72.11.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Pettican P. Vasopressin stimulation of mouse 3T3 cell growth. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1284–1287. doi: 10.1073/pnas.76.3.1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Legg A., Strang G., Courtenay-Luck N. Cyclic AMP: a mitogenic signal for Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4392–4396. doi: 10.1073/pnas.78.7.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shechter Y., Hernaez L., Cuatrecasas P. Epidermal growth factor: biological activity requires persistent occupation of high-affinity cell surface receptors. Proc Natl Acad Sci U S A. 1978 Dec;75(12):5788–5791. doi: 10.1073/pnas.75.12.5788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith J. B., Rozengurt E. Serum stimulates the Na+,K+ pump in quiescent fibroblasts by increasing Na+ entry. Proc Natl Acad Sci U S A. 1978 Nov;75(11):5560–5564. doi: 10.1073/pnas.75.11.5560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tell G. P., Haour F., Saez J. M. Hormonal regulation of membrane receptors and cell responsiveness: a review. Metabolism. 1978 Oct;27(10):1566–1592. doi: 10.1016/s0026-0495(78)80029-8. [DOI] [PubMed] [Google Scholar]


