Abstract
Preconditioning is a phenomenon in which brief episodes of a sublethal insult induce robust protection against subsequent lethal injuries. Preconditioning has been observed in multiple organisms and can occur in the brain as well as other tissues. Extensive animal studies suggest that the brain can be preconditioned to resist acute injuries, such as ischemic stroke, neonatal hypoxia/ischemia, trauma, and agents that are used in models of neurodegenerative diseases, such as Parkinson’s disease and Alzheimer’s disease. Effective preconditioning stimuli are numerous and diverse, ranging from transient ischemia, hypoxia, hyperbaric oxygen, hypothermia and hyperthermia, to exposure to neurotoxins and pharmacological agents. The phenomenon of “cross-tolerance,” in which a sublethal stress protects against a different type of injury, suggests that different preconditioning stimuli may confer protection against a wide range of injuries. Research conducted over the past few decades indicates that brain preconditioning is complex, involving multiple effectors such as metabolic inhibition, activation of extra- and intracellular defense mechanisms, a shift in the neuronal excitatory/inhibitory balance, and reduction in inflammatory sequelae. An improved understanding of brain preconditioning should help us identify innovative therapeutic strategies that prevent or at least reduce neuronal damage in susceptible patients. In this review, we focus on the experimental evidence of preconditioning in the brain and systematically survey the models used to develop paradigms for neuroprotection, and then discuss the clinical potential of brain preconditioning. In a subsequent components of this two-part series, we will discuss the cellular and molecular events that are likely to underlie these phenomena.
1.0 Introduction
Until now, few pharmacological agents have been successfully translated to human acute brain injury (e.g., stroke or traumatic brain injury (TBI)) and other neurodegenerative diseases, even though many molecules have initially appeared promising in animal models. Although the reason for these failures is unclear, we believe that understanding the means and mechanisms of stimuli that trigger an endogenous and pluripotent neuroprotective response in the brain will aid in the translation of interventions to prevent and/or limit many human disorders. As long ago as the 16th century, the toxicologist Paracelsus observed, “The dose makes the poison.” A corollary is that subtoxic doses of cellular stress can lead to the generation of a protective state, termed “preconditioning.” Preconditioning has been most commonly employed in studies of ischemia. In stroke models, sublethal ischemic episodes reduce the size of an infarct in response to a subsequent longer duration ischemic challenge (Kirino et al., 1991; Kitagawa et al., 1990; Liu et al., 1992). Consistently, evidence suggests that transient ischemic attacks may precondition humans against stroke (Moncayo et al., 2000; Weih et al., 1999). Such preconditioning can be elicited throughout the body, but it is of particular clinical interest in brain where the majority of neurons cannot regenerate and strokes can leave humans severely disabled.
The purpose of this two-part review is to demonstrate that preconditioning is actually a widely applicable phenomenon that can be applied to many disease states in addition to ischemia. Neurodegenerative diseases in particular involve a lengthy prodromal phase during which there may be subtoxic stress caused by a wide variety of events, some of which are similar to subtoxic insults prevalent in the brain of individuals suffering from ischemic stroke. In a widely cited review, it was argued that preconditioning is stereotypical in nature, that is, it follows a similar pattern in both cause and effect (Dirnagl et al., 2003). If true, many findings from stroke models might be generalized to other disease models, including models of neurodegeneration. This is of obvious clinical importance because it would broaden the array of possible therapeutic targets for a multiplicity of diseases.
In order to review both the basic scientific literature as well as clinical applications, this review will explore the various preconditioning protocols currently used. In a second review we will focus on the underlying cellular mechanisms identified to date. In this first part of the two-part review, we will detail the preconditioning paradigms to induce brain tolerance against ischemic stroke and other acute brain injuries as well as neurodegenerative states that have been employed in animal models by categorizing the preconditioning stimuli. Part two of the review will deal with the mechanisms thus far understood to contribute to the preconditioned, or tolerant, state.
2.0 Relevant models of CNS diseases
In order to conceptualize the neural diseases that have appeared to be sensitive to preconditioning-induced protection, we will first describe the model systems of the major disease states within this section. The major models will be briefly described, not as a conclusive survey, but rather as a foundation upon which one can understand the disease context in which various preconditioning stimuli were found to be protective.
2.1 Global/focal ischemia
Ischemic brain injuries, resulting either from global or focal decreases in perfusion, are one of the most common causes of death and the leading cause of adult disability worldwide. Although many neuroprotective drugs have been shown to reduce infarction and improve neurological functioning in animal models of stroke, only tissue plasminogen activator (tPA) has been successfully translated into clinical application. The negative results of these attempts have prompted research of endogenous modulators of neuroprotection as an alternative approach to therapy. Such endogenous modulators mediate the ischemic tolerance phenomenon, which was first identified in brain in the early 1990s and shown to have a neuroprotective role in cerebral ischemia (Kitagawa et al., 1991; Kitagawa et al., 1990).
The primary model of stroke used in basic research targets the region of the middle cerebral artery, an area highly sensitive to thrombotic occlusion in humans. The rodent model of middle cerebral artery occlusion (MCAO) can be performed by insertion of an intraluminal suture leading from the carotid artery to the juncture of the middle cerebral artery. In both preconditioning scenarios, as well as in severe ischemic insults, the suture is removed after a period of ischemia (transient focal ischemia), allowing reperfusion of cerebral blood flow. This MCAO model yields fairly reproducible injury in the ischemic core (typically within the striatum), as well as an extensive ipsilateral cortical penumbral region. Severe cerebral ischemic insult may also be achieved by permanent ligation or electrocoagulation of the middle cerebral artery.
Alternatively, brain ischemia can occur in the context of arrested blood circulation (often termed a “heart attack”). In order to model this scenario with the cerebral focus, models of global forebrain ischemia have been developed. In normotensive rats or mice, the vertebral arteries are permanently coagulated followed by transient ligation of the carotid arteries, typically lasting 8–10 min. This model leads to specific damage that is most noticeable in the CA1 region of the hippocampus. A similar model can be created through the transient ligation of the carotid arteries alone in either gerbils, which lack the circle of Willis formation for compensatory blood flow via the vertebral arteries, or spontaneously hypertensive rats.
Overall, global and focal ischemic models have been the most comprehensively examined, in terms of severe ischemic insults and sublethal ischemic preconditioning paradigms. However, several models of thrombotic stroke have been recently developed using either a photothrombotic technique or coagulation injury. While these thrombotic models better represent the natural formation of ischemic regions, they are more difficult to control and analyze. Although emerging data suggest that thrombotic models may be helpful in assessing therapeutic strategies in the preclinical settings, they have not yet been widely applied to preconditioning models.
Ischemia has also been widely modeled in vitro, and the effects of preconditioning stimuli have been studied in a variety of ischemic culture model systems. The most widely used system in preconditioning against ischemia is the use of the oxygen/glucose deprivation model, accomplished by replacing the culture medium with glucose-free medium and incubating the cultures in a hypoxia chamber (flushed with 95% argon and 5% CO2). Enriched neuronal cultures are most sensitive to OGD, where 60–90 minutes of OGD yields significant cell death. Conversely, astrocytes are fairly resistant to OGD when cultured alone, requiring more than 6 hours of OGD to incur a significant amount of cell loss. Mixed neuron/astrocyte cultures have also been used, but again require significantly extended OGD parameters to induce cell toxicity.
2.2 Neonatal hypoxia/ischemia
Neonatal hypoxia/ischemia (HI) remains a clinical problem, as brain damage occurring in the perinatal period imposes a high risk of acute mortality and chronic disability (Vannucci and Vannucci, 1997). The immature brain paradoxically exhibits both sensitivity and resistance to HI injury, likely dependent on the stage of cerebral development and injury location (Vannucci and Hagberg, 2004). Cerebral blood flow in neonatal brain is particularly sensitive to changes in systemic blood pressure, and it appears that cerebral blood flow is not autoregulated beyond a 10–20 mm Hg change in systemic pressure (Gill and Perez-Polo, 2008). This results in neonates being more susceptible to systemic hypoxia, as hypoxemia causes rapidly dysregulated systemic blood pressure and also disrupts the Circle of Willis. Once the systemic blood pressure drops, cerebral blood flow becomes acutely disrupted and ischemia ensues, leading the immature brain to be highly susceptible to hypoxic injury. When combined with unilateral ligation of the carotid artery, blood flow in the ipsilateral hemisphere drops by 40–60% of the contralateral side (Vannucci and Hagberg, 2004). Rat models of perinatal HI typically utilize neonatal pups at postnatal day 7 (P7), as the developmental state of the brain is estimated to be roughly equivalent to that of a human fetus or infant at 32–34 weeks of gestation (Vannucci and Vannucci, 1997). The neonatal HI model has several variations, but the most commonly used models combine unilateral carotid artery coagulation (compensated by collateral blood flow under normoxic conditions) and exposure to 8% O2 (hypoxia) at normothermic temperature.
2.3 Surgical-related brain injury
Surgical brain injury (SBI) refers to the neurologic injuries caused by cardiopulmonary bypass surgery, with or without the use of hypothermic circulatory arrest, or neurosurgery itself. While each of these surgical scenarios are used to the benefit of the patient, they also carry risk of neurological injury. Minimizing such risks is of great interest as we attempt to improve surgical outcomes and overall quality of life for the patient.
Cardiopulmonary bypass is a technique widely used in cardiac surgeries to facilitate the surgical exposure of the heart by temporarily taking over the function of the heart and lungs during surgery. This technique thus maintains the circulation of blood and the oxygen content of the body. It is. However, cerebral injury is a significant risk for patients undergoing cardiopulmonary bypass surgeries. In order to reduce that risk, hypothermic circulatory arrest (HCA) has become a frequently used adjunct method to suspend blood flow during major vascular operations, such as repair of the aortic arch or neurosurgical excision of aneurysms of the vertebrobasilar circulation (McCullough et al., 1999). The technique leads to both a relatively bloodless surgical area and a spontaneous decrease in brain metabolism due to the hypothermic component, leading to cerebral protection in the context of decreased blood flow. HCA, widely used clinically, has also been modeled in animals in order to determine additional strategies that further diminish potential for cerebral injury and lengthen the surgical time windows. Briefly, pigs are subjected to cooling of the brain core temperature to 18°C for 60 minutes or more (Hickey et al., 2007; McCullough et al., 1999; Yannopoulos et al., 2010).
In addition to indirect cerebral injury from distal surgical procedures, most neurosurgeries are accompanied by unavoidable cortical and parenchymal incisions, intraoperative hemorrhage, brain lobe retraction, and thermal injuries from electrocautery, all of which are associated with brain damage (Jadhav and Zhang, 2008). These injuries have been modeled in mice and rats by excising a discreet region of the frontal lobe designed to model the general effects of brain tissue loss (Jadhav et al., 2007).
2.4 Traumatic brain injury
Traumatic brain injury (TBI) is a major cause of death and disability worldwide, especially in young people (Prins and Giza, 2011). It can cause impairment of both sensory and motor functions that often persist for months and even years. As most TBI is accidental, investigators have tended to focus on therapies provided after the brain injury. However, with the emergence of sport- and military-related head injuries occurring in a more predictable and orchestrated timeframe, TBI now includes the potential to view the damage as a manifestation of a chronic process, and thus it may benefit from preventative or preconditioning strategies. Examples of chronic TBI degeneration in humans include diffuse axonal injury, which is largely responsible for the persistent vegetative state after head trauma (Graham et al., 2005), and chronic traumatic encephalopathy (CTE), which results from multiple concussive episodes of mild TBI (McKee et al., 2013).
Recent studies in animal models of TBI have noted that several preconditioning scenarios can serve as a potential neuroprotective strategy (Costa et al., 2010; Hu et al., 2010b; Hu et al., 2008). Although several TBI models have been developed in rats and mice, to date the most studied are the controlled cortical impact (CCI) model, fluid percussion injury (FPI), and the weight drop model (reviewed in Albert-Weissenberger and Siren, 2010; Cernak, 2005). Both CCI and FPI inflict impact or pressure directly on the intact dura following craniotomy. In contrast, the weight drop model can be delivered either to the closed skull or the directly to the intact dura. The particular models and forces applied generate different degrees of severity.
2.5 Chronic neurodegenerative diseases
Neurodegenerative diseases generally progress slowly and in most cases do not even emerge as clinical entities until relatively late in life. Recently, hypotheses regarding the underlying cause of neurodegenerative diseases have been extended to include two related concepts. First, the preconditioning or adaptive response to initial threats may be impaired (Texel and Mattson, 2011) or wane with age, impairing the cellular ability to adapt to a toxic insult. Second, the primary insult causing neurodegeneration may fail to elicit a preconditioning response. At this point, these scenarios remain largely hypothetical, but emerging preliminary evidence may lay the groundwork for bolstering preconditioning responses as possible targets for neuroprotection in chronic diseases.
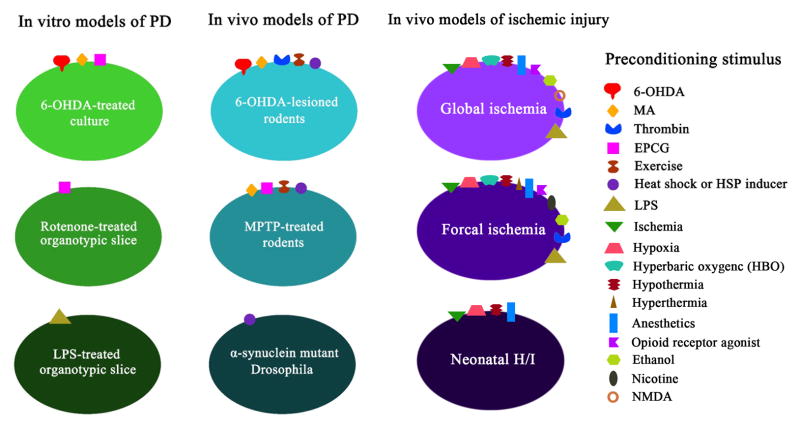
Only a small number of studies have reproducibly established the preconditioning phenomenon in animal and cellular models of chronic neurodegenerative diseases such as Parkinson’s disease (PD) and Alzheimer’s disease (AD) (Figure 1). A large problem in determining the effects of preconditioning in such diseases lies in the limitations of the models themselves. Although several toxins can be used to selectively target and kill the affected neurons in humans, this does not typically mimic what we know (and, more likely, what we do not know) about the underlying pathology. PD studies have largely relied on rodent models in which 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) or 6-hydroxydopamine (6-OHDA) were used to destroy dopamine (DA) neurons, and more recently have included rotenone and known genetic mutations that lead to a small subset of familial PD (reviewed in Jackson-Lewis et al., 2012). These models have some characteristics that reflect the clinical state, but, particularly with the former toxin models, the injury tends to be acute rather than chronic. Thus, developing preconditioning paradigms for a relatively acute toxicity model is likely to be inherently distinct from preconditioning stages in a chronic disease setting. As preconditioning stimuli typically elicit only transient protection and neurodegenerative diseases are chronic, long-term conditions, expansion of the limited studies in repetitive preconditioning stimuli is necessary. As will be discussed below, we propose that long-term preconditioning is also possible in neurodegenerative conditions and that adaptations against cellular stress can be long lasting provided the stimulus is applied continuously and is sublethal in intensity.
Figure 1. Preconditioning paradigms in different animal and cellular models of brain injury.
Several kind of preconditioning stimuli, including neurotoxins, thrombin, EPCG, exercise and HSP inducers, have been reported to establish preconditioning against PD or stroke models. Commonly used PD models for preconditioning studies include 6-OHDA or MPTP-lesioned rodents and 6-OHDA-treated dopaminergic cells. Low-dose endotoxin LPS treatment was also applied to establish preconditioning in an in vitro PD model. The major animal models of stroke models used to study preconditioning include focal and global ischemia in adult rodents, and neonatal hypoxia/ischemia.
Despite the scarcity in preconditioning studies in neurodegenerative diseases, several in vitro models have established that preconditioning can protect against PD- and AD-related toxicity. In a variety of dopaminergic cultures, rotenone, 6-OHDA, and MPP+ toxicity models have been developed to support PD animal models. AD in vitro models primarily focus on amyloid beta toxicity in organotypic hippocampal-entorhinal slice cultures and neuronal cultures. The translation of these in vitro studies to chronic degenerative animal models will significantly aid in the understanding of sublethal stress and disease progression.
3.0 Preconditioning stimuli
The preconditioned state is typically defined by the response to a subtoxic stimulus that extends beyond its presence in the system, and that would become toxic if applied at higher doses and/or for longer durations. This review will focus primarily on the stimuli that are normally viewed as deleterious (e.g., ischemia, inflammation and oxidative stress), but will also include stimuli such as anesthesia and exercise. The latter are commonplace, but induce cellular environments that fall under the definition of a preconditioned state. The underlying specific mechanisms of preconditioning stimuli will be detailed in the second component of our overall review. Here, we will describe known stimuli that induce preconditioning against specific injury models.
3.1 Ischemic preconditioning
3.1.1 Ischemic paradigms for studying preconditioning
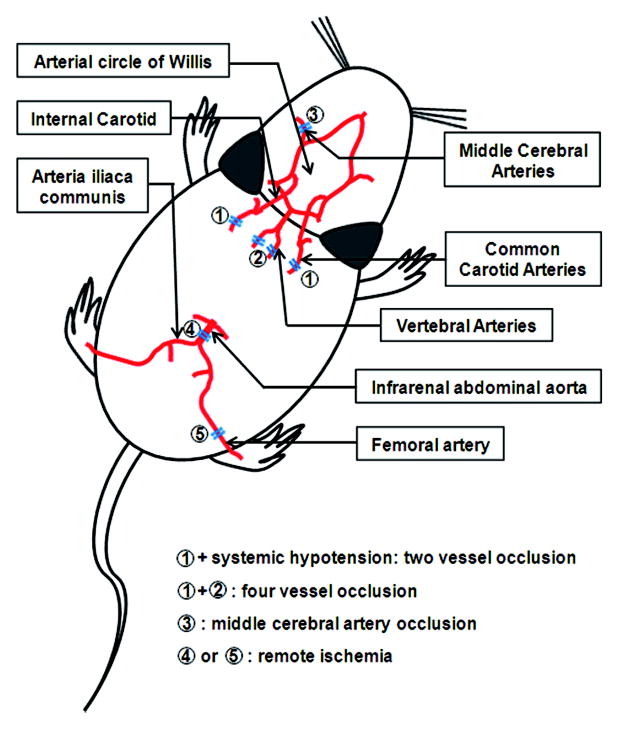
Ischemic preconditioning has been extensively studied and reviewed over the past 20 years. Thus, we will briefly describe the most common paradigms. Two major models of cerebral ischemia are typically used, both as preconditioning stimuli and as brain injury models. Forebrain, or global, ischemic preconditioning has been effectively used to induce tolerance to severe focal or global ischemia (Liu et al., 1992; Matsushima and Hakim, 1995; Zhou et al., 2004). In such studies, brief occlusion of the bilateral common carotid arteries is combined with either vertebral artery coagulation (four-vessel occlusion, or 4VO) or systemic hypotension (two-vessel occlusion, or 2VO) (Figure 2). Severe global ischemic injury produced by extended occlusion (e.g., 7–10 minutes) yields profound damage in the CA1 region of the hippocampus as well as in other regions. In contrast, no grossly observable damage occurs following the preconditioning stimulus (e.g., 2–3 minutes occlusion) during the tolerant window (Perez-Pinzon et al., 1997). Such “global/global” models of tolerance are well established to confer neuroprotection following the severe ischemic challenge. Bilateral common carotid artery occlusion (BCCAO) itself is also protective in mice (Speetzen et al., 2013; Wu et al., 2001). Either a single occlusion of 6 minutes or three 1 minute periods of BCCAO resulted in a transient reduction of cerebral blood flow, yet decreased neuronal damage following subsequent severe BCCAO (18 minutes) or MCAO (45 minute) (Speetzen et al., 2013).
Figure 2. Ischemic paradigms to induce protection against cerebral ischemia in rodents.
Ischemic preconditioning has been effectively used to induce protection against severe cerebral ischemia. Four paradigms have been tested in rodent models: 1. Brief occlusion of the bilateral common carotid arteries combined with either vertebral artery coagulation (4VO, ➀+➁); 2. Brief occlusion of the bilateral common carotid arteries with systemic hypotension (2VO, ➀+systemic hypotension); 3. Brief occlusion of one middle cerebral artery (MCAO, ➂); 4. Occlusion of unilateral femoral artery or the infrarenal abdominal aorta (remote ischemia, ➃ or ➄).
Focal ischemic preconditioning has been also been demonstrated to protect against either permanent or transient focal ischemia (Cardenas et al., 2002; Stagliano et al., 1999). The exact number of minutes of occlusion necessary to produce a tolerant state depends both on the animal species and strain and the downstream insult.
Subischemic paradigms, such as unilateral carotid arterial ligation or cortical spreading depression (CSD), may produce a tolerant state with only minor effects on blood flow. Unilateral carotid arterial ligation in and of itself is capable of producing a preconditioned state against subsequent forebrain global ischemia in rats (Bronner et al., 1998). Moreover, with only moderate alterations in blood flow during the unilateral carotid arterial ligation (Coyle and Panzenbeck, 1990; De Ley et al., 1985), an apparent vascular adaptive response was elicited and proposed to lead to better-maintained blood flow during a subsequent hypoxic/ischemic event (Bronner et al., 1998). CSD has been used as a preconditioning stimulus against focal or global ischemia in rats (Matsushima et al., 1996; Taga et al., 1997), cardiac arrest cerebral ischemia (Kawahara et al., 1995), or subsequent CSD or OGD in organotypic cultures (Gniel and Martin, 2013). CSD induces a significant decrease in baseline cerebral blood flow over an extended period (Bronner et al., 1998), and thus does to some extent mimic ischemic preconditioning. Infarct volume or hippocampal CA1 cell loss was reduced by CSD evoked in vivo by 2 hours of cortical application of potassium chloride occurring 3 days prior to focal ischemia (Matsushima et al., 1996), 1, 3, or 7 days prior to forebrain ischemia (Taga et al., 1997), or 3 days prior to cardiac arrest cerebral ischemia (Kawahara et al., 1995). Organotypic cultures exposed to a 2-minute potassium chloride bath 7 minutes prior to OGD decreased intracellular calcium influx when compared to slices exposed to OGD alone (Gniel and Martin, 2013).
Although not commonly used to achieve preconditioning against neonatal hypoxia/ischemia (HI), ischemic preconditioning can be induced in neonates (P6–7) by 2 hours of ipsilateral reversible carotid artery occlusion followed by 6–22 hours of reperfusion before subsequent HI (Lin et al., 2009b). Using this paradigm, protection was evident according to both histopathological and neurobehavioral outcomes. In addition to postnatal ischemic preconditioning, intrauterine sublethal ischemic preconditioning has also been reported to reduce brain injury following neonatal HI (Xiao et al., 2000). In these studies uterine vasculature was clamped for 30 minutes at embryonic day 17 (E17), and HI was induced at P7 following normal delivery. Brain size in the ipsilateral hemisphere was closer to the contralateral hemisphere, and decreased cell injury was noted. These two models of ischemic preconditioning against neonatal HI target different brain developmental stages, and thus are likely to be mediated by different mechanisms. Analyses of different physiological, molecular, and cellular brain responses will provide important insights into the unique environment of the developing brain, and may provide clues for therapeutics in the adult scenario, as well.
An important point to consider in the effects of ischemic preconditioning is the precise definition of “protection.” Most studies have focused on increased cell survival, spine density, or infarct volume and have correlated that protection with an improvement in behavioral performance at a later time (Corbett et al., 2006). However, an acute impairment in behavior has been observed following 15 minutes of sublethal preconditioning focal ischemia (Hua et al., 2005), and structural changes have become evident several weeks after the preconditioning ischemic event (Sommer, 2008; Tanay et al., 2006). The implication of subtle structural or acute behavioral changes due to the preconditioning stimulus – whether it represents a mechanism for protection or a potentially debilitating artifact is – still unknown.
3.1.2 Timeframe for ischemic preconditioning – rapid and delayed windows of tolerance
The actual paradigms of preconditioning stimuli used in experimental studies vary greatly in terms of time and duration. Nuances have emerged using ischemic preconditioning paradigms that indicate protection can be achieved in two distinct timeframes. “Delayed” ischemic preconditioning refers to the induction of the preconditioning stimulus hours to days prior to the severe insult (e.g., 30-minute occlusion followed by 4 days of reperfusion prior to severe ischemia (Matsushima and Hakim, 1995). In comparison, “rapid” tolerance occurs when the window between the ischemic preconditioning stimulus and the severe insult is on the order of minutes. For example, in the model of global ischemic preconditioning followed by severe global ischemia, carotid arteries are clamped for several minutes and then allowed only a brief reperfusion (e.g., 2 minutes of occlusion followed by a 30-minute reperfusion (Perez-Pinzon et al., 1997)) prior to induction of the severe insult. If the severe ischemic insult occurs between these two windows (i.e., from several hours to 24 hours following preconditioning), no protection is apparent. However, tolerance can be re-induced once the neuroprotective time window has passed (Chen et al., 1994).
Delayed preconditioning is associated with longer-lasting molecular changes compared to the rapid preconditioning paradigm (Durukan and Tatlisumak, 2010). Rapid preconditioning, on the other hand, typically confers transient and less robust neuroprotection compared to delayed preconditioning, but does not appear to require de novo protein synthesis (Barone et al., 1998; Dirnagl et al., 2003). These two well-defined time windows of tolerance that open after a preconditioning event – one within minutes of the event that lasts 1–2 hour, and one 1–7 days following preconditioning (Figure 3) –are pivotal in understanding the cellular response to sublethal injury and the range of protection afforded against the subsequent severe insult that likely occur by the induction of differential and likely non-overlapping cellular mechanisms.
Figure 3. Timeframe for ischemic preconditioning in stroke models.
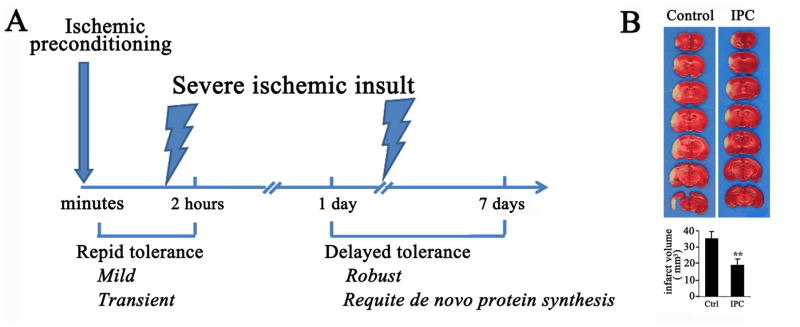
A. There are two time windows of ischemic tolerance that open after a preconditioning stimulus. “Rapid” tolerance develops within minutes and lasts only 1–2 hours after preconditioning. Rapid tolerance typically confers transient and less robust neuroprotection compared to “delayed” tolerance, which develops 1–7 days following preconditioning and requires de novo protein synthesis. If the severe ischemic insult occurs between these two windows, no protection is typically elicited. B. Protection afforded by delayed ischemic preconditioning. Mice were subjected to ischemic preconditioning (IPC, 12 minutes of MCAO) or sham preconditioning (Control), allowed to recover for 48 h, and then subjected to 60 minutes of MCAO. Infarct size was determined by TTC staining. P<0.01.
Improved outcomes following ischemic preconditioning against neonatal HI were observed in rapid, intermediate, and delayed preconditioned groups, and persisted for an extended time (to P42) (Lin et al., 2009b). These findings draw a clear distinction with adult ischemic preconditioning models, where only limited and transient protection was observed in rapid preconditioned groups.
While the multiple windows of ischemic preconditioning have been fairly well established, it remains unknown if this phenomenon is unique to ischemic preconditioning or if it extends to other preconditioning stimuli. More comprehensive studies to determine time window and alternative timeframe responses in non-ischemic preconditioning stimuli may help to better understand generalized versus stimulus-specific responses to preconditioning.
3.1.3 Remote ischemic preconditioning
Remote ischemic preconditioning presents an interesting alternative to cerebral preconditioning because it is significantly less invasive and thus has a much higher potential for clinical translation. To date, the major paradigm establishing remote preconditioning uses subtoxic levels of ischemic preconditioning in extra-neural tissue. However, of particular interest, non-invasive, repeated acute constriction of a limb (upper arm in humans, hind- or forelimbs in rodents) also yielded a tolerant state in the brain. For example, transient (15–30 min) tourniquet application of the hind limb conferred neuroprotection following asphyxial cardiac arrest (Dave et al., 2006) or transient focal ischemia (Hu et al., 2012).
In addition to limb constriction, remote preconditioning against cerebral ischemic insults has also been achieved by occlusion of a major artery (e.g., unilateral femoral artery (Ren et al., 2008) or the infra-renal abdominal aorta (Malhotra et al., 2011) (Figure 2), allowing for precisely controlled preconditioning protocols. In addition, transient occlusion of arteries supplying blood flow to major organs conferred a preconditioned state against stroke models. Silachev et al. demonstrated that remote renal ischemic preconditioning – attained by microvascular clamping of the unilateral renal arteries three times for 5 minutes each cycle – significantly decreased cerebral ischemic injury when MCAO was induced 24 hours following preconditioning (Silachev et al., 2012). This protection was dependent on nephritic function, as pretreatment with the nephrotoxic drug gentamicin abrogated any renal preconditioning effect on cerebral ischemia.
Remote ischemic preconditioning was recently evaluated in SBI paradigms such as hypothermic circulatory arrest and following carotid endarterectomy. Hypothermic circulatory arrest, an increasingly used adjunct in vascular surgeries, incurs significant cerebral dysfunction (Jensen et al., 2011). In a pig model of hypothermic circulatory arrest, remote preconditioning by limb ischemia was induced by applying a static pressure of 230 mm Hg with a blood pressure cuff wrapped around the right hind leg of pig, in 4 cycles of 5-minutes ischemia intermittent with 3 x 5-minutes reperfusion periods completed 1 hour before initiation of cardiopulmonary bypass. This protocol led to a faster recovery of cortical neuronal activity as well as improved histological outcomes (Jensen et al., 2011; Yannopoulos et al., 2010).
When compared to brain ischemic preconditioning, remote ischemic preconditioning may have a wider therapeutic window (Ren et al., 2008), although this observation is likely dependent on which remote preconditioning paradigm is used (Malhotra et al., 2011). For example, remote preconditioning paradigms with limb tourniquets varied in the time between the preconditioning stimulus and the toxicity model from minutes to days (Dave et al., 2006; Hu et al., 2012; Ren et al., 2008). Furthermore, remote ischemic post-conditioning via femoral artery occlusion for two to three cycles of 10–15 minutes has been observed to have neuroprotective effects in models of transient and permanent focal ischemia (Ren et al., 2011; Ren et al., 2008), as well as neonatal hypoxia/ischemia (Zhou et al., 2011b).
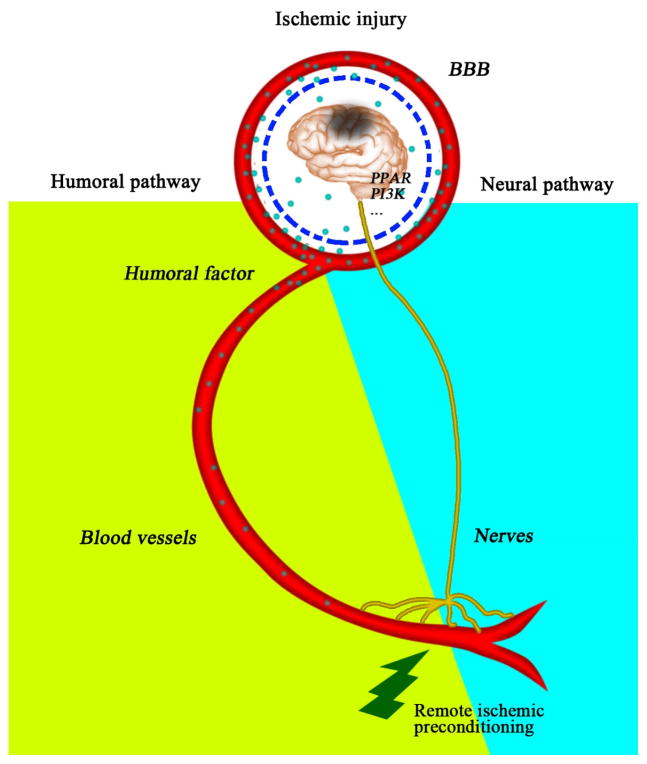
The observation that remote preconditioning can lead to protection against subsequent ischemic insult in heart and brain broadens the pertinent systemic partners to include neural or humoral elements (Lim et al., 2010). Remote preconditioning effects on neurological injury in theory would necessitate a breakdown in the blood-brain barrier (BBB) and/or the release of brain-permeable factors, or alternatively involve distal neurotransmission (Figure 4). Two major scenarios – humoral- and neurotransmission-based – have been proposed that could be translated into preconditioning models. First, humoral alterations were proposed as a logical extension of the sentinel system present in blood flow and the ready access to remote regions to which circulating factors have privilege. Evidence for this exists in models of remote preconditioning against myocardial infarction, where the cardioprotective effects of remote ischemic preconditioning were completely abolished by femoral vein occlusion (Lim et al., 2010), indicating that humoral (i.e., circulatory) pathways were critical. A role for humoral contributions in the establishment of the preconditioned state protective against subsequent cerebral ischemia has also emerged. Renal function was critical in the neuroprotective action of both remote renal ischemic preconditioning and the systemic injection of the mitochondrial-targeted cationic antioxidant SkQR1 (which accumulates in the kidneys), as nephrotoxicity or bilateral nephrectomy abolished the protective effects (Silachev et al., 2012).
Figure 4. Predicted pathways underlying remote ischemia-induced protection against cerebral ischemic injury.
Remote ischemic preconditioning may induce neuroprotection through neural as well as humoral pathways. Humoral effects would necessitate either a breach in the integrity of the BBB or involve brain permeable factors. Both humoral and neural elements may eventually trigger neuroprotective signaling, such as PPAR and PI3K pathways, in ischemic brain.
Neurotransmission may also be critical to remote preconditioning scenarios, either in conjunction with or independent of humoral components. Remote ischemic preconditioning against myocardial ischemia was partially abrogated by sciatic nerve resection, suggesting neural transmission plays a role in remote preconditioning (Lim et al., 2010). Likewise, the sensory nerve inhibitors hexamethonium and capsaicin attenuated the neuroprotection afforded by remote limb ischemic preconditioning against subsequent transient cerebral ischemia (Malhotra et al., 2011; Wei et al., 2012a). Thus, the roles for neural and humoral elements – either separately or as a coordinated effort – in neuroprotection represent an exciting field of study for determining the mechanisms underlying the endogenous response to remote preconditioning.
Taken together, these studies suggest that remote ischemic preconditioning is emerging as a clinically feasible concept to deter brain injury, particularly in cases of surgical injury. Several preliminary studies have begun to evaluate the safety and efficacy in clinical settings; these are described below under the section “Clinical Potential of Preconditioning.”
3.2 Oxygen preconditioning – hypoxic and hyperbaric conditions
Altitude changes and/or atmospheric pressure represent conditions to which the human body can adapt. Consistent with the concept that “the dose makes the poison,” extreme changes in atmospheric oxygen are lethal. However, adaptation to controlled and limited changes can be managed. The physical adaptations to alterations in atmospheric oxygen appear to extend not only to survival, but also to a preconditioned state.
3.2.1 Hypoxia
Hypoxic preconditioning has been modeled both in vitro and in vivo, and effectively preconditions against ischemic brain injury in both adults and neonates. Cultures or animals are placed in an airtight hypoxia chamber, where oxygen is replaced by nitrogen, typically to normobaric hypoxic conditions (8% oxygen). Initially described in neonatal ischemic tolerance models, neuroprotection via hypoxic preconditioning has been extended to multiple cultures and ex vivo conditions such as hippocampal organotypic cultures (Bickler and Fahlman, 2009; Bickler et al., 2009; Gage and Stanton, 1996), primary neuronal cultures (Arthur et al., 2004; Bruer et al., 1997) or astrocytic cultures (Liu and Alkayed, 2005). These findings were later further extended to adult stroke tolerance models (Bernaudin et al., 2002; Fan et al., 2011; Lin et al., 2003; Miller et al., 2001; Tang et al., 2006; Zhan et al., 2010), with important distinctions from ischemic preconditioning. Specifically, the tolerant state induced by normobaric hypoxia appears to exist in a fairly rapid and limited timeframe, lasting only approximately 72 hours (Bernaudin et al., 2002; Prass et al., 2003; Zhan et al., 2010). Conversely, sustained tolerance (7 d) was achieved in female rats by 4 weeks of daily exposure to hypoxic conditions in an altitude chamber (Lin et al., 2003). The implications of preconditioning in female stroke models are as yet unknown, but likely to be distinct from male models. While incidence of stroke in women overall is lower than in men (age<85 years), the severity of outcome is higher (Appelros et al., 2009; Persky et al., 2010).
In addition to gender differences, a distinction in hypoxic preconditioning has emerged that indicates the phenomenon may also be age-dependent. In the neonatal HI brain, hypoxia also confers a preconditioned state (Gidday et al., 1994; Ota et al., 1998). Hypoxic preconditioning can be achieved by exposing neonatal rats (P6–7) to sustained hypoxia (e.g., 3 hours at 8% O2/92% N2) in a humidified chamber held at 37°C. Disruption of systemic pH is avoided in neonatal hypoxia due to the increased respiration (hypocapnia). Preconditioning is then followed by normoxia for 24 hours, and then pathological HI. Neural and behavioral outcomes following this paradigm demonstrate substantial protection compared to non-preconditioned groups, and last for an extended period of time (e.g., 7–8 weeks) (Gustavsson et al., 2005). Indeed, the recovery following HI in the preconditioned groups can be near complete. However, in slices derived from aged animals (>2 years of age), hypoxic preconditioning did not confer protection against subsequent severe oxygen/glucose deprivation (OGD) (Bickler et al., 2010). This discrepancy has also been observed in an ischemic preconditioning model against global ischemia (He et al., 2005). These findings are in line with observations that neonates recover better from hypoxic/ischemic injury, and support the concept that aged brain either is more susceptible to oxidative injury (Xu et al., 2007) or has diminished capacity to repair itself after sublethal or lethal injury (Mattson et al., 2002). Further research is needed to determine the influence of age and gender on preconditioning in stroke models.
Hypoxic preconditioning was found to be protective against ischemic injury in cultured human brain endothelial cells (Zhang et al., 2007), and drug inhibition of endothelial sphingosine kinase upregulation abrogated the neuroprotection afforded by hypoxic preconditioning against transient MCAO (Wacker et al., 2009). Thus, hypoxic preconditioning could represent a relatively broad phenomenon that may extend to inclusion of preconditioning the cerebrovasculature. These observations have spurred major interest in identifying the multiple signaling pathways and physiological alterations conferring hypoxia-mediated protection against HI.
3.2.2 Hyperbaric oxygen preconditioning
Hyperbaric oxygen (HBO), a condition obtained by containment in a pressurized chamber infused with 100% O2 flow, can protect against subsequent transient MCAO and global ischemia, as well as SBI and TBI. Similar to hypoxic and ischemic preconditioning, many different paradigms have been used to demonstrate that either rapid or delayed tolerance is affected by HBO. Preconditioning with pressures of 2 atmospheres absolute (ATA) every other day for 3 or 5 sessions was effective at inducing tolerance against global ischemia in the gerbil (Wada et al., 1996; Wada et al., 2001). Similarly, 2.5 ATA preconditioning 1 hour daily for 5 days protected against subsequent global ischemic injury in rat (Cheng et al., 2011). When air (20% oxygen) rather than 100% O2 was infused into the hyperbaric chamber the tolerance was negated, demonstrating a requirement for O2 in the hyperbaric preconditioned state (Wada et al., 2001).
As mentioned above, longer-term HBO preconditioning paradigms appear more effective at establishing tolerance than do acute paradigms. For example, preconditioning at 2.5 ATA for 1 hour per day for 5 days ending 24 hours prior to injury was effective at establishing tolerance in rats against global cerebral ischemia, transient focal ischemia with hemorrhagic transformation, and SBI (Jadhav et al., 2010; Ostrowski et al., 2008; Soejima et al., 2012), whereas acute preconditioning (3 treatments of 1 hour at 2.5 ATA occurring 24, 12 and 6 hours prior to MCAO) was less effective, reaching significance only when assessed 24 hours following MCAO (Ostrowski et al., 2008). Similarly, protection was observed following a regimen of 1-hour treatments at 2.5 ATA every 12 hours, beginning 2 days before ischemia (Li et al., 2009). Under such conditions, improved outcomes were observed at 24 hours following ischemia.
In addition to conferring tolerance in adult models, HBO preconditioning has been found to be protective against neonatal HI (Freiberger et al., 2006; Li et al., 2008b). HBO conditioning was accomplished by exposure to 2.5 ATA for 2.5 hours, completed 24 hours prior to the HI injury. Less intense HBO (2 ATA for 1 hour) did not appear to confer a tolerant state, even with successive treatments or an extended timeframe (Freiberger et al., 2006).
HBO preconditioning also leads to delayed neuroprotection against a weight drop model of TBI (Hu et al., 2010b; Hu et al., 2008), as well as improved neurological outcomes following SBI (cortical excision model) (Jadhav et al., 2010; Jadhav et al., 2009). In both models, HBO preconditioning was established by subjecting animals to 5 sessions of 2.5 ATA, 100% O2 for 1 hour per day. Prior to TBI, the preconditioned animals were kept in a hyperbaric chamber (0.6 ATA) at a simulated pressure of 4000 m altitude for 3 days following HBO and then subjected to TBI 72 hours later (Hu et al., 2008). HBO preconditioning improved regional cerebral blood flow and brain tissue O2 pressure following TBI (Hu et al., 2010b), and attenuated post-operative brain edema following SBI (Jadhav et al., 2010; Jadhav et al., 2009). Neurological outcomes following TBI or SBI were significantly improved when preceded by HBO preconditioning (Hu et al., 2010b; Hu et al., 2008; Jadhav et al., 2010; Jadhav et al., 2009). Further studies using more sensitive neurobehavioral or cognitive assays as well as improved histological assessments should further specify the protection conferred by HBO against TBI or SBI.
3.3 Temperature preconditioning – hypothermia and hyperthermia
Similar to oxygen modulations, temperatures above or below homeostatic temperatures can induce a tolerant state. Although the mechanisms are likely to be dissimilar, the concept remains that limited deviations from homeostasis appear to induce protective responses.
3.3.1 Hypothermia
Brain cooling (hypothermia) has been recommended in emergency medicine in the protection against neurological injury following cardiac arrest due to ventricular fibrillation (Azmoon et al., 2011; Bernard, 2009). A brief period of hypothermia can also lead to preconditioning-like neuroprotection. Twenty minutes of hypothermic exposure (31–32°C) induced a delayed ischemic tolerance window that was initiated 6 hours after preconditioning and persisted for 2 days (Nishio et al., 1999; Nishio et al., 2000). Focal hypothermic preconditioning by selectively cooling the head was as effective as whole-body cooling for eliciting tolerance (Yunoki et al., 2002). Similar mild hypothermic exposure was also reported to have a preconditioning effect at an acute phase of tolerance in in vitro models of ischemia. Hypothermic preconditioning (33°C for 20 minutes) applied 0 to 3 hours before OGD effectively prevented OGD-induced Purkinje cell death in rat cerebellar slices (Yuan et al., 2004, 2006). This rapid neuroprotection induced by hypothermic preconditioning appeared dependent on the activation of signaling molecules and increased gene expression (Yuan et al., 2004, 2006).
3.3.2 Hyperthermia
Brief hyperthermic exposure can also induce brain tolerance against ischemic injuries. In such studies, adult rats are placed in a water bath set at 42°C for 15 minutes or wrapped in a temperature-controlled heating pad until rectal temperature reaches 42°C. When examined 18 or 24 hours later, rats suffered a smaller infarct volume after MCAO (Xu et al., 2002) and showed improved learning abilities and memory following diffuse axonal injury (Su et al., 2009). The preconditioning effect against MCAO was not observed either at an earlier phase (6 hours after preconditioning) or at a later phase (48 hours after preconditioning) (Xu et al., 2002). In addition, heat acclimation, a unique preconditioning paradigm established by chronic exposure to moderate heat, was also reported to induce endogenous protection against TBI (Shein et al., 2007; Umschweif et al., 2013; Umschwief et al., 2010).
As will be discussed in detail in the second part of this series, heat stress is associated with the upregulation of heat shock proteins. Overexpression of heat shock proteins is protective in models of PD (Fan et al., 2005; Fan et al., 2006; Gorman et al., 2005). Furthermore, heat shock preconditioning is protective against dopaminergic toxins (MPP+) in culture models (Fan et al., 2005; Quigney et al., 2003).
3.4 Pharmacological preconditioning
Pharmacological preconditioning is a clinically feasible paradigm that can be elicited by a wide variety of drugs. The classification of pharmacological preconditioning versus pharmacological pretreatment is somewhat nebulous, with the major distinction being that in the preconditioned state protection is afforded in a timeframe beyond agent elimination. Due to its ease of establishment compared to the more invasive or elaborate preconditioning protocols described above, pharmacological preconditioning has been accepted as a promising paradigm to combat cerebral ischemic injury in the clinic. However, many limitations still need to be overcome, such as understanding and thus titrating the indirect effects of the agent that elicits the preconditioned state. Potential mechanisms and downstream targets of pharmacological preconditioning will be discussed in the second component of this series.
3.4.1 Anesthetics/analgesics
Following the demonstration of anesthetic preconditioning in an animal model of myocardial infarction (Kersten et al., 1997), accumulating evidence supports the protective capacity of anesthetic preconditioning against cerebral ischemic injury as well. Furthermore, a recent study suggests a preconditioning-like effect against an AD model. The dose, time window of tolerance and preconditioning paradigms vary with agent and model. We will discuss here various aspects of these paradigms in the context of neurological injury.
3.4.1.1 Inhalational anesthetics
Currently used inhalational anesthetic agents include isoflurane, sevoflurane, and desflurane. Isoflurane is a potent anesthetic agent that has been used for decades in clinical anesthesia, but is currently being replaced by sevoflurane and desflurane due to the low blood:gas partition coefficient of the latter two drugs, resulting in both relatively rapid anesthesia induction and recovery from anesthesia. Both sevoflurane and isoflurane are accepted as having neuroprotective properties against adult and neonatal cerebral ischemic injury when used in preconditioning. Accumulating evidence indicates that isoflurane preconditioning (exposure to 1% to 2% isoflurane) provides a delayed protective effect (usually 24 hours after preconditioning) against ischemic neuronal injury both in in vitro models (Bickler and Fahlman, 2009; Bickler et al., 2005; Li et al., 2008a) and in adult and neonate in vivo models (Kitano et al., 2007; Li and Zuo, 2009; McAuliffe et al., 2007; Zhao et al., 2007; Zhao and Zuo, 2004; Zheng and Zuo, 2004; Zhu et al., 2010).
The timeframe used for isoflurane preconditioning varies between models. In ischemic neuronal culture model systems, 15 minutes to 2 hours of anesthetic preconditioning was used (Bickler et al., 2005; Li et al., 2008a), whereas 30 minutes to 4 hours were applied under different isoflurane concentrations in MCAO models (Kitano et al., 2007; Li and Zuo, 2009; Zheng and Zuo, 2004; Zhu et al., 2010). Repetitive exposure, which is achieved by isoflurane exposure for 1 hour per day for 5 days, induced ischemic tolerance in a time- and dose-dependent manner against MCAO (Xiong et al., 2003), improved motor function and decreased the number of degenerating neurons following global ischemia (Zhang et al., 2010). In addition to the delayed preconditioning observed in the adult, isoflurane preconditioning induced both rapid and delayed phases of ischemic tolerance in neonatal brains (McAuliffe et al., 2007; Sasaoka et al., 2009; Zhao et al., 2007; Zhao and Zuo, 2004). P7 pups exposed to 1% or 2% isoflurane ending 15 minutes before the HI injury were protected against HI injury (Sasaoka et al., 2009), as were pups exposed to isoflurane 24 hours before HI (McAuliffe et al., 2007; Zhao et al., 2007; Zhao and Zuo, 2004). The protection conferred by preconditioning 24 hours before HI was long-lasting, as improved functional outcomes were observed in adult mice that experienced preconditioning followed by neonatal HI injury (McAuliffe et al., 2007; Zhao et al., 2007).
Sevoflurane is also a volatile anesthetic that is now replacing isoflurane in modern anesthesiology due to the reduction in mucosal membrane irritation and the faster onset and offset. A large body of evidence indicates that sevoflurane is able to protect the brain against ischemic injury in various models (Warner et al., 1993; Werner et al., 1995). In preconditioning paradigms, sevoflurane elicited a rapid neuroprotection within 3 hours after preconditioning against OGD in neuronal or hippocampal slice cultures (Sigaut et al., 2009; Velly et al., 2009). This neuroprotective effect of sevoflurane preconditioning appeared at concentrations higher than 0.07 mM (Velly et al., 2009) and only under modest OGD challenges (30 minutes or less for hippocampal slices) (Sigaut et al., 2009). In animal models, sevoflurane preconditioning has been examined in both the adult global and focal cerebral ischemia models, as well as in neonatal models. Rapid preconditioning can be induced by sevoflurane exposure within 1 hour before ischemic injury in rats (Codaccioni et al., 2009; Payne et al., 2005; Wang et al., 2007a), more clinically relevant for SBI., Sevoflurane exposure also conferred neuroprotection against either global (Payne et al., 2005) or focal ischemia (Adamczyk et al., 2010; Ye et al., 2012a) during a late time window (from 1 to 3 days after preconditioning). In neonatal brain injury models, preconditioning with 1.5% sevoflurane alone or 0.75% sevoflurane in combination with 20% xenon significantly reduced infarct size in the model of neonatal asphyxia (Luo et al., 2008). Likewise, delayed preconditioning with 8.4% desflurane or 3.1% sevoflurane improved selective behavioral performance of adult mice previously subjected to neonatal HI injury (McAuliffe et al., 2009).
In a transgenic model of AD (PS1/Swe/tau), female mice exposed to halothane in the presymptomatic stage performed better in a spatial memory test (Morris water maze) compared to un-exposed or isoflurane-exposed females (Tang et al., 2011). The effect was short lived, but may suggest a preconditioning-like phenomenon possible in an AD model.
3.4.1.2 Opioid receptor agonists
Opioid receptors are associated with preconditioning-induced cardioprotection (Liang and Gross, 1999), and are widely expressed in the central nervous system. These receptors have been found to contribute to preconditioning models in brain, including hypoxia preconditioning (Gao et al., 2012; Zhang et al., 2006), and their agonists confer a preconditioned-like state. Morphine is a potent non-selective opioid receptor agonist and is frequently used as an analgesic conjunctive therapy in clinical anesthesiology. Morphine pretreatment provided both a rapid and delayed neuroprotection against OGD-induced neuronal injury in hippocampal slice cultures (Liu et al., 2008a; Zhao et al., 2006b). A delayed preconditioning effect was achieved in animals by a single injection of morphine (2 mg/kg or 8 mg/kg, i.p.) 24 hours before ischemic injury (Rehni et al., 2008; Zhao et al., 2006b). Morphine-induced pharmacological preconditioning not only reduced ischemic infarct size, but also reversed the impairment of memory and motor coordination induced by global and permanent focal ischemia (Rehni et al., 2008; Zhao et al., 2006b).
3.4.2 Ethanol
Light to moderate alcohol consumption has recently been associated with reduced risk of AD, stroke and cardiovascular disease (Belmadani et al., 2004; Berger et al., 1999; Krenz and Korthuis, 2012; Mehlig et al., 2008; Mukamal et al., 2003; Orgogozo et al., 1997; Stampfer et al., 1988). Heavy consumption of alcohol, on the contrary, is associated with increased stroke severity (Ducroquet et al., 2013). Epidemiological data with alcohol consumption is difficult to interpret, as other components of alcoholic beverages (notably, resveratrol in red wine) have also been demonstrated to exert a preconditioning effect (Pinder and Sandler, 2004; Raval et al., 2006; Singh et al., 2013), and individual differences in alcohol metabolism may alter absorption and clearance rates. However, recent studies using animal models have demonstrated a role for ethanol in protecting against brain disease.
In animal models of cerebral ischemia, ethanol preconditioning protected against ischemic brain damage (Liao et al., 2003; Wang et al., 2010b; Wang et al., 2007b). For example, it was shown that ethanol preconditioning, administered by gavage to gerbils with a moderate dose of ethanol as a single bolus (producing a peak plasma concentration of 42–46 mg/dl) 24 hours before ischemia, offered a number of beneficial effects against ischemic injuries, including behavioral deficit, delayed neuronal death, neuronal and dendritic degeneration, and oxidative DNA damage (Wang et al., 2007b). Both pre- and post-treatment with intracerebroventricular infusion of ethanol (0.1%, 60 minutes prior or 30 minutes after ischemia) attenuated transient focal ischemic infarct (Liao et al., 2003). Finally, alcohol can protect against NMDA receptor-mediated neurodegeneration in neuronal cultures in vitro (Cebere and Liljequist, 2003; Chandler et al., 1993; Wegelius and Korpi, 1995).
Models of inflammatory neural diseases also benefit from pre-exposure to ethanol, which may allow some extrapolation to the inflammatory state in stroke or chronic neurodegeneration. Chronic ethanol intake (70 days) protected neurons from subsequent intraperitoneal LPS exposure (Singh et al., 2007). In hippocampal-entorhinal cortical organotypic cultures, ethanol pre-exposure (20–30 mM, 6 days) significantly decreased neuronal degeneration due to exposure of the neuroinflammatory HIV-1 envelope glycoprotein 120 (Belmadani et al., 2001; Collins et al., 2000). These studies demonstrate a possible role for ethanol in effecting a preconditioned state against neuroinflammatory processes.
Epidemiological studies focusing on AD risk factors have indicated that alcohol, if consumed in moderation, is inversely correlated with developing either cardiovascular disease or AD, two conditions that share many common risk factors (Collins et al., 2009; Mehlig et al., 2008; Mukamal et al., 2003; Orgogozo et al., 1997; Peters et al., 2008). Specifically, 1–6 drinks per week were associated with reduced risk for developing dementia (Collins et al., 2009; Mukamal et al., 2003). Concurring with these observations, rat brain cerebellar cultures and hippocampal-entorhinal cortical organotypic cultures were protected against Aβ toxicity by moderate ethanol exposure prior to the insult (Belmadani et al., 2004; Collins et al., 2010; Mitchell et al., 2009).
3.4.3 Stimulants
Several other drugs commonly used have had some association with a possible preconditioning effect. For example, although cigarette smoking is highly toxic and often leads to heart disease and cancer, smoking and ingestion of dietary nicotine is negatively correlated with PD (Baron, 1986; Nielsen et al., 2013; Ross and Petrovitch, 2001). Nicotine has the ability to stimulate DA release (Giorguieff-Chesselet et al., 1979; Grenhoff and Svensson, 1989), and thus may not be acting as a preconditioning stimulus but rather as a mask of the symptoms. Additionally, nicotine may also improve PD-related dyskinesias by acting on nicotinic, cholinergic receptors(Quik et al., 2013; Quik et al., 2009). Nicotine pretreatment (1.2 mg/kg, i.p. 2 hours prior to MCAO) reduced infarct volume when assessed a day after stroke (Chen et al., 2013). However, smoking has long been associated with an increased risk in stroke, and consistent with this, several studies have conclusively demonstrated the deleterious effects of nicotine in a variety of ischemia models (Bradford et al., 2011; Li et al., 2012; Paulson et al., 2010; Raval et al., 2011).
On the other hand, in the context of this review, one might also consider a quite different hypothesis, namely that nicotine is neuroprotective at low doses by triggering a variety of cellular defenses, including those involving mitochondrial energy production and the inflammatory response. This proposal deserves future research, particularly in that it might suggest the development of other approaches to trigger similar cellular defenses without being either carcinogenic or a stress to the cardiovascular system.
Another neurotoxin that has been shown to have neuroprotective properties in low concentrations is methamphetamine. In a recent study using a dopaminergic cell line, we were able to show that although high concentrations of methamphetamine (2–3 mM) can be cytotoxic, exposure to lower concentrations (0.5–1.0 mM) for 24 hours prior to a 20-minute exposure to 6-OHDA (100 μM) was cytoprotective (El Ayadi and Zigmond, 2011). These findings with methamphetamine are consistent with many previous reports. For example, methamphetamine has also been shown to induce a tolerance to the neurotoxic effects of 3,4-methylenedioxymethamphetamine (Shankaran and Gudelsky, 1998), methamphetamine (Cadet et al., 2009), other amphetamines (Abekawa et al., 1997; Danaceau et al., 2007; Graham et al., 2008; Johnson-Davis et al., 2003; Johnson-Davis et al., 2004), and MPTP oxidation products (Kita et al., 2003; Park et al., 2002) as well as to 6-OHDA in an animal model of PD (Moroz et al., 2004). Moreover, preconditioning and cross-tolerance using several different drugs have been shown by us and others in various in vitro models of the DA deficiency seen in PD (Leak et al., 2006; Tai and Truong, 2002; Tang et al., 2005).
3.5 Neurotoxic or neuroinflammatory agent preconditioning
Exposure to low doses of chemicals that are toxic at moderate concentrations may induce neuroprotection against ischemia, which reduces infarct size and improves functional outcome in both cultured neuronal cells and animal stroke models (Marini and Novelli, 1991; Marini and Paul, 1992). The drugs included in the context of chemical preconditioning include primarily those whose known targets are typically detrimental in nature (e.g., mitochondrial respiration inhibitors), that are excitotoxic or inflammatory in nature (e.g., glutamate receptor agonists or toll-like receptor ligands) or that have been identified as critical elements of injurious states (e.g., thrombin). In the context of this review, these chemicals have no therapeutic or social use, in contrast to the preconditioning stimuli described under pharmacological preconditioning.
3.5.1 Chemical preconditioning with drugs used for injury models
3.5.1.1 NMDA
Glutamate excitotoxicity contributes significantly to the cell death induced by ischemia and can be modeled with the use of high doses of glutamate receptor agonists, such as NMDA (Mandir et al., 2000). However, subtoxic doses of NMDA-receptor agonists have been found to lead to the activation of intracellular pro-survival signaling in the absence of cell death (Lipsky et al., 2001; Marini and Paul, 1992; Marini et al., 1998; Ogita et al., 2003; Zhu et al., 2005). The neuroprotective effect of NMDA preconditioning has been examined in both in vitro and in vivo models of ischemia. Cultured neurons can be protected from an excitotoxic concentration of glutamate or exposure to lethal OGD by pretreatment with 50 μM NMDA (Grabb and Choi, 1999; Head et al., 2008; Jiang et al., 2005). In addition, preconditioning with a micro-injection of 10 μM NMDA into the prefrontal cortex induced a rapid protection against permanent occlusion of the middle cerebral artery in rats (Saleh et al., 2009).
The excessive activation of NMDA receptor activity is also elemental in the cascade of events subsequent to TBI. Although NMDA receptor antagonists have been used as a possible neuroprotective agent in both animal TBI models and clinical trials (Muir, 2006; Rao et al., 2001; Yurkewicz et al., 2005), longterm therapy may reduce the capacity of the brain to recover, as glutamate exerts trophic effects contributing to neuronal reorganization after brain injury (Billard and Rouaud, 2007; Lo, 2008). Sublethal, transient NMDA preconditioning may therefore be a promising protective strategy in a mouse TBI model. Preconditioning by administration of a nonconvulsant dose of NMDA (75 mg/kg, i.p.) was induced 24 hours before mild TBI (Costa et al., 2010), and selectively improved motor deficits (revealed by footprint tests) in a short timeframe after injury (Costa et al., 2010).
3.5.1.2 Thrombin
Thrombin, a trypsin-like serine protease protein, converts soluble fibrinogen into insoluble strands of fibrin, as well as catalyzing many other coagulation-related reactions. Thrombin can be released from a ruptured cerebral aneurysm clot around a cerebral artery, and leading to the prolonged narrowing of blood vessels, and cerebral ischemia and infarction. In this manner, it has been used as a model for ischemic stroke (El Amki et al., 2012; Sun et al., 2011). In addition to vascular actions inducing cerebral ischemia, thrombin formation also contributes to brain edema after intracerebral hemorrhage (Lee et al., 1995; Lee et al., 1996a; Lee et al., 1996b, Lee et al., 1997), leading to worsened brain injury and poorer neurological outcomes. High concentrations of thrombin infused into the caudate nucleus causes inflammation, edema, reactive gliosis, and scar formation (Lee et al., 1995; Lee et al., 1996a; Lee et al., 1996b; Nishino et al., 1993). Despite these detrimental effects of thrombin, low concentrations of thrombin exert protective effects in neural cells against hypoglycemia and ischemia (Vaughan et al., 1995). Following cerebrovascular injury, thrombin is produced immediately upon breakdown of the BBB and regulates gene expression and process outgrowth in neural cells. These effects were blocked by the brain thrombin inhibitor, protease nexin-1 (Vaughan et al., 1995). Thus, the immediate presence of low levels of thrombin may aid in faster post-injury repair.
Prior studies indicated that a state of ischemic tolerance can be induced by thrombin both in organotypic hippocampal slice cultures and in MCAO mice. Low doses of thrombin (0.01 U/ml thrombin injected intracerebroventricularly) protected neurons against severe OGD and transient focal ischemia (Granziera et al., 2007). Thrombin pretreatment also can significantly attenuate brain edema (Xi et al., 1999).
Thrombin has also been investigated in a PD model. High doses of thrombin (20 U) cause degeneration of dopaminergic neurons and microglial activation in the substantia nigra (Carreno-Muller et al., 2003; Choi et al., 2003). However, stereotaxic infusions of low doses of thrombin (1 U) administered above the medial forebrain bundle though which DA neurons project from the ventral mesencephalon to the striatum and other telencephalic structures protect rats against subsequent infusions of 6-OHDA, a classic model of PD (Cannon et al., 2005). Protection was evident in assessments of motor behavior, TH immunoreactive terminals in the striatum, and ventricular enlargement. Surprisingly, however, thrombin did not protect against DA depletion in this model of preconditioning, possibly suggesting that a defect in DA storage remained despite the sparing of DA terminals. The effects of thrombin, however, depended closely on the time of administration relative to 6-OHDA. If co-infused with 6-OHDA, thrombin at low doses actually increased behavioral deficits (Cannon et al., 2007). In contrast, if thrombin was administered 7 days after 6-OHDA, it had protective behavioral and neurochemical effects (Cannon et al., 2007). Further studies are warranted on whether the timing effects of thrombin fall within classical rapid or delayed preconditioning paradigms, and on elucidating the contrast between the thrombin-induced protection of TH-immunoreactive terminals without protection of DA stores.
3.5.1.3 6-OHDA
Most models of PD have focused on the use of toxins that target DA neurons. To use an analogy with the ischemic preconditioning literature, the simplest paradigm to establish preconditioning in PD models is to use a low dose of one of these toxins to protect against a subsequent challenge with a higher dose of the same toxin. Indeed, we have found that low concentrations of 6-OHDA protect dopaminergic MN9D cells against a high concentration of 6-OHDA applied 6 hours later (Leak et al., 2006). Similarly, in a preliminary study in vivo, we have observed that a low dose of 6-OHDA (0.15 μg into the striatum) significantly reduced the loss of TH immunoreactive terminals in the striatum in response to a much higher dose of 6-OHDA (3 μg) administered 4 days later (R. Leak and M. Zigmond, unpublished observations).
3.5.2 Preconditioning with inflammation
3.5.2.1 Toll-like receptor ligands
Toll-like receptors (TLRs) are archetypal pattern recognition receptors that play essential roles in the innate immune response to foreign pathogens. TLRs are expressed in a variety of immunocytes such as B cells, dendritic cells, neutrophils, macrophages, and monocytes. TLRs are also present in the cerebral endothelium and in almost all types of brain parenchymal cells, including microglia (Olson and Miller, 2004), astrocytes (Bowman et al., 2003), oligodendrocytes (Bsibsi et al., 2002), and neurons (Tang et al., 2007). At least 13 TLRs have been identified in mammals. Each TLR recognizes invaders through different exogenous pathogen-associated molecular patterns such as bacterial cell wall lipoprotein (TLR2) and lipopolysaccharide (LPS) (TLR4), bacterial DNA containing particular nonmethylated cytosine-guanine motifs (TLR9), viral double-strand RNA (TLR3), and single-strand RNA (TLR7) (Vance et al., 2009). In addition to their role in recognition of pathogen-associated molecular patterns, TLRs can also sense cell damage through endogenous molecules that have been termed DAMPs (damage-associated molecular patterns) and initiate inflammatory responses to aseptic tissue injury (Kim et al., 2009b; Yang et al., 2010a; Yang et al., 2010b).
Excessive inflammation after stroke can cause secondary neuronal injury and long-term neurological deficits (Barone and Feuerstein, 1999; Dirnagl et al., 1999). Consistent with this, prolonged post-ischemic systemic administration of LPS, the most potent bacterial ligand of TLR4 receptor, is devastating to stroke outcome in the absence of a prolonged febrile response (Langdon et al., 2010). Genetic ablation of either TLR2 or TLR4 conferred protection against brain damage following both transient and permanent focal cerebral ischemia (Caso et al., 2007; Tang et al., 2007; Ziegler et al., 2007). Despite the detrimental role of TLRs in ischemia, stimulation of these receptors with a low/sublethal dose of their ligands prior to ischemia provides robust neuroprotection. For example, preconditioning with a low dose of LPS protected the brain against subsequent ischemic damage (Bordet et al., 2000; Dawson et al., 1999; Furuya et al., 2005; Kunz et al., 2007; Marsh et al., 2009; Rosenzweig et al., 2004; Yu et al., 2010). Some other TLRs have also been suggested to be neuroprotective. For example, it has been shown that priming of TLR2 or TLR9 with their respective agonists can serve as a preconditioning stimulus and provide protection against brain ischemia (Hua et al., 2008; Stevens et al., 2008).
3.5.2.2 LPS
The hypothesis underlying the preconditioning effect of the potent TLR4 ligand LPS is that a small inflammatory stimulus dampens subsequent inflammatory responses associated with cerebral ischemia and mitigates against brain injury. The protective effect of LPS against cerebral ischemia is both dose- and time-dependent. The optimal preconditioning dose and duration of LPS depend on multiple factors, including the animal model, the route of LPS application, and the quantity of LPS. In mice, LPS is administered prior to ischemic challenge in experimental animals in a single systemic dose of 0.05 to 1 mg/kg, which would be a highly toxic dose in a human but is a low dose for mice. In a mouse transient MCAO model, the tolerance window is quite extended, appearing within 24 hours after LPS administration (0.2 mg/kg, i.p.) and extending through 7 days following LPS pretreatment (Rosenzweig et al., 2007). Similarly, in a rat model, the neuroprotective effect can be observed with LPS treatment (0.9 mg/kg, i.v.) at least 2 days and up to 4 days prior to permanent MCAO (Tasaki et al., 1997). Although the potential mechanisms will be discussed in in a second review, LPS preconditioning was also associated with decreased endothelium relaxation following transient MCAO (Bastide et al., 2003), and with – improvement albeit delayed – in cerebral blood flow following permanent MCAO (Dawson et al., 1999). These results suggest that LPS preconditioning may function on the cerebrovasculature as well as directly on neuronal tissue.
The protective effect of LPS preconditioning has also been demonstrated in in vitro models of ischemia. Low-dose LPS (1 μg/ml) pretreatment for 24 hours protected cortical neurons from OGD-induced cell death (Rosenzweig et al., 2007). Application of LPS (1 μg/ml) or conditioned media from LPS-treated mixed glial cultures 24 hours before can also protect cerebellar granule neurons against the ischemic injury induced by mitochondrial toxin 3-NPA (Lastres-Becker et al., 2006).
Preconditioning induced by LPS was observed not only in adult stroke models, as previously noted, but also in neonatal HI (Eklind et al., 2005; Lin et al., 2009a). However, uniquely to the neonates, the effect of LPS on immature brain varies depending on the interval between LPS application and HI and the intensity of HI (Eklind et al., 2005). For example, a low dose of LPS (0.3 mg/kg, i.p.) administered 24 h before hypoxia-ischemia in P7 rats provided neuroprotection to the immature brain (Eklind et al., 2005). Further study demonstrated that systemic LPS preconditioning (0.05 mg/kg, i.p.) in P7 rats 24 h prior to HI greatly reduced HI-induced cerebral inflammation and conferred long-term neuroprotection against brain damage and behavioral abnormalities (Lin et al., 2009a). However, when administered 6 hours or 72 hours prior to HI, LPS exacerbated post-HI brain injury (Eklind et al., 2005). It is possible that the brain development at the time of infection induction determines the sensitizing or preconditioning effects of LPS; future studies geared at exploring this possibility may provide useful data on inflammatory responses in the different neonatal stages.
At higher doses, LPS is also highly toxic to dopaminergic cells. Prenatal or adult LPS exposure at higher doses consistently led to permanent DA neuronal loss in the substantia nigra (Carvey et al., 2003; Ling et al., 2004; Ling et al., 2002) (Serotonin neurons, which are also affected in PD (Wang et al., 2009), are also lost.) In wild-type adult male mice, intraperitoneal LPS by itself at a dose of 5 mg/kg can also cause delayed and progressive loss of DA neurons (Liu et al., 2008b). Female mice are more resistant, reminiscent of the pattern in PD in which males have a somewhat higher likelihood of developing the disease than do females (Liu et al., 2008b). LPS infusions directly into the striatum also caused progressive DA neuron degeneration and accumulation of α-synuclein and ubiquitin in remaining DA neurons (Choi et al., 2009; Hunter et al., 2009; Hunter et al., 2007). Behavioral deficits were elicited in this model with loss of striatal DA and were alleviated with L-DOPA (Hunter et al., 2009).
Despite the toxicity of LPS at higher doses on dopaminergic cells, LPS has also been used as a preconditioning stimulus at low concentrations in the context of dopaminergic loss. Pretreatment of organotypic slices of ventral midbrain with 1–10 ng/ml LPS protected against subsequent treatment with 100 ng/ml LPS (Ding and Li, 2008). This raises the interesting possibility that whereas a major inflammatory stress could be a risk factor for PD, a more minor inflammatory stimulus might actually be neuroprotective. Further studies of this hypothesis seem warranted.
Low-dose LPS preconditioning has also been reported to provide a delayed protection against brain injuries related to hypothermic circulatory arrest. In a piglet model of hypothermic circulatory arrest, preconditioning with 20 μg/kg LPS 3 days before surgery significantly reduced histological injury in the cortex, basal ganglia, and hippocampus, indicating that systemic LPS preconditioning induces global cerebral protection against brain injury from hypothermic circulatory arrest (Hickey et al., 2007).
3.5.2.3 Other TLRs ligands
Preconditioning with other TLRs ligands uses similar paradigms. TLR9 preconditioning against transient focal ischemia could be achieved in experimental animals by systemic administration of its specific ligand, unmethylated CpG oligodeoxynucleotide (Stevens et al., 2008). A concentration range from 20 μg to 40 μg for a single i.p. injection of CpG oligodeoxynucleotide 72 hours prior to transient MCAO conferred robust cerebral protection with a tolerance window beginning 1 day after injection and lasting at least 7 days (Stevens et al., 2008). For in vitro studies, CpG oligodeoxynucleotide (0.5 to 5 μg/ml) pretreatment for 24 hours protected cortical neurons against OGD-induced cell death. A TLR2 agonist, Pam3Cys-Ser-Lys4, at 2 mg/kg has been shown to be protective to MCAO mice when applied i.p. 24 hours before insult (Hua et al., 2008). Likewise, the TLR7 agonist Gardiquimod (40 μg) administered subcutaneously 72 hours prior to transient focal ischemia conferred neuroprotection (Leung et al., 2012). As more is known about the effect of TLR ligand preconditioning, prophylactic TLRs stimulation may become a new paradigm for stroke management.
3.6 Systemic stress preconditioning
Most preconditioning studies deliver acute stressors to elicit neuroprotection. However, we have noted above that even chronic stress can be protective provided it is sublethal in concentration. Chronic organism stress, such as during physical exercise and caloric restriction or intermittent fasting appear to induce a preconditioned state.
3.6.1 Physical exercise
Exercise can be considered a form of mild stress (Arumugam et al., 2006; Morton et al., 2009), despite its popular view as being innocuous. Exercise raises levels of reactive oxygen species (ROS) and subsequent antioxidant defenses to deal with this subtoxic challenge (Morton et al., 2009; Radak et al., 2008; Radak et al., 2001), just as would be expected of a prototypical preconditioning stimulus. Furthermore, exercise may function as a stressor simply by heating the organism (Lim et al., 2008; Whitham and Fortes, 2008), and, thus, leading to mild heat stress, a preconditioning stimulus by itself, as described above. Animal studies demonstrate that physical exercise can reduce behavioral and neuropathological deficits after ischemic stroke (Ding et al., 2005; Hu et al., 2010c; Li et al., 2004), and clinical studies are consistent with these findings. In the animal studies of this phenomenon, mice or rats experience either voluntary exercise such as running in a wheel (Hu et al., 2010c) or forced exercise on a treadmill (Liebelt et al., 2010) prior to ischemic insults. It was observed that a threshold of exercise must be reached to achieve benefits to the ischemic brain. For example, it has been shown that at least 2 or 3 weeks of pre-training on a treadmill is necessary to exert neuroprotection against ischemic stroke in rats (Jia et al., 2009; Liebelt et al., 2010). The nature of exercise-conferred neuroprotection seems to be multisystemic, including maintaining BBB and neurovascular integrity (Ding et al., 2006), reducing cerebral inflammation (Ding et al., 2005), inhibiting neuronal apoptosis (Liebelt et al., 2010) and stimulating angiogenesis/neurogenesis (Brandt et al., 2010).
There has been a great deal of interest in exercise as a protective factor against neurodegeneration. Significant evidence exists that exercise protects the nigrostriatal pathway against DA neuron toxins in animal studies (Petzinger et al., 2010; Zigmond et al., 2009; Zigmond et al., 2012). Only a few of these studies will be highlighted below, with an emphasis on the more detailed studies.
Several studies have shown that exercise (and environmental enrichment) protects DA neurons in an MPTP mouse model. For example, 3 months of access to a running wheel reduced the loss of DA cells in the substantia nigra (Faherty et al., 2005). Like other preconditioning interventions, the effect was dose-dependent, significant only after 2 months of exercise and maximal after 3 months of exercise (Gerecke et al., 2010). Further support for the preconditioning effects of exercise comes from a recent study of chronic MPTP. In this case, 18 weeks of exercise for 1 hr 5 days a week on a treadmill improved balance and movement indicators, mitochondrial function, and effected protection against DA neuronal loss (Lau et al., 2011). Furthermore, 4 weeks of running exercise prevented LPS-induced loss of DA neurons and motor dysfunction (Wu et al., 2011). Not surprisingly, treadmill exercise also promoted angiogenesis in the striatum of MPTP-treated mice (Al-Jarrah et al., 2010). It has been suggested that exercise may act to mitigate cortically-driven hyper-excitability in the basal ganglia (Gerecke et al., 2010; Petzinger et al., 2010). Our preliminary observations with 16–20-year-old female rhesus treated with MPTP indicate both a behavioral and neurobiological protective effect of 3 months of treadmill running (Zigmond et al., 2009; Zigmond et al., 2012). We have also used another model of exercise, casting a forelimb is associated with increased use (or exercise) of the contralateral forelimb, which would otherwise have been reduced by the unilateral toxin (e.g., 6-OHDA) treatment (Tillerson et al., 2001; Tillerson et al., 2002). We found that forelimb casting for 7 days prior to infusion of 6-OHDA in the ipsilateral medial forebrain bundle dramatically reduced behavioral deficits and neurodegeneration (Tillerson et al., 2001; Tillerson et al., 2002).
3.6.2 Caloric restriction
Caloric intake – both excessive and restricted – exerts multiple effects on cellular systems and thus may affect neurological function and susceptibility to injury or degeneration. The role of caloric restriction (CR) in aging protection has now been fairly well established, and is associated with increased antioxidant capacity. The categorization of CR as a preconditioning stimulus is derived from two major concepts. First, CR presents a spectrum of toxicity; severe CR (fasting, <50% caloric needs) has been found in increased oxidative stress in liver, whereas moderate CR (60–70% caloric needs) increased antioxidant capacity and effectively decreased oxidative stress. Secondly, moderate CR has been associated with increased mitochondrial respiration (Cerqueira et al., 2012; Lin et al., 2002), which increases ROS generation (Turrens, 2003). Whether moderate CR-mediated protection is due to the subsequent upregulation of antioxidant capacity and severe CR leads to overwhelming of such capacity has not yet been explored. A discussion of the mechanistic implications of CR are planned for a later review.
Moderate CR has recently been studied in the protection against global ischemia and neonatal HI. In the neonatal HI model, pregnant dams were subjected to normal diet or moderate or extreme CR, with no resulting differences in the CA1 of the pups or learning tasks in adulthood (Tu et al., 2012). However, following HI, moderate CR significantly decreased brain volume loss and neurovascular damage (Tu et al., 2012). Moderate CR also improved functional recovery following global ischemia in adult rats, despite not significantly improving CA1 cell death (Roberge et al., 2008).
Epidemiological studies suggest that AD is more prevalent in countries with high-calorie diets, and that conditions promoting insulin-resistance and high levels of cholesterol raise the risk for the disease (Kalmijn et al., 1997; Leibson et al., 1997; Notkola et al., 1998). Animals fed high-fat diets were also more vulnerable to some of the behavioral deficits that are observed in transgenic mouse models of AD (Schroeder et al., 2010), and had disturbances in cerebrovascular integrity, synaptic density and reactive gliosis (Pepping et al., 2013). On the other hand, caloric restriction or reducing meal frequency can extend lifespan and protect against various insults, including stroke, in diverse organisms ranging from worms and flies to mammals (Ingram et al., 2007; Mattson et al., 2004; Schroeder et al., 2010; Yu and Mattson, 1999). Early studies extended the protective role of caloric restriction to neurodegenerative disease (Ingram et al., 1987). Caloric restriction attenuated amyloid deposition, phospho-tau levels and neurological dysfunction in transgenic mouse models of AD (Halagappa et al., 2007; Love, 2005; Mouton et al., 2009; Wang et al., 2005; Wu et al., 2008; Zhu et al., 1999), as well as amyloidosis in naturally aged squirrel monkeys (Qin et al., 2006). An influence of caloric restriction on other neurodegenerative conditions, including PD, has also been reported (Duan and Ross, 2010). Intermittent fasting, a non-chronic form of caloric restriction, also demonstrated some benefit in transgenic AD mouse models (Halagappa et al., 2007), as well was neuroprotective against seizures in hippocampal neurons (Duan et al., 2001).
Caloric restriction has been reported to reduce behavioral and/or neurobiological abnormalities in C. elegans, rodent, and monkey models of PD (Duan and Mattson, 1999; Holmer et al., 2005; Jadiya et al., 2011; Maswood et al., 2004) and to be efficacious in clinical PD, as well (Logroscino et al., 1996).
It has been argued that restriction of caloric intake to 50–70% of normal is a form of mild stress that exerts preconditioning effects because it activates stress responses (Arumugam et al., 2006; Kouda and Iki, 2010; Mattson et al., 2002; Mattson et al., 2004). Calorie-restricted animals also lower their metabolic rates, which could provide direct protection against damage to DNA and other insults (Duffy et al., 1990; Duffy et al., 1989). Another study found that caloric restriction altered the expression of mammalian genes associated with autophagy and lifespan (Hands et al., 2009; Honjoh and Nishida, 2011; Jia et al., 2004; Jung et al., 2010; Kenyon, 2011; Paradis and Ruvkun, 1998; Tissenbaum and Ruvkun, 1998; Wu et al., 2009), leading to the hypothesis that autophagy takes a “front seat in lifespan extension” (Petrovski and Das, 2010). Since the biggest risk factor for AD is aging, these studies on dietary-induced changes in lifespan may be highly relevant to this form of neurodegeneration. Finally, it seems likely that the effects of caloric restriction are also related to the fact that calorie-restricted animals tend to exhibit more spontaneous locomotor activity (Duffy et al., 1989; Poehlman et al., 2001), which can be protective in its own right as noted above.
3.7 Subcellular stress – mitochondrial, proteotoxic and ER preconditioning
Subcellular stressors, including proteotoxic stress, ER stress, and mitochondrial stress, can be both endogenously and chemically induced and, under the latter situation, controlled in a dose-dependent manner. Proteotoxic stress arises from a long-term burden of misfolded proteins and proteasome inhibition such as occurs in PD (Braak and Del Tredici, 2009; McNaught et al., 2003). We have shown that long-term proteasome inhibition with sublethal MG132 (2 weeks to 6 months duration of treatment) raised defensive proteins in dopaminergic cells (Leak et al., 2008) and elicited long-term protection against oxidative toxicity from 6-OHDA (Leak et al., 2006). Likewise, induction of ER stress with thapsigargin protected against 6-OHDA in SH-SY5Y cells (Hara et al., 2011). Thus, it appears possible that even chronic subcellular stress might elicit long-term adaptations in chronic neurodegenerative conditions.
Mitochondrial stress represents perhaps the best studied of subcellular stressors in the context of preconditioning. Neurons have particular sensitivity to mitochondrial function, as neuronal function is highly regulated by mitochondrial energetic stores and calcium buffering capacity. In addition, mitochondria are a major source of ROS as byproducts of oxidative phosphorylation. Several compounds that target mitochondrial function when used at subtoxic doses confer a preconditioned state in neurons.
3-NPA is an irreversible inhibitor of the mitochondrial protein succinate dehydrogenase (respiratory Complex II), an enzyme complex that participates in both the citric acid cycle and the electron transport chain, catalyzing the oxidation of succinate to fumarate with the reduction of ubiquinone to ubiquinol (Marini et al., 2008). Inhibition of Complex II is associated with increased production of cellular ROS (Mandavilli et al., 2005) and, in models of acute and chronic toxicity, 3-NPA can produce striatal neural damage similar to that observed in Huntington’s disease (Brouillet et al., 2005). However, subtoxic doses of 3-NPA (20 mg/kg) can also induce delayed (1–3 days) ischemic tolerance in rodent brains. 3-NPA significantly decreased neuronal apoptosis and reduced the subsequent neurological deficits and infarct volume within 24 to 72 hours after transient ischemia (Hoshi et al., 2006; Kuroiwa et al., 2000; Pera et al., 2004; Zhu et al., 2004), and also prevented delayed neurological deterioration at 7 days following ischemia (Kuroiwa et al., 2000). The critical threshold in the dose needed to obtain preconditioning versus dosing that does not confer tolerance or causes toxicity is fairly narrow. In a model of 3-NPA preconditioning against permanent focal ischemia in rats, 10–15 mg/kg systemically administered 3 days prior to ischemia effectively afforded neuroprotection, but 5 or 20 mg/kg 3-NPA did not, and 25 mg/kg resulted in significant mortality (Hoshi et al., 2005). The narrowness of the critical threshold for preconditioning and potential strain/ischemic model differences may explain why, in a gerbil model, 3–10 mg/kg 3-NPA did not confer tolerance against global ischemia, whereas another study found that low doses (3–4 mg/kg) were protective (Sugino et al., 1999).
Several studies in both heart and neural settings have suggested that opening of the mitochondrial adenosine triphosphate-sensitive potassium (mtK+ATP) channel correlated with the preconditioned state (Oldenburg et al., 2002; Ye et al., 2012b), and may, in fact, underlie the tolerant state conferred by 3-NPA (Horiguchi et al., 2003). Taken a step further, chemical preconditioning with compounds that directly open mtK+ATP have now been identified as providing tolerance to subsequent ischemic challenges.
Diazoxide is a prototypic mtK+ATP channel opener that causes local relaxation in smooth muscle and vasodilation in the treatment of malignant hypertension or acute hypertension (van Hamersvelt et al., 1996). The preconditioning effects of diazoxide have been well documented in the heart and other organs, and have been further observed in ischemic neural injury (Kis et al., 2003; Lenzser et al., 2005; Rajapakse et al., 2003; Wang et al., 2011b) and glutamate-induced excitotoxicity (Nagy et al., 2004). Administration of 20 or 40 mg/kg diazoxide, i.p., for 3 days prior to global cerebral ischemia conferred partial protection against brain edema in rats (Lenzser et al., 2005). In addition, rapid preconditioning (15–30 minutes prior to injury) was achieved with diazoxide in a model of subdural hematoma (Nakagawa et al., 2013), a model of photochemical thrombotic venous ischemia (Nakagawa et al., 2005) and global cerebral ischemia in gerbils (Wang et al., 2011b). An inhibitor of the mtK+ATP channel abolished the preconditioning effect in both of these models.
Several other small-molecule compounds that target the mtK+ATP channel have also been demonstrated to confer preconditioning against neural ischemic challenges. BMS-191095 is another mtK+ATP channel opener that has fewer known side effects compared to diazoxide (Kis et al., 2004). In cultured cortical neurons, BMS-191095 effectively induced neuronal preconditioning against subsequent OGD (Kis et al., 2004). Consistently, BMS-191095 also conferred neuroprotection against subsequent transient focal ischemia in rats (Mayanagi et al., 2007). The non-selective K+ATP channel opener pinacidil was effective at establishing a preconditioned state against rotenone toxicity in the catecholaminergic PC12 cell line (Tai and Truong, 2002), a model that is somewhat related to PD. Together, these data suggest that mitochondrial K+ATP channel opening may be an avenue for establishing drug-mediated preconditioning.
4.0 Clinical potential of preconditioning
The extensive literature on preconditioning or tolerance suggests that exposure to a low level of cellular stress can reduce the vulnerability of cells to an otherwise toxic, or challenge, level of stress. As we have noted, the preconditioning stressor can be either the same as the challenge stress or different (termed “cross tolerance”). These phenomena have primarily been the subject of laboratory studies employing either in vitro or in vivo models. However, the reproducibility of the preconditioning phenomenon has led to studies on its application and significance in clinical settings.
4.1 Incidental preconditioning
Preconditioning may be induced quite naturally by a variety of sublethal insults that the patient experiences. For example, a patient might be exposed to a transient episode of hypoxia, which could result in cells responding to the mild stress by increasing their defenses and thereby raising the level of stress required for subsequent toxicity. This unplanned preconditioning can be termed “incidental,” and may help to explain the slow progression of neurodegenerative diseases such as PD and AD to which we have already alluded. Another example of incidental preconditioning might be the result from neuronal death. As neurons die they are likely to exert a stress on neighboring neurons by stimulating an inflammatory response that could affect healthy bystanders. Whereas that inflammatory response has been proposed as one of the causes of subsequent cell death in neurodegenerative diseases (Qian et al., 2010), it is also possible that cell death can act to precondition the remaining neurons and in this way slow the progression of a disease, much as appears to occur in response to exogenous LPS (see above). There is already some precedence for the belief that preconditioning may occur naturally in humans with vascular disease. For example, humans suffering periods of angina within 7 days of myocardial infarction had lower mortality than those without previous angina (Kloner et al., 1995; Muller et al., 1990). Furthermore, transient ischemic attacks may help precondition the human brain against subsequent ischemia (Moncayo et al., 2000; Wegener et al., 2004; Weih et al., 1999). Notably, patients with preexisting steno-occlusive vascular disease have reduced subarachnoid hemorrhage-induced cerebral vasospasms (Kim et al., 2012), a correlation that is suggestive of a preconditioned state. Thus, it also seems likely that mild stress can have positive clinical effects through preconditioning. Indeed, as previously discussed, perhaps preconditioning can also explain the seemingly paradoxical negative correlation between tobacco smoking and the incidence of PD (Allam et al., 2004) or the apparently neuroprotective effects of alcohol (see above).
4.2 Purposeful preconditioning
Some forms of preconditioning might actually be purposefully introduced to prevent or retard disease. Epidemiological data suggest that individuals who engage in intense physical exercise are less likely to develop PD (Speelman et al., 2011; Xu et al., 2010), and an inverse correlation between activity levels and risk of developing PD and point to the benefits of exercise programs in improving postural control, balance and mobility (Allen et al., 2010; Archer et al., 2011; Chen et al., 2005; Gobbi et al., 2009; Nocera et al., 2009; Xu et al., 2010). Of course, one cannot rule out the potential confound that humans in the early prodromal phase of PD are less likely to stick to an exercise regime, thereby confounding the data. However, physical exercise is now widely recommended for patients with PD, although research is still required to differentiate between symptomatic effects and the true disease-modifying effects that are suggested by our animal studies, as well as to determine the optimal type and duration of exercise.
Since exercise stimulates mitochondrial respiration and oxidative stress (Di Meo and Venditti, 2001), it seems likely that these effects precondition neurons, reducing their vulnerability to subsequent stress. Caloric restriction or intermittent fasting is another example of preconditioning that might be applied to the clinic, particularly in individuals who are overweight (Mercken et al., 2012). Other examples of stimuli that may help to precondition humans include dietary phytochemicals, anesthetics, and inflammatory mediators.
The concept of purposeful preconditioning with sublethal injury in the clinical setting has been explored primarily in the treatment of cardiac ischemic events (Hausenloy and Yellon, 2011) and may in turn be applicable to the brain during vascular neurosurgery or during surgical procedures with a high risk of cerebral ischemia. Indeed, cardiac surgery itself, such as coronary artery bypass graft surgery, can pose a risk to the brain. Alex and colleagues found some evidence of protection against neuropsychometric dysfunction following coronary artery bypass graft surgery with hyperbaric oxygen preconditioning (Alex et al., 2005). Notably, repeated hyperbaric oxygen preconditioning before on-pump coronary artery bypass graft surgery reduced biochemical markers of neuronal injury (S100B protein and neuron-specific enolase release) and improved clinical outcomes in that it reduced intensive care stay and decreased use of inotropic drugs (Li et al., 2011). Whereas the focus of preconditioning in the cardiac setting logically has rested on cardiac tissue, there is room to explore the effects on cognitive and cerebral functional outcomes.
In addition, surgical procedures on major vascular sites often carry significant risk of secondary cerebral ischemia or cognitive dysfunction. A small clinical study (12 patients) reported that ischemic preconditioning of the proximal artery (2 minutes followed by 30 minutes of reperfusion) prior to clipping of cerebral aneurysms attenuated tissue hypoxia (Chan et al., 2005). Arterial stenoses represent a severe risk for ischemic stroke in humans, and remote limb preconditioning (RIPC) induced by bilateral upper limb ischemia (5 cycles, twice daily over 300 consecutive days) was recently found to significantly decrease the incidence of recurrent stroke in patients with intracranial arterial stenosis (Meng et al., 2012). For carotid stenosis >70%, carotid endarterectomy (CEA) is the preferred treatment, but carries with it significant risk of neurocognitive deficits, which occur in approximately 20% of patients (Heyer et al., 2002). Whether or not RIPC can reduce surgery-related neurocognitive deficits is still questionable (Meybohm et al., 2013), but a pilot clinical trial has suggested that the procedure is safe in CEA patients (Walsh et al., 2010). In addition, one study of patients undergoing cervical decompression surgery found that remote ischemic preconditioning reduced injury markers and improved recovery rates (Hu et al., 2010a). Remote limb preconditioning in patients with subarachnoid hemorrhage was also found to be safe and tolerated in individuals with subarachnoid hemorrhage (Koch et al., 2011), although no studies have yet indicated whether remote preconditioning confers neuroprotection in models of subarachnoid hemorrhage.
An important caveat is that ischemic preconditioning in humans may depend on the patient’s tolerance of occlusion of a selected vessel and that the preconditioning ischemia may induce atrioventricular block (Apostolakis et al., 2012). More evidence is thus required before a conclusion on preconditioning safety and efficacy in cardiovascular surgery can be drawn. Nonetheless, the studies so far seem promising and we hold out hope for a positive impact of purposeful preconditioning on the brain during neurosurgery.
4.3 Post-conditioning
Post-conditioning has become a major line of research in recent years and deserves a review in its own right. However, due to its highly relevant clinical applicability, we include mention of it within this section. The evidence for clinical efficacy of preconditioning is less compelling in the case of neurodegenerative disease as contrasted, for example, with timed neurosurgery. Keep and colleagues have discussed the difficulties associated with preconditioning against chronic brain diseases, in part due to a narrow therapeutic window when both stimulus dose and duration are considered (Keep et al., 2010). However, combining the insights gained from preconditioning in surgical or controlled scenarios with emerging post-conditioning settings may help to identify situations advantageous for conditioning-induced neuroprotection.
Most cases of brain damage cannot be anticipated or are relatively rare. For example, PD affects only 1% of those at or above the age of 60, and TBI affects only about 1.5 million Americans each year, or less than 0.5% of the total population. For these reasons it would be extremely valuable if one could apply a conditioning stimulus after the initial toxic insult. Although post-conditioning will not protect against the initial damage produced by ischemia, TBI, or the onset of neurodegenerative diseases, a considerable amount of damage occurs in the period immediately after ischemia and TBI, and diseases such as PD and AD develop gradually over many years. Might post-conditioning protect against these sequelae?
Post-conditioning is an area of active investigation. In the case of post-conditioning of ischemia, the majority of the initial work was established in models of myocardial injury. However, recent advances have been made in establishing ischemic post-conditioning models against cerebral ischemia (Zhao, 2009; Zhao et al., 2012). Thus far, the most robust ischemic post-conditioning paradigm involves repeated cycles of rapid reocclusion during the onset of reperfusion (Zhao et al., 2006a). The exact parameters of the cycles appear to be highly sensitive. For example, 3 cycles, but not 10 cycles, of 10-second re-occlusion/30-second reperfusion effectively reduced infarct volume (Gao et al., 2008), and 10 cycles, but not 3 cycles, of 10-second re-occlusion/10-second reperfusion reduced infarct (Gao et al., 2008). In a global ischemic model, rapid preconditioning (administered at the onset of reperfusion) by six cycles of 10-second reperfusion/10-second re-occlusion significantly decreased TUNEL labeling and caspase-3 immunoreactivity 24–72 hours following ischemia (Zhang et al., 2012). Surprisingly, post-conditioning induced 2 days following moderate (10 minute) global ischemia conferred protection, as well (Burda et al., 2006; Zhou et al., 2011a), but not against slightly more severe ischemic insults (15 minutes global ischemia) (Burda et al., 2006).The sensitivity of these paradigms correlates well with ischemic preconditioning, and with the concept that the “dose” of the preconditioning stimulus is critical to its beneficial effects.
As mentioned above, remote ischemic post-conditioning via femoral artery occlusion for two to three cycles of 10–15 minutes confers neuroprotective effects in models of transient and permanent focal ischemia (Ren et al., 2011; Ren et al., 2008), as well as neonatal HI (Zhou et al., 2011b). The effect of remote ischemic post-conditioning in neonatal HI is interesting, as direct ischemic post-conditioning by common carotid artery did not appear to reduce infarct in neonates following HI (Leger et al., 2012). Remote femoral ischemic preconditioning did not improve outcomes following ICH (Geng et al., 2012).
Post-conditioning against myocardial injury can be elicited by non-ischemic stimuli that overlap with established preconditioning stimuli, including inhalational anesthetics, G-protein coupled receptor ligands such as opioids, adenosine, bradykinin, insulin, erythropoietin, adipocytokines, and statins (Hausenloy and Yellon, 2009, 2011). Likewise, recent studies have included non-ischemic post-conditioning stimuli in neurological injury models. Anesthetics including isoflurane, sevoflurane and propofol have been found to be useful as post-conditioning stimuli. Isoflurane post-conditioning decreased infarct in rat corticostriatal slice cultures subjected to OGD and improved neurological outcomes following transient focal ischemia (Lee et al., 2008). Administration of the anesthetic propofol for 4 hours at the onset of reperfusion led to long-term improvements in recovery following focal ischemia (Wang et al., 2011a). Sevoflurane administered at the onset of reperfusion following focal ischemia reduced infarct and improved memory task performance (Adamczyk et al., 2010; Wang et al., 2010a), and improved neurological outcomes and CA1 cell survival in a model of BCCAO with hypotension (Jeon et al., 2013). Again, the administration necessary for neuroprotection may be highly sensitive. When sevoflurane was administered at later time points following focal ischemia (5 minutes post-reperfusion) or following a model of incomplete ischemia (occlusion of right carotid artery combined with hypotension), protection was lost (Adamczyk et al., 2010; Lee et al., 2010).
Other common preconditioning stimuli are now demonstrating effects when administered in a post-conditioning paradigm. Hypothermia (33°C for 24 hours) following cardiopulmonary resuscitation decreased inflammation in the brain in pigs (Meybohm et al., 2010). Normobaric hyperoxia (intermittent or continuous occurring during the ischemic period) reduced infarct volume and neurological deficits (Liu et al., 2012). Intraventricular injection of LPS or lipooligosaccharide significantly decreased inflammation when administered 2 hours following instrastriatal injection of IL-1beta (Bingham et al., 2013; Davis et al., 2005). Hypoxic post-conditioning (three cycles of 15-minutes hypoxia/15-minutes reoxygenation) decreased cell death in cultured neurons exposed to toxic hypoxia (Yao et al., 2011). Taken together, these results suggest that the post-conditioned state appears to be attained in a parallel fashion to the preconditioned state.
4.4 Preconditioning in therapeutic tool development
In addition to possibly promoting the tolerant state, preconditioning of cultured stem cells may provide a critical tool in the use of stem cells as therapeutics. To date, stem cell delivery into infarcted regions has been hampered by poor graft survival and/or differentiation. Although full differentiation and restoration of lost neurons appears unlikely, stem cells could be used as conduits for the release of trophic factors to minimize secondary or prolonged damage. Thus far, the application of preconditioning stem cells for therapeutic purposes in neuronal models in vivo has produced some positive results. Hypoxic preconditioning of MSCs improved cell migration to the ischemic cortex when delivered intranasally 24 hours following transient focal ischemia (Wei et al., 2013). The preconditioned MCSs also reduced ischemic infarct and improved behavioral outcomes better than non-preconditioned MCSs (Wei et al., 2013), and intravenous injection 24 hours following transient focal ischemia led to decreased microglial activity and improved performance on the rotarod (Wei et al., 2012b). Preconditioning of neural stem cells with minocycline or interleukin 6 in vitro led to improved graft survival and increased secretion of trophic factors when transplanted into ischemic brains following transient focal ischemia, as well as improved histological and behavioral outcomes (Sakata et al., 2012a; Sakata et al., 2012b). In models of TBI, tail vein injection of MSCs or the MSC culture medium (secretome) alone was found to be protective (Chuang et al., 2012; Kim et al., 2010). Hypoxic preconditioning of MSCs significantly improved the release of trophic factors from cultured MCSs, and improved the behavior and histological outcomes following secretome injection (Chang et al., 2013). These studies have just begun to explore preconditioning in the development of a therapeutic tool, and will likely lead to further interesting applications of preconditioning.
5.0 Caveats: Have we been doing the right experiments?
Despite the promising animal studies outlined above, there remain significant hurdles before preconditioning can be more widely, safely, and effectively translated into the clinic. Limitations to clinical translation include the weaknesses inherent in most experimental studies. For example, although age and gender constitute major risk factors in stroke as in neurodegenerative diseases, studies in models of these conditions typically use young adult or even “teenage” rodents. In fact, aged animals may not precondition as well as do younger animals, which may suggest an important limitation to introducing preconditioning into some clinical situations (He et al., 2005). A second concern relates to strain differences that have been observed in animal studies (Purcell et al., 2003), which may indicate that unique genetic characteristics of humans might reduce the generalizability of animal studies to patients, or that subpopulations of humans may respond differently to preconditioning stimuli.
Another consideration is that many clinical trials in human conditions have relied upon end-stage patient populations. However, clinical trials that focus upon milder cases are likely to have a far greater chance of success. Furthermore, common comorbidities such as hypertension, diabetes, and metabolic syndrome may well derail efforts to elicit endogenous neuroprotection because such conditions can reduce the effects of preconditioning, as has been shown in ischemia (Purcell et al., 2003; Wang et al., 2002). As suggested by Keep, Xi, and colleagues, the effects of gender, inflammation, smoking, atherosclerosis, aging, hypertension, and diabetes on human preconditioning all need to be factored into the final equation when assessing clinical outcomes (Keep et al., 2010). Similar to pharmacogenomics, tailoring ischemic preconditioning to the patient population will be critical if preconditioning is ever to become widely used in the clinic. These considerations emphasize the importance of a bidirectional flow of information in translational studies, with clinical trials guiding animal studies as well as the reverse.
As mentioned above, in the case of ischemic preconditioning, questions have been raised because of a narrow therapeutic window (Keep et al., 2010). Both the duration of the preconditioning stimulus and the time between the end of the preconditioning stimulus and the final stroke can have a significant impact on outcome. One of the inherent weaknesses of ischemic preconditioning is that the preconditioning response usually fades within a week or two. Thus, to maintain patients in a preconditioned state, one would have to re-apply the stimulus at set intervals. Whereas this may seem an insurmountable requirement, there is some evidence that long-term preconditioning is feasible. For example, long-term hypo-perfusion protects against acute focal ischemia, improves motor function, and elicits structural changes in the brain, such as a rise in blood vessel density (Kim et al., 2008). Moreover, dietary phytochemicals that can also precondition, such as resveratrol from grapes, can be ingested over the long term. Similarly, in principle, the preconditioning elicited by exercise can also be maintained chronically. In contrast, induction of direct ischemic preconditioning in humans is necessarily limited to those cases where we know the timing of the ischemic event, such as during interventions, including heart bypass, angioplasty, and neurosurgery. Also, it should be noted that the temporal kinetics of preconditioning efficacy are typically garnered from experimental models and may therefore need to be refined after gathering more clinical data. Because of the limited time window between preconditioning and a subsequent ischemic challenge, ischemic post-conditioning and remote preconditioning have also generated much research interest. Furthermore, remote preconditioning of a limb could perhaps occur at repeated intervals over the long term.
Of course, patients may already be preconditioned by spontaneously occurring transient ischemic attacks (Moncayo et al., 2000; Wegener et al., 2004; Weih et al., 1999), perhaps even sleep apnea, dietary factors, physical activity, and the like. This would mean that purposeful induction of ischemia might push the brain towards a different location on the dose-response or duration-response curve than expected by the medical team. Indeed, this may explain failure in some clinical trials. This may detrimentally affect the safety profile of ischemic preconditioning, which is already of significant concern. Preconditioning stimuli by definition do not elicit cell death because they are sublethal in nature, but they can cause subtle behavioral deficits even in the absence of overt brain injury (Hua et al., 2005). This finding underscores the importance of having patients in clinical trials for preconditioning also undergo cognitive testing. Finally, the lack of consensus in how to image and quantify human strokes is also likely to retard progress.
Despite all these limitations, the failure of many clinical trials of pharmacological interventions that were effective in animals has generated much interest in promoting endogenous protection in the human brain. Preconditioning (remote or direct) and post-conditioning still promise to be of clinical utility. Although two studies on transient ischemic attacks in humans did not gather evidence for preconditioning (Della Morte et al., 2008; Johnston, 2004), a meta-analysis of preconditioning in patients undergoing cardiac surgery suggests that cardiac preconditioning reduced ventricular arrhythmias and inotrope requirements, as well as length of intensive care unit stay (Walsh et al., 2008). A second meta-analysis of remote ischemic preconditioning in cardiac surgery patients also gathered evidence of protection (Takagi et al., 2008). Further purposeful preconditioning studies for stroke as well as for other conditions in humans therefore seem warranted despite the limitations outlined here, particularly by changes in lifestyle (e.g., increased exercise and dieting) that are likely to promote incidental conditioning.
In addition to direct or remote preconditioning, another potential use of preconditioning lies in the enhancement of stem cell survival in transplantation. Ischemic preconditioning of MSCs increased graft survival in infarcted heart (Kim et al., 2009a), and oxytocin preconditioning appeared to improve functionality of MSCs related to ischemic protection of cultured rat cardiomyocytes (Noiseux et al., 2012). Diazoxide – a common preconditioning stimulus described above – also improved the survival of MSCs (Suzuki et al., 2010). Hypoxic preconditioning of the neonatal piglet brain led to increased proliferation of cultured SVZ-derived neural stem cells (Ara and De Montpellier, 2013), suggesting that the protective effects observed in tolerance models may be due at least in part to an improved capacity for repair using preconditioned stem cells. These studies indicate that stem cells respond robustly to preconditioning stimuli, and thus may play a role in tolerance.
6.0 Summary
A central theme that emerges from synthesizing the preconditioning literature above is that preconditioning stimuli, though highly diverse, exert protective effects across a wide range of neurological injury. It is not likely that any one drug by itself will protect neurons from stroke as effectively as nature is able to accomplish through preconditioning. Instead, as has already been argued for many disease states, a “cocktail therapy” approach will likely be needed to mimic the manifold natural defenses that preconditioning elicits. In addition to a cocktail approach, the effect of the drugs will be influenced by lifestyle factors that affect preconditioning and stress, such as exercise, diet, smoking, sleep, social interactions, environmental complexity, and cognitive activity, to name only a few. Studying these factors in conjunction with preconditioning, to see if synergistic protective responses can perhaps be elicited by combining various protective regimens would be worthwhile for future investigations.
Preconditioning is defined as the same process that can be both protective and harmful to neurons. At this point it can be speculated that normal cellular processes that are protective in moderation can turn lethal when engaged in excess of normal homeostatic levels. It is important to note that this dose-dependency is not unlike preconditioning itself, where the dose of the initial stimulus tips the balance in favor of either adaptation or cell death in response to a second challenge. However, one must superimpose on this complexity the cell type, the duration and intensity of the stressful stimulus, the previous history of the cell (exposure to previous stressors), and genetic predispositions to weak or strong preconditioning responses. Given all of these complex variables, the dual nature of many cellular processes is perhaps not a paradox at all. However, much more needs to be understood about the context in which the conditioning stimulus is being applied. Particularly in the case of chronic neurodegenerative disease, it is possible that the application of a mild stress onto chronically stressed cells may exacerbate the disease state, rather than provide a therapy. This then requires advancement of knowledge not only into the mechanism of preconditioning stimuli and disease but also into the response of a diseased cell/tissue/organ to mild stress at different stages of the disease.
Finally, given the observation that the same sets of proteins are often involved in various types of neuroprotection and that the same sets of insults are present in both the ischemic and the chronically degenerating brain, it is likely that the specific mechanisms of ischemic preconditioning can be generalized to neurodegeneration. As argued above, the phenomenon of cross-tolerance – systemic, pharmacological, or remote preconditioning of a peripheral limb – may even protect the brain from neurodegenerative diseases. It is our hope that this review and its companion not only synthesize much of what is known about preconditioning but also generate fresh interest in exploring the brain’s powerful system of natural defenses.
Table 1.
Preconditioning paradigms which induce ischemic tolerance in adults
Highlights.
Comprehensive overview of evidence related to neuroprotective effects of preconditioning paradigms in CNS disease models.
Thorough discussion of potential clinical significance derived from experimental preconditioning studies
Extension from preconditioning neuroprotection in stroke models to non-stroke models, including neurodegenerative diseases.
Acknowledgments
Dr. Yu Gan’s current address: State Key Laboratory of Oncogenes and Related Genes, Shanghai Cancer Institute, Renji Hospital, Shanghai Jiao-Tong University School of Medicine, 2200–25 Xietu Road, Shanghai 200032, China. This work was supported by National Institutes of Health/NINDS Grants NS36736, NS43802, NS56118, and NS45048 (to JC) and NS070825 (to MZ and JC), the VA Merit Review Grant (to JC), and Chinese Natural Science Foundation grants 81020108021, 8117111149 and 30870794 (to YG); 81228008 (to JC). J.C. is a recipient of the VA Career Scientist Award, the RK Mellon Endowed Chair from the University of Pittsburgh Medical Center, and the Changjiang Chair Professorship from the Chinese Ministry of Education. M.Z. is the recipient of the International Distinguished Professorship from the Chinese Ministry of Education (MS2011FDDX025). We thank Ms. Carol Culver for excellent editorial assistance and Ms. Pat Strickler for secretarial support.
Abbreviations
- MCAO
middle cerebral artery occlusion
- 2VO
two vessel occlusion
- 3-NPA
3-Nitropropionic acid
- 4VO
four vessel occlusion
- BCCAO
bilateral common carotid artery occlusion
- CSD
cortical spreading depression
- 6-OHDA
6-hydroxydopamine
- Aβ
amyloid-beta
- AD
Alzheimer’s disease
- ATA
atmospheres absolute
- BBB
blood brain barrier
- DA
dopamine
- HBO
hyperbaric oxygen
- HI
hypoxia/ischemia
- HMGB1
high-mobility group protein box-1
- HSP
heat shock proteins
- LPS
lipopolysaccharide
- MPTP
1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine
- mtK+ATP channel
mitochondrial adenosine triphosphate-sensitive potassium channel
- NMDA
N-methyl-D-aspartate
- NOS
nitric oxide synthase
- OGD
oxygen/glucose deprivation
- PD
Parkinson’s disease
- ROS
reactive oxygen species
- SBI
surgical brain injury
- TBI
traumatic brain injury
- TH
tyrosine hydroxylase
- TLR
toll-like receptor
- MSCs
mesenchymal stem cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Abekawa T, Ohmori T, Koyama T. Tolerance to the neurotoxic effect of methamphetamine in rats behaviorally sensitized to methamphetamine or amphetamine. Brain Res. 1997;767:34–44. doi: 10.1016/s0006-8993(97)00542-8. [DOI] [PubMed] [Google Scholar]
- Adamczyk S, Robin E, Simerabet M, Kipnis E, Tavernier B, Vallet B, Bordet R, Lebuffe G. Sevoflurane pre- and post-conditioning protect the brain via the mitochondrial K ATP channel. Br J Anaesth. 2010;104:191–200. doi: 10.1093/bja/aep365. [DOI] [PubMed] [Google Scholar]
- Al-Jarrah M, Jamous M, Al Zailaey K, Bweir SO. Endurance exercise training promotes angiogenesis in the brain of chronic/progressive mouse model of Parkinson's Disease. NeuroRehabilitation. 2010;26:369–373. doi: 10.3233/NRE-2010-0574. [DOI] [PubMed] [Google Scholar]
- Albert-Weissenberger C, Siren AL. Experimental traumatic brain injury. Exp Transl Stroke Med. 2010;2:16. doi: 10.1186/2040-7378-2-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alex J, Laden G, Cale AR, Bennett S, Flowers K, Madden L, Gardiner E, McCollum PT, Griffin SC. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: a prospective randomized double-blind trial. J Thorac Cardiovasc Surg. 2005;130:1623–1630. doi: 10.1016/j.jtcvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Allam MF, Campbell MJ, Hofman A, Del Castillo AS, Fernandez-Crehuet Navajas R. Smoking and Parkinson's disease: systematic review of prospective studies. Mov Disord. 2004;19:614–621. doi: 10.1002/mds.20029. [DOI] [PubMed] [Google Scholar]
- Allen NE, Canning CG, Sherrington C, Lord SR, Latt MD, Close JC, O'Rourke SD, Murray SM, Fung VS. The effects of an exercise program on fall risk factors in people with Parkinson's disease: a randomized controlled trial. Mov Disord. 2010;25:1217–1225. doi: 10.1002/mds.23082. [DOI] [PubMed] [Google Scholar]
- Apostolakis E, Baikoussis NG, Papakonstantinou NA. The role of myocardial ischaemic preconditioning during beating heart surgery: biological aspect and clinical outcome. Interact Cardiovasc Thorac Surg. 2012;14:68–71. doi: 10.1093/icvts/ivr024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appelros P, Stegmayr B, Terent A. Sex differences in stroke epidemiology: a systematic review. Stroke. 2009;40:1082–1090. doi: 10.1161/STROKEAHA.108.540781. [DOI] [PubMed] [Google Scholar]
- Ara J, De Montpellier S. Hypoxic-preconditioning enhances the regenerative capacity of neural stem/progenitors in subventricular zone of newborn piglet brain. Stem Cell Res. 2013;11:669–686. doi: 10.1016/j.scr.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Archer T, Fredriksson A, Johansson B. Exercise alleviates Parkinsonism: clinical and laboratory evidence. Acta Neurol Scand. 2011;123:73–84. doi: 10.1111/j.1600-0404.2010.01360.x. [DOI] [PubMed] [Google Scholar]
- Arthur PG, Lim SC, Meloni BP, Munns SE, Chan A, Knuckey NW. The protective effect of hypoxic preconditioning on cortical neuronal cultures is associated with increases in the activity of several antioxidant enzymes. Brain Res. 2004;1017:146–154. doi: 10.1016/j.brainres.2004.05.031. [DOI] [PubMed] [Google Scholar]
- Arumugam TV, Gleichmann M, Tang SC, Mattson MP. Hormesis/preconditioning mechanisms, the nervous system and aging. Ageing Res Rev. 2006;5:165–178. doi: 10.1016/j.arr.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Azmoon S, Demarest C, Pucillo AL, Hjemdahl-Monsen C, Kay R, Ahmadi N, Aronow WS, Frishman WH. Neurologic and cardiac benefits of therapeutic hypothermia. Cardiol Rev. 2011;19:108–114. doi: 10.1097/CRD.0b013e31820828af. [DOI] [PubMed] [Google Scholar]
- Baron JA. Cigarette smoking and Parkinson's disease. Neurology. 1986;36:1490–1496. doi: 10.1212/wnl.36.11.1490. [DOI] [PubMed] [Google Scholar]
- Barone FC, Feuerstein GZ. Inflammatory mediators and stroke: new opportunities for novel therapeutics. J Cereb Blood Flow Metab. 1999;19:819–834. doi: 10.1097/00004647-199908000-00001. [DOI] [PubMed] [Google Scholar]
- Barone FC, White RF, Spera PA, Ellison J, Currie RW, Wang X, Feuerstein GZ. Ischemic preconditioning and brain tolerance: temporal histological and functional outcomes, protein synthesis requirement, and interleukin-1 receptor antagonist and early gene expression. Stroke. 1998;29:1937–1950. doi: 10.1161/01.str.29.9.1937. discussion 1950–1931. [DOI] [PubMed] [Google Scholar]
- Bastide M, Gele P, Petrault O, Pu Q, Caliez A, Robin E, Deplanque D, Duriez P, Bordet R. Delayed cerebrovascular protective effect of lipopolysaccharide in parallel to brain ischemic tolerance. J Cereb Blood Flow Metab. 2003;23:399–405. doi: 10.1097/01.WCB.0000050064.57184.F2. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Kumar S, Schipma M, Collins MA, Neafsey EJ. Inhibition of amyloid-beta-induced neurotoxicity and apoptosis by moderate ethanol preconditioning. Neuroreport. 2004;15:2093–2096. doi: 10.1097/00001756-200409150-00019. [DOI] [PubMed] [Google Scholar]
- Belmadani A, Zou JY, Schipma MJ, Neafsey EJ, Collins MA. Ethanol pre-exposure suppresses HIV-1 glycoprotein 120-induced neuronal degeneration by abrogating endogenous glutamate/Ca2+-mediated neurotoxicity. Neuroscience. 2001;104:769–781. doi: 10.1016/s0306-4522(01)00139-7. [DOI] [PubMed] [Google Scholar]
- Berger K, Ajani UA, Kase CS, Gaziano JM, Buring JE, Glynn RJ, Hennekens CH. Light-to-moderate alcohol consumption and risk of stroke among U.S. male physicians. N Engl J Med. 1999;341:1557–1564. doi: 10.1056/NEJM199911183412101. [DOI] [PubMed] [Google Scholar]
- Bernard S. Hypothermia after cardiac arrest: expanding the therapeutic scope. Crit Care Med. 2009;37:S227–233. doi: 10.1097/CCM.0b013e3181aa5d0c. [DOI] [PubMed] [Google Scholar]
- Bernaudin M, Nedelec AS, Divoux D, MacKenzie ET, Petit E, Schumann-Bard P. Normobaric hypoxia induces tolerance to focal permanent cerebral ischemia in association with an increased expression of hypoxia-inducible factor-1 and its target genes, erythropoietin and VEGF, in the adult mouse brain. J Cereb Blood Flow Metab. 2002;22:393–403. doi: 10.1097/00004647-200204000-00003. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS. Expression of signal transduction genes differs after hypoxic or isoflurane preconditioning of rat hippocampal slice cultures. Anesthesiology. 2009;111:258–266. doi: 10.1097/ALN.0b013e3181a8647f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS, Gray J, McKleroy W. Inositol 1,4,5-triphosphate receptors and NAD(P)H mediate Ca2+ signaling required for hypoxic preconditioning of hippocampal neurons. Neuroscience. 2009;160:51–60. doi: 10.1016/j.neuroscience.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickler PE, Fahlman CS, Gray JJ. Hypoxic preconditioning failure in aging hippocampal neurons: impaired gene expression and rescue with intracellular calcium chelation. J Neurosci Res. 2010;88:3520–3529. doi: 10.1002/jnr.22508. [DOI] [PubMed] [Google Scholar]
- Bickler PE, Zhan X, Fahlman CS. Isoflurane preconditions hippocampal neurons against oxygen-glucose deprivation: role of intracellular Ca2+ and mitogen-activated protein kinase signaling. Anesthesiology. 2005;103:532–539. doi: 10.1097/00000542-200509000-00016. [DOI] [PubMed] [Google Scholar]
- Billard JM, Rouaud E. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by D-cycloserine. The European journal of neuroscience. 2007;25:2260–2268. doi: 10.1111/j.1460-9568.2007.05488.x. [DOI] [PubMed] [Google Scholar]
- Bingham D, John CM, Levin J, Panter SS, Jarvis GA. Post-injury conditioning with lipopolysaccharide or lipooligosaccharide reduces inflammation in the brain. Journal of neuroimmunology. 2013;256:28–37. doi: 10.1016/j.jneuroim.2012.12.009. [DOI] [PubMed] [Google Scholar]
- Bordet R, Deplanque D, Maboudou P, Puisieux F, Pu Q, Robin E, Martin A, Bastide M, Leys D, Lhermitte M, Dupuis B. Increase in endogenous brain superoxide dismutase as a potential mechanism of lipopolysaccharide-induced brain ischemic tolerance. J Cereb Blood Flow Metab. 2000;20:1190–1196. doi: 10.1097/00004647-200008000-00004. [DOI] [PubMed] [Google Scholar]
- Bowman CC, Rasley A, Tranguch SL, Marriott I. Cultured astrocytes express toll-like receptors for bacterial products. Glia. 2003;43:281–291. doi: 10.1002/glia.10256. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K. Neuroanatomy and pathology of sporadic Parkinson's disease. Adv Anat Embryol Cell Biol. 2009;201:1–119. [PubMed] [Google Scholar]
- Bradford ST, Stamatovic SM, Dondeti RS, Keep RF, Andjelkovic AV. Nicotine aggravates the brain postischemic inflammatory response. Am J Physiol Heart Circ Physiol. 2011;300:H1518–1529. doi: 10.1152/ajpheart.00928.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt MD, Maass A, Kempermann G, Storch A. Physical exercise increases Notch activity, proliferation and cell cycle exit of type-3 progenitor cells in adult hippocampal neurogenesis. The European journal of neuroscience. 2010;32:1256–1264. doi: 10.1111/j.1460-9568.2010.07410.x. [DOI] [PubMed] [Google Scholar]
- Bronner G, Mitchell K, Welsh FA. Cerebrovascular adaptation after unilateral carotid artery ligation in the rat: preservation of blood flow and ATP during forebrain ischemia. J Cereb Blood Flow Metab. 1998;18:118–121. doi: 10.1097/00004647-199801000-00012. [DOI] [PubMed] [Google Scholar]
- Brouillet E, Jacquard C, Bizat N, Blum D. 3-Nitropropionic acid: a mitochondrial toxin to uncover physiopathological mechanisms underlying striatal degeneration in Huntington's disease. J Neurochem. 2005;95:1521–1540. doi: 10.1111/j.1471-4159.2005.03515.x. [DOI] [PubMed] [Google Scholar]
- Bruer U, Weih MK, Isaev NK, Meisel A, Ruscher K, Bergk A, Trendelenburg G, Wiegand F, Victorov IV, Dirnagl U. Induction of tolerance in rat cortical neurons: hypoxic preconditioning. FEBS Lett. 1997;414:117–121. doi: 10.1016/s0014-5793(97)00954-x. [DOI] [PubMed] [Google Scholar]
- Bsibsi M, Ravid R, Gveric D, van Noort JM. Broad expression of Toll-like receptors in the human central nervous system. J Neuropathol Exp Neurol. 2002;61:1013–1021. doi: 10.1093/jnen/61.11.1013. [DOI] [PubMed] [Google Scholar]
- Burda J, Danielisova V, Nemethova M, Gottlieb M, Matiasova M, Domorakova I, Mechirova E, Ferikova M, Salinas M, Burda R. Delayed postconditionig initiates additive mechanism necessary for survival of selectively vulnerable neurons after transient ischemia in rat brain. Cell Mol Neurobiol. 2006;26:1141–1151. doi: 10.1007/s10571-006-9036-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadet JL, McCoy MT, Cai NS, Krasnova IN, Ladenheim B, Beauvais G, Wilson N, Wood W, Becker KG, Hodges AB. Methamphetamine preconditioning alters midbrain transcriptional responses to methamphetamine-induced injury in the rat striatum. PLoS One. 2009;4:e7812. doi: 10.1371/journal.pone.0007812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Hua Y, Richardson RJ, Xi G, Keep RF, Schallert T. The effect of thrombin on a 6-hydroxydopamine model of Parkinson's disease depends on timing. Behav Brain Res. 2007;183:161–168. doi: 10.1016/j.bbr.2007.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon JR, Keep RF, Hua Y, Richardson RJ, Schallert T, Xi G. Thrombin preconditioning provides protection in a 6-hydroxydopamine Parkinson's disease model. Neurosci Lett. 2005;373:189–194. doi: 10.1016/j.neulet.2004.10.089. [DOI] [PubMed] [Google Scholar]
- Cardenas A, Moro MA, Leza JC, O'Shea E, Davalos A, Castillo J, Lorenzo P, Lizasoain I. Upregulation of TACE/ADAM17 after ischemic preconditioning is involved in brain tolerance. J Cereb Blood Flow Metab. 2002;22:1297–1302. doi: 10.1097/01.WCB.0000033968.83623.D0. [DOI] [PubMed] [Google Scholar]
- Carreno-Muller E, Herrera AJ, de Pablos RM, Tomas-Camardiel M, Venero JL, Cano J, Machado A. Thrombin induces in vivo degeneration of nigral dopaminergic neurones along with the activation of microglia. J Neurochem. 2003;84:1201–1214. doi: 10.1046/j.1471-4159.2003.01634.x. [DOI] [PubMed] [Google Scholar]
- Carvey PM, Chang Q, Lipton JW, Ling Z. Prenatal exposure to the bacteriotoxin lipopolysaccharide leads to long-term losses of dopamine neurons in offspring: a potential, new model of Parkinson's disease. Front Biosci. 2003;8:s826–837. doi: 10.2741/1158. [DOI] [PubMed] [Google Scholar]
- Caso JR, Pradillo JM, Hurtado O, Lorenzo P, Moro MA, Lizasoain I. Toll-like receptor 4 is involved in brain damage and inflammation after experimental stroke. Circulation. 2007;115:1599–1608. doi: 10.1161/CIRCULATIONAHA.106.603431. [DOI] [PubMed] [Google Scholar]
- Cebere A, Liljequist S. Ethanol differentially inhibits homoquinolinic acid- and NMDA-induced neurotoxicity in primary cultures of cerebellar granule cells. Neurochem Res. 2003;28:1193–1199. doi: 10.1023/a:1024228412198. [DOI] [PubMed] [Google Scholar]
- Cernak I. Animal models of head trauma. NeuroRx. 2005;2:410–422. doi: 10.1602/neurorx.2.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira FM, Cunha FM, Laurindo FR, Kowaltowski AJ. Calorie restriction increases cerebral mitochondrial respiratory capacity in a NO*-mediated mechanism: impact on neuronal survival. Free Radic Biol Med. 2012;52:1236–1241. doi: 10.1016/j.freeradbiomed.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Chan MT, Boet R, Ng SC, Poon WS, Gin T. Effect of ischemic preconditioning on brain tissue gases and pH during temporary cerebral artery occlusion. Acta Neurochir Suppl. 2005;95:93–96. doi: 10.1007/3-211-32318-x_20. [DOI] [PubMed] [Google Scholar]
- Chandler LJ, Sumners C, Crews FT. Ethanol inhibits NMDA receptor-mediated excitotoxicity in rat primary neuronal cultures. Alcohol Clin Exp Res. 1993;17:54–60. doi: 10.1111/j.1530-0277.1993.tb00726.x. [DOI] [PubMed] [Google Scholar]
- Chang CP, Chio CC, Cheong CU, Chao CM, Cheng BC, Lin MT. Hypoxic preconditioning enhances the therapeutic potential of the secretome from cultured human mesenchymal stem cells in experimental traumatic brain injury. Clin Sci (Lond) 2013;124:165–176. doi: 10.1042/CS20120226. [DOI] [PubMed] [Google Scholar]
- Chen H, Zhang SM, Schwarzschild MA, Hernan MA, Ascherio A. Physical activity and the risk of Parkinson disease. Neurology. 2005;64:664–669. doi: 10.1212/01.WNL.0000151960.28687.93. [DOI] [PubMed] [Google Scholar]
- Chen T, Kato H, Liu XH, Araki T, Itoyama Y, Kogure K. Ischemic tolerance can be induced repeatedly in the gerbil hippocampal neurons. Neurosci Lett. 1994;177:159–161. doi: 10.1016/0304-3940(94)90891-5. [DOI] [PubMed] [Google Scholar]
- Chen Y, Nie H, Tian L, Tong L, Yang L, Lao N, Dong H, Sang H, Xiong L. Nicotine-induced neuroprotection against ischemic injury involves activation of endocannabinoid system in rats. Neurochem Res. 2013;38:364–370. doi: 10.1007/s11064-012-0927-6. [DOI] [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DY, Liu M, Hunter RL, Cass WA, Pandya JD, Sullivan PG, Shin EJ, Kim HC, Gash DM, Bing G. Striatal neuroinflammation promotes Parkinsonism in rats. PLoS One. 2009;4:e5482. doi: 10.1371/journal.pone.0005482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SH, Joe EH, Kim SU, Jin BK. Thrombin-induced microglial activation produces degeneration of nigral dopaminergic neurons in vivo. J Neurosci. 2003;23:5877–5886. doi: 10.1523/JNEUROSCI.23-13-05877.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang TJ, Lin KC, Chio CC, Wang CC, Chang CP, Kuo JR. Effects of secretome obtained from normoxia-preconditioned human mesenchymal stem cells in traumatic brain injury rats. J Trauma Acute Care Surg. 2012;73:1161–1167. doi: 10.1097/TA.0b013e318265d128. [DOI] [PubMed] [Google Scholar]
- Codaccioni JL, Velly LJ, Moubarik C, Bruder NJ, Pisano PS, Guillet BA. Sevoflurane preconditioning against focal cerebral ischemia: inhibition of apoptosis in the face of transient improvement of neurological outcome. Anesthesiology. 2009;110:1271–1278. doi: 10.1097/ALN.0b013e3181a1fe68. [DOI] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Mukamal KJ, Gray MO, Parks DA, Das DK, Korthuis RJ. Alcohol in moderation, cardioprotection, and neuroprotection: epidemiological considerations and mechanistic studies. Alcohol Clin Exp Res. 2009;33:206–219. doi: 10.1111/j.1530-0277.2008.00828.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Wang K, Achille NJ, Mitchell RM, Sivaswamy S. Moderate ethanol preconditioning of rat brain cultures engenders neuroprotection against dementia-inducing neuroinflammatory proteins: possible signaling mechanisms. Mol Neurobiol. 2010;41:420–425. doi: 10.1007/s12035-010-8138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins MA, Neafsey EJ, Zou JY. HIV-I gpI20 neurotoxicity in brain cultures is prevented by moderate ethanol pretreatment. Neuroreport. 2000;11:1219–1222. doi: 10.1097/00001756-200004270-00015. [DOI] [PubMed] [Google Scholar]
- Corbett D, Giles T, Evans S, McLean J, Biernaskie J. Dynamic changes in CA1 dendritic spines associated with ischemic tolerance. Exp Neurol. 2006;202:133–138. doi: 10.1016/j.expneurol.2006.05.020. [DOI] [PubMed] [Google Scholar]
- Costa T, Constantino LC, Mendonca BP, Pereira JG, Herculano B, Tasca CI, Boeck CR. N-methyl-D-aspartate preconditioning improves short-term motor deficits outcome after mild traumatic brain injury in mice. J Neurosci Res. 2010;88:1329–1337. doi: 10.1002/jnr.22300. [DOI] [PubMed] [Google Scholar]
- Coyle P, Panzenbeck MJ. Collateral development after carotid artery occlusion in Fischer 344 rats. Stroke. 1990;21:316–321. doi: 10.1161/01.str.21.2.316. [DOI] [PubMed] [Google Scholar]
- Danaceau JP, Deering CE, Day JE, Smeal SJ, Johnson-Davis KL, Fleckenstein AE, Wilkins DG. Persistence of tolerance to methamphetamine-induced monoamine deficits. Eur J Pharmacol. 2007;559:46–54. doi: 10.1016/j.ejphar.2006.11.045. [DOI] [PubMed] [Google Scholar]
- Dave KR, Saul I, Prado R, Busto R, Perez-Pinzon MA. Remote organ ischemic preconditioning protect brain from ischemic damage following asphyxial cardiac arrest. Neurosci Lett. 2006;404:170–175. doi: 10.1016/j.neulet.2006.05.037. [DOI] [PubMed] [Google Scholar]
- Davis AE, Campbell SJ, Wilainam P, Anthony DC. Post-conditioning with lipopolysaccharide reduces the inflammatory infiltrate to the injured brain and spinal cord: a potential neuroprotective treatment. The European journal of neuroscience. 2005;22:2441–2450. doi: 10.1111/j.1460-9568.2005.04447.x. [DOI] [PubMed] [Google Scholar]
- Dawson DA, Furuya K, Gotoh J, Nakao Y, Hallenbeck JM. Cerebrovascular hemodynamics and ischemic tolerance: lipopolysaccharide-induced resistance to focal cerebral ischemia is not due to changes in severity of the initial ischemic insult, but is associated with preservation of microvascular perfusion. J Cereb Blood Flow Metab. 1999;19:616–623. doi: 10.1097/00004647-199906000-00004. [DOI] [PubMed] [Google Scholar]
- De Ley G, Nshimyumuremyi JB, Leusen I. Hemispheric blood flow in the rat after unilateral common carotid occlusion: evolution with time. Stroke. 1985;16:69–73. doi: 10.1161/01.str.16.1.69. [DOI] [PubMed] [Google Scholar]
- Della Morte D, Abete P, Gallucci F, Scaglione A, D'Ambrosio D, Gargiulo G, De Rosa G, Dave KR, Lin HW, Cacciatore F, Mazzella F, Uomo G, Rundek T, Perez-Pinzon MA, Rengo F. Transient ischemic attack before nonlacunar ischemic stroke in the elderly. J Stroke Cerebrovasc Dis. 2008;17:257–262. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Meo S, Venditti P. Mitochondria in exercise-induced oxidative stress. Biol Signals Recept. 2001;10:125–140. doi: 10.1159/000046880. [DOI] [PubMed] [Google Scholar]
- Ding Y, Li L. Lipopolysaccharide preconditioning induces protection against lipopolysaccharide-induced neurotoxicity in organotypic midbrain slice culture. Neurosci Bull. 2008;24:209–218. doi: 10.1007/s12264-008-0408-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding YH, Li J, Yao WX, Rafols JA, Clark JC, Ding Y. Exercise preconditioning upregulates cerebral integrins and enhances cerebrovascular integrity in ischemic rats. Acta neuropathologica. 2006;112:74–84. doi: 10.1007/s00401-006-0076-6. [DOI] [PubMed] [Google Scholar]
- Ding YH, Young CN, Luan X, Li J, Rafols JA, Clark JC, McAllister JP, 2nd, Ding Y. Exercise preconditioning ameliorates inflammatory injury in ischemic rats during reperfusion. Acta neuropathologica. 2005;109:237–246. doi: 10.1007/s00401-004-0943-y. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Iadecola C, Moskowitz MA. Pathobiology of ischaemic stroke: an integrated view. Trends Neurosci. 1999;22:391–397. doi: 10.1016/s0166-2236(99)01401-0. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Simon RP, Hallenbeck JM. Ischemic tolerance and endogenous neuroprotection. Trends Neurosci. 2003;26:248–254. doi: 10.1016/S0166-2236(03)00071-7. [DOI] [PubMed] [Google Scholar]
- Duan W, Lee J, Guo Z, Mattson MP. Dietary restriction stimulates BDNF production in the brain and thereby protects neurons against excitotoxic injury. J Mol Neurosci. 2001;16:1–12. doi: 10.1385/JMN:16:1:1. [DOI] [PubMed] [Google Scholar]
- Duan W, Mattson MP. Dietary restriction and 2-deoxyglucose administration improve behavioral outcome and reduce degeneration of dopaminergic neurons in models of Parkinson's disease. J Neurosci Res. 1999;57:195–206. doi: 10.1002/(SICI)1097-4547(19990715)57:2<195::AID-JNR5>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Duan W, Ross CA. Potential therapeutic targets for neurodegenerative diseases: lessons learned from calorie restriction. Curr Drug Targets. 2010;11:1281–1292. doi: 10.2174/1389450111007011281. [DOI] [PubMed] [Google Scholar]
- Ducroquet A, Leys D, Saabi AA, Richard F, Cordonnier C, Girot M, Deplanque D, Casolla B, Allorge D, Bordet R. Influence of chronic ethanol consumption on the neurological severity in patients with acute cerebral ischemia. Stroke. 2013;44:2324–2326. doi: 10.1161/STROKEAHA.113.001355. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Feuers R, Nakamura KD, Leakey J, Hart RW. Effect of chronic caloric restriction on the synchronization of various physiological measures in old female Fischer 344 rats. Chronobiol Int. 1990;7:113–124. doi: 10.3109/07420529009056963. [DOI] [PubMed] [Google Scholar]
- Duffy PH, Feuers RJ, Leakey JA, Nakamura K, Turturro A, Hart RW. Effect of chronic caloric restriction on physiological variables related to energy metabolism in the male Fischer 344 rat. Mech Ageing Dev. 1989;48:117–133. doi: 10.1016/0047-6374(89)90044-4. [DOI] [PubMed] [Google Scholar]
- Durukan A, Tatlisumak T. Preconditioning-induced ischemic tolerance: a window into endogenous gearing for cerebroprotection. Exp Transl Stroke Med. 2010;2:2. doi: 10.1186/2040-7378-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklind S, Mallard C, Arvidsson P, Hagberg H. Lipopolysaccharide induces both a primary and a secondary phase of sensitization in the developing rat brain. Pediatr Res. 2005;58:112–116. doi: 10.1203/01.PDR.0000163513.03619.8D. [DOI] [PubMed] [Google Scholar]
- El Amki M, Lerouet D, Coqueran B, Curis E, Orset C, Vivien D, Plotkine M, Marchand-Leroux C, Margaill I. Experimental modeling of recombinant tissue plasminogen activator effects after ischemic stroke. Exp Neurol. 2012;238:138–144. doi: 10.1016/j.expneurol.2012.08.005. [DOI] [PubMed] [Google Scholar]
- El Ayadi A, Zigmond MJ. Low Concentrations of Methamphetamine Can Protect Dopaminergic Cells against a Larger Oxidative Stress Injury: Mechanistic Study. PLoS One. 2011;6:e24722. doi: 10.1371/journal.pone.0024722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faherty CJ, Raviie Shepherd K, Herasimtschuk A, Smeyne RJ. Environmental enrichment in adulthood eliminates neuronal death in experimental Parkinsonism. Brain Res Mol Brain Res. 2005;134:170–179. doi: 10.1016/j.molbrainres.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Fan GH, Qi C, Chen SD. Heat shock proteins reduce toxicity of 1-methyl-4-phenylpyridinium ion in SK-N-SH cells. J Neurosci Res. 2005;82:551–562. doi: 10.1002/jnr.20656. [DOI] [PubMed] [Google Scholar]
- Fan GH, Zhou HY, Yang H, Chen SD. Heat shock proteins reduce alpha-synuclein aggregation induced by MPP+ in SK-N-SH cells. FEBS Lett. 2006;580:3091–3098. doi: 10.1016/j.febslet.2006.04.057. [DOI] [PubMed] [Google Scholar]
- Fan YY, Hu WW, Dai HB, Zhang JX, Zhang LY, He P, Shen Y, Ohtsu H, Wei EQ, Chen Z. Activation of the central histaminergic system is involved in hypoxia-induced stroke tolerance in adult mice. J Cereb Blood Flow Metab. 2011;31:305–314. doi: 10.1038/jcbfm.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freiberger JJ, Suliman HB, Sheng H, McAdoo J, Piantadosi CA, Warner DS. A comparison of hyperbaric oxygen versus hypoxic cerebral preconditioning in neonatal rats. Brain Res. 2006;1075:213–222. doi: 10.1016/j.brainres.2005.12.088. [DOI] [PubMed] [Google Scholar]
- Furuya K, Zhu L, Kawahara N, Abe O, Kirino T. Differences in infarct evolution between lipopolysaccharide-induced tolerant and nontolerant conditions to focal cerebral ischemia. J Neurosurg. 2005;103:715–723. doi: 10.3171/jns.2005.103.4.0715. [DOI] [PubMed] [Google Scholar]
- Gage AT, Stanton PK. Hypoxia triggers neuroprotective alterations in hippocampal gene expression via a heme-containing sensor. Brain Res. 1996;719:172–178. doi: 10.1016/0006-8993(96)00092-3. [DOI] [PubMed] [Google Scholar]
- Gao CJ, Niu L, Ren PC, Wang W, Zhu C, Li YQ, Chai W, Sun XD. Hypoxic preconditioning attenuates global cerebral ischemic injury following asphyxial cardiac arrest through regulation of delta opioid receptor system. Neuroscience. 2012;202:352–362. doi: 10.1016/j.neuroscience.2011.11.060. [DOI] [PubMed] [Google Scholar]
- Gao X, Ren C, Zhao H. Protective effects of ischemic postconditioning compared with gradual reperfusion or preconditioning. J Neurosci Res. 2008;86:2505–2511. doi: 10.1002/jnr.21703. [DOI] [PubMed] [Google Scholar]
- Geng X, Ren C, Wang T, Fu P, Luo Y, Liu X, Yan F, Ling F, Jia J, Du H, Ji X, Ding Y. Effect of remote ischemic postconditioning on an intracerebral hemorrhage stroke model in rats. Neurol Res. 2012;34:143–148. doi: 10.1179/1743132811Y.0000000073. [DOI] [PubMed] [Google Scholar]
- Gerecke KM, Jiao Y, Pani A, Pagala V, Smeyne RJ. Exercise protects against MPTP-induced neurotoxicity in mice. Brain Res. 2010;1341:72–83. doi: 10.1016/j.brainres.2010.01.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gidday JM, Fitzgibbons JC, Shah AR, Park TS. Neuroprotection from ischemic brain injury by hypoxic preconditioning in the neonatal rat. Neurosci Lett. 1994;168:221–224. doi: 10.1016/0304-3940(94)90455-3. [DOI] [PubMed] [Google Scholar]
- Gill MB, Perez-Polo JR. Hypoxia ischemia-mediated cell death in neonatal rat brain. Neurochem Res. 2008;33:2379–2389. doi: 10.1007/s11064-008-9649-1. [DOI] [PubMed] [Google Scholar]
- Giorguieff-Chesselet MF, Kemel ML, Wandscheer D, Glowinski J. Regulation of dopamine release by presynaptic nicotinic receptors in rat striatal slices: effect of nicotine in a low concentration. Life Sci. 1979;25:1257–1262. doi: 10.1016/0024-3205(79)90469-7. [DOI] [PubMed] [Google Scholar]
- Gniel HM, Martin RL. Cortical spreading depression-induced preconditioning in mouse neocortex is lamina specific. J Neurophysiol. 2013;109:2923–2936. doi: 10.1152/jn.00855.2011. [DOI] [PubMed] [Google Scholar]
- Gobbi LT, Oliveira-Ferreira MD, Caetano MJ, Lirani-Silva E, Barbieri FA, Stella F, Gobbi S. Exercise programs improve mobility and balance in people with Parkinson's disease. Parkinsonism Relat Disord. 2009;15(Suppl 3):S49–52. doi: 10.1016/S1353-8020(09)70780-1. [DOI] [PubMed] [Google Scholar]
- Gorman AM, Szegezdi E, Quigney DJ, Samali A. Hsp27 inhibits 6-hydroxydopamine-induced cytochrome c release and apoptosis in PC12 cells. Biochemical and biophysical research communications. 2005;327:801–810. doi: 10.1016/j.bbrc.2004.12.066. [DOI] [PubMed] [Google Scholar]
- Grabb MC, Choi DW. Ischemic tolerance in murine cortical cell culture: critical role for NMDA receptors. J Neurosci. 1999;19:1657–1662. doi: 10.1523/JNEUROSCI.19-05-01657.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham DI, Adams JH, Murray LS, Jennett B. Neuropathology of the vegetative state after head injury. Neuropsychol Rehabil. 2005;15:198–213. doi: 10.1080/09602010443000452. [DOI] [PubMed] [Google Scholar]
- Graham DL, Noailles PA, Cadet JL. Differential neurochemical consequences of an escalating dose-binge regimen followed by single-day multiple-dose methamphetamine challenges. J Neurochem. 2008;105:1873–1885. doi: 10.1111/j.1471-4159.2008.05269.x. [DOI] [PubMed] [Google Scholar]
- Granziera C, Thevenet J, Price M, Wiegler K, Magistretti PJ, Badaut J, Hirt L. Thrombin-induced ischemic tolerance is prevented by inhibiting c-jun N-terminal kinase. Brain Res. 2007;1148:217–225. doi: 10.1016/j.brainres.2007.02.025. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Pharmacology of nicotine. Br J Addict. 1989;84:477–492. doi: 10.1111/j.1360-0443.1989.tb00604.x. [DOI] [PubMed] [Google Scholar]
- Gustavsson M, Anderson MF, Mallard C, Hagberg H. Hypoxic preconditioning confers long-term reduction of brain injury and improvement of neurological ability in immature rats. Pediatr Res. 2005;57:305–309. doi: 10.1203/01.PDR.0000151122.58665.70. [DOI] [PubMed] [Google Scholar]
- Halagappa VK, Guo Z, Pearson M, Matsuoka Y, Cutler RG, Laferla FM, Mattson MP. Intermittent fasting and caloric restriction ameliorate age-related behavioral deficits in the triple-transgenic mouse model of Alzheimer's disease. Neurobiol Dis. 2007;26:212–220. doi: 10.1016/j.nbd.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Hands SL, Proud CG, Wyttenbach A. mTOR's role in ageing: protein synthesis or autophagy? Aging (Albany NY) 2009;1:586–597. doi: 10.18632/aging.100070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara H, Kamiya T, Adachi T. Endoplasmic reticulum stress inducers provide protection against 6-hydroxydopamine-induced cytotoxicity. Neurochem Int. 2011;58:35–43. doi: 10.1016/j.neuint.2010.10.006. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. Preconditioning and postconditioning: underlying mechanisms and clinical application. Atherosclerosis. 2009;204:334–341. doi: 10.1016/j.atherosclerosis.2008.10.029. [DOI] [PubMed] [Google Scholar]
- Hausenloy DJ, Yellon DM. The therapeutic potential of ischemic conditioning: an update. Nat Rev Cardiol. 2011;8:619–629. doi: 10.1038/nrcardio.2011.85. [DOI] [PubMed] [Google Scholar]
- He Z, Crook JE, Meschia JF, Brott TG, Dickson DW, McKinney M. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2005;2:365–374. doi: 10.2174/156720205774962674. [DOI] [PubMed] [Google Scholar]
- Head BP, Patel HH, Tsutsumi YM, Hu Y, Mejia T, Mora RC, Insel PA, Roth DM, Drummond JC, Patel PM. Caveolin-1 expression is essential for N-methyl-D-aspartate receptor-mediated Src and extracellular signal-regulated kinase 1/2 activation and protection of primary neurons from ischemic cell death. FASEB J. 2008;22:828–840. doi: 10.1096/fj.07-9299com. [DOI] [PubMed] [Google Scholar]
- Heyer EJ, Sharma R, Rampersad A, Winfree CJ, Mack WJ, Solomon RA, Todd GJ, McCormick PC, McMurtry JG, Quest DO, Stern Y, Lazar RM, Connolly ES. A controlled prospective study of neuropsychological dysfunction following carotid endarterectomy. Arch Neurol. 2002;59:217–222. doi: 10.1001/archneur.59.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickey EJ, You X, Kaimaktchiev V, Stenzel-Poore M, Ungerleider RM. Lipopolysaccharide preconditioning induces robust protection against brain injury resulting from deep hypothermic circulatory arrest. J Thorac Cardiovasc Surg. 2007;133:1588–1596. doi: 10.1016/j.jtcvs.2006.12.056. [DOI] [PubMed] [Google Scholar]
- Holmer HK, Keyghobadi M, Moore C, Menashe RA, Meshul CK. Dietary restriction affects striatal glutamate in the MPTP-induced mouse model of nigrostriatal degeneration. Synapse. 2005;57:100–112. doi: 10.1002/syn.20163. [DOI] [PubMed] [Google Scholar]
- Honjoh S, Nishida E. Two sides of lifespan regulating genes: pro-longevity or anti-longevity? J Biochem. 2011;149:381–388. doi: 10.1093/jb/mvr026. [DOI] [PubMed] [Google Scholar]
- Horiguchi T, Kis B, Rajapakse N, Shimizu K, Busija DW. Opening of mitochondrial ATP-sensitive potassium channels is a trigger of 3-nitropropionic acid-induced tolerance to transient focal cerebral ischemia in rats. Stroke. 2003;34:1015–1020. doi: 10.1161/01.STR.0000063404.27912.5B. [DOI] [PubMed] [Google Scholar]
- Hoshi A, Nakahara T, Kayama H, Yamamoto T. Ischemic tolerance in chemical preconditioning: possible role of astrocytic glutamine synthetase buffering glutamate-mediated neurotoxicity. J Neurosci Res. 2006;84:130–141. doi: 10.1002/jnr.20869. [DOI] [PubMed] [Google Scholar]
- Hoshi A, Nakahara T, Ogata M, Yamamoto T. The critical threshold of 3-nitropropionic acid-induced ischemic tolerance in the rat. Brain Res. 2005;1050:33–39. doi: 10.1016/j.brainres.2005.05.028. [DOI] [PubMed] [Google Scholar]
- Hu S, Dong H, Zhang H, Wang S, Hou L, Chen S, Zhang J, Xiong L. Noninvasive limb remote ischemic preconditioning contributes neuroprotective effects via activation of adenosine A1 receptor and redox status after transient focal cerebral ischemia in rats. Brain Res. 2012;1459:81–90. doi: 10.1016/j.brainres.2012.04.017. [DOI] [PubMed] [Google Scholar]
- Hu S, Dong HL, Li YZ, Luo ZJ, Sun L, Yang QZ, Yang LF, Xiong L. Effects of remote ischemic preconditioning on biochemical markers and neurologic outcomes in patients undergoing elective cervical decompression surgery: a prospective randomized controlled trial. J Neurosurg Anesthesiol. 2010a;22:46–52. doi: 10.1097/ANA.0b013e3181c572bd. [DOI] [PubMed] [Google Scholar]
- Hu S, Li F, Luo H, Xia Y, Zhang J, Hu R, Cui G, Meng H, Feng H. Amelioration of rCBF and PbtO2 following TBI at high altitude by hyperbaric oxygen pre-conditioning. Neurol Res. 2010b;32:173–178. doi: 10.1179/174313209X414524. [DOI] [PubMed] [Google Scholar]
- Hu SL, Hu R, Li F, Liu Z, Xia YZ, Cui GY, Feng H. Hyperbaric oxygen preconditioning protects against traumatic brain injury at high altitude. Acta Neurochir Suppl. 2008;105:191–196. doi: 10.1007/978-3-211-09469-3_37. [DOI] [PubMed] [Google Scholar]
- Hu X, Zheng H, Yan T, Pan S, Fang J, Jiang R, Ma S. Physical exercise induces expression of CD31 and facilitates neural function recovery in rats with focal cerebral infarction. Neurol Res. 2010c;32:397–402. doi: 10.1179/016164110X12670144526309. [DOI] [PubMed] [Google Scholar]
- Hua F, Ma J, Ha T, Kelley J, Williams DL, Kao RL, Kalbfleisch JH, Browder IW, Li C. Preconditioning with a TLR2 specific ligand increases resistance to cerebral ischemia/reperfusion injury. Journal of neuroimmunology. 2008;199:75–82. doi: 10.1016/j.jneuroim.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua Y, Wu J, Pecina S, Yang S, Schallert T, Keep RF, Xi G. Ischemic preconditioning procedure induces behavioral deficits in the absence of brain injury? Neurol Res. 2005;27:261–267. doi: 10.1179/016164105X25270. [DOI] [PubMed] [Google Scholar]
- Hunter RL, Cheng B, Choi DY, Liu M, Liu S, Cass WA, Bing G. Intrastriatal lipopolysaccharide injection induces parkinsonism in C57/B6 mice. J Neurosci Res. 2009;87:1913–1921. doi: 10.1002/jnr.22012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter RL, Dragicevic N, Seifert K, Choi DY, Liu M, Kim HC, Cass WA, Sullivan PG, Bing G. Inflammation induces mitochondrial dysfunction and dopaminergic neurodegeneration in the nigrostriatal system. J Neurochem. 2007;100:1375–1386. doi: 10.1111/j.1471-4159.2006.04327.x. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Weindruch R, Spangler EL, Freeman JR, Walford RL. Dietary restriction benefits learning and motor performance of aged mice. J Gerontol. 1987;42:78–81. doi: 10.1093/geronj/42.1.78. [DOI] [PubMed] [Google Scholar]
- Ingram DK, Young J, Mattison JA. Calorie restriction in nonhuman primates: assessing effects on brain and behavioral aging. Neuroscience. 2007;145:1359–1364. doi: 10.1016/j.neuroscience.2006.10.031. [DOI] [PubMed] [Google Scholar]
- Jackson-Lewis V, Blesa J, Przedborski S. Animal models of Parkinson's disease. Parkinsonism Relat Disord. 2012;18(Suppl 1):S183–185. doi: 10.1016/S1353-8020(11)70057-8. [DOI] [PubMed] [Google Scholar]
- Jadhav V, Ostrowski RP, Tong W, Matus B, Chang C, Zhang JH. Hyperbaric oxygen preconditioning reduces postoperative brain edema and improves neurological outcomes after surgical brain injury. Acta Neurochir Suppl. 2010;106:217–220. doi: 10.1007/978-3-211-98811-4_40. [DOI] [PubMed] [Google Scholar]
- Jadhav V, Ostrowski RP, Tong W, Matus B, Jesunathadas R, Zhang JH. Cyclo-oxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke. 2009;40:3139–3142. doi: 10.1161/STROKEAHA.109.549774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav V, Solaroglu I, Obenaus A, Zhang JH. Neuroprotection against surgically induced brain injury. Surg Neurol. 2007;67:15–20. doi: 10.1016/j.surneu.2006.07.014. discussion 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav V, Zhang JH. Surgical brain injury: prevention is better than cure. Front Biosci. 2008;13:3793–3797. doi: 10.2741/2968. [DOI] [PubMed] [Google Scholar]
- Jadiya P, Chatterjee M, Sammi SR, Kaur S, Palit G, Nazir A. Sir-2.1 modulates 'calorie-restriction-mediated' prevention of neurodegeneration in Caenorhabditis elegans: implications for Parkinson's disease. Biochemical and biophysical research communications. 2011;413:306–310. doi: 10.1016/j.bbrc.2011.08.092. [DOI] [PubMed] [Google Scholar]
- Jensen HA, Loukogeorgakis S, Yannopoulos F, Rimpilainen E, Petzold A, Tuominen H, Lepola P, Macallister RJ, Deanfield JE, Makela T, Alestalo K, Kiviluoma K, Anttila V, Tsang V, Juvonen T. Remote ischemic preconditioning protects the brain against injury after hypothermic circulatory arrest. Circulation. 2011;123:714–721. doi: 10.1161/CIRCULATIONAHA.110.986497. [DOI] [PubMed] [Google Scholar]
- Jeon YT, Hwang JW, Lim YJ, Kim AN, Park HP. A combination of sevoflurane postconditioning and albumin increases Bcl-2 expression after transient global cerebral ischemia compared with either sevoflurane postconditioning or albumin alone. J Neurosurg Anesthesiol. 2013;25:43–50. doi: 10.1097/ANA.0b013e31826ca3bc. [DOI] [PubMed] [Google Scholar]
- Jia J, Hu YS, Wu Y, Liu G, Yu HX, Zheng QP, Zhu DN, Xia CM, Cao ZJ. Pre-ischemic treadmill training affects glutamate and gamma aminobutyric acid levels in the striatal dialysate of a rat model of cerebral ischemia. Life Sci. 2009;84:505–511. doi: 10.1016/j.lfs.2009.01.015. [DOI] [PubMed] [Google Scholar]
- Jia K, Chen D, Riddle DL. The TOR pathway interacts with the insulin signaling pathway to regulate C. elegans larval development, metabolism and life span. Development. 2004;131:3897–3906. doi: 10.1242/dev.01255. [DOI] [PubMed] [Google Scholar]
- Jiang X, Tian F, Mearow K, Okagaki P, Lipsky RH, Marini AM. The excitoprotective effect of N-methyl-D-aspartate receptors is mediated by a brain-derived neurotrophic factor autocrine loop in cultured hippocampal neurons. J Neurochem. 2005;94:713–722. doi: 10.1111/j.1471-4159.2005.03200.x. [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Fleckenstein AE, Wilkins DG. The role of hyperthermia and metabolism as mechanisms of tolerance to methamphetamine neurotoxicity. Eur J Pharmacol. 2003;482:151–154. doi: 10.1016/j.ejphar.2003.09.063. [DOI] [PubMed] [Google Scholar]
- Johnson-Davis KL, Truong JG, Fleckenstein AE, Wilkins DG. Alterations in vesicular dopamine uptake contribute to tolerance to the neurotoxic effects of methamphetamine. J Pharmacol Exp Ther. 2004;309:578–586. doi: 10.1124/jpet.103.062695. [DOI] [PubMed] [Google Scholar]
- Johnston SC. Ischemic preconditioning from transient ischemic attacks? Data from the Northern California TIA Study. Stroke. 2004;35:2680–2682. doi: 10.1161/01.STR.0000143322.20491.0f. [DOI] [PubMed] [Google Scholar]
- Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287–1295. doi: 10.1016/j.febslet.2010.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM. Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol. 1997;42:776–782. doi: 10.1002/ana.410420514. [DOI] [PubMed] [Google Scholar]
- Kawahara N, Ruetzler CA, Klatzo I. Protective effect of spreading depression against neuronal damage following cardiac arrest cerebral ischaemia. Neurol Res. 1995;17:9–16. doi: 10.1080/01616412.1995.11740281. [DOI] [PubMed] [Google Scholar]
- Keep RF, Wang MM, Xiang J, Hua Y, Xi G. Is There A Place For Cerebral Preconditioning In The Clinic? Transl Stroke Res. 2010;1:4–18. doi: 10.1007/s12975-009-0007-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenyon C. The first long-lived mutants: discovery of the insulin/IGF-1 pathway for ageing. Philos Trans R Soc Lond B Biol Sci. 2011;366:9–16. doi: 10.1098/rstb.2010.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kersten JR, Schmeling TJ, Pagel PS, Gross GJ, Warltier DC. Isoflurane mimics ischemic preconditioning via activation of K(ATP) channels: reduction of myocardial infarct size with an acute memory phase. Anesthesiology. 1997;87:361–370. doi: 10.1097/00000542-199708000-00024. [DOI] [PubMed] [Google Scholar]
- Kim HJ, Lee JH, Kim SH. Therapeutic effects of human mesenchymal stem cells on traumatic brain injury in rats: secretion of neurotrophic factors and inhibition of apoptosis. J Neurotrauma. 2010;27:131–138. doi: 10.1089/neu.2008.0818. [DOI] [PubMed] [Google Scholar]
- Kim HW, Haider HK, Jiang S, Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009a;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SC, Stice JP, Chen L, Jung JS, Gupta S, Wang Y, Baumgarten G, Trial J, Knowlton AA. Extracellular heat shock protein 60, cardiac myocytes, and apoptosis. Circ Res. 2009b;105:1186–1195. doi: 10.1161/CIRCRESAHA.109.209643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim EH, Lee BI, Heo JH. Chronic cerebral hypoperfusion protects against acute focal ischemia, improves motor function, and results in vascular remodeling. Curr Neurovasc Res. 2008;5:28–36. doi: 10.2174/156720208783565627. [DOI] [PubMed] [Google Scholar]
- Kim YW, Ogilvy CS, Pricola K, Reavey-Cantwell JF, Kelman CR, Zipfel GJ, Washington C, Albuquerque F, Kalani MYS, Hoh BL. Evidence for an ischemic preconditioning effect on cerebral vasospasm in patients with aneurysmal subarachnoid hemorrhage. Congress of Neurological Surgeons; Chicago, IL. 2012. p. E553. [DOI] [PubMed] [Google Scholar]
- Kirino T, Tsujita Y, Tamura A. Induced tolerance to ischemia in gerbil hippocampal neurons. J Cereb Blood Flow Metab. 1991;11:299–307. doi: 10.1038/jcbfm.1991.62. [DOI] [PubMed] [Google Scholar]
- Kis B, Nagy K, Snipes JA, Rajapakse NC, Horiguchi T, Grover GJ, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 induces neuronal preconditioning. Neuroreport. 2004;15:345–349. doi: 10.1097/00001756-200402090-00027. [DOI] [PubMed] [Google Scholar]
- Kis B, Rajapakse NC, Snipes JA, Nagy K, Horiguchi T, Busija DW. Diazoxide induces delayed pre-conditioning in cultured rat cortical neurons. J Neurochem. 2003;87:969–980. doi: 10.1046/j.1471-4159.2003.02072.x. [DOI] [PubMed] [Google Scholar]
- Kita T, Wagner GC, Nakashima T. Current research on methamphetamine-induced neurotoxicity: animal models of monoamine disruption. J Pharmacol Sci. 2003;92:178–195. doi: 10.1254/jphs.92.178. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. 'Ischemic tolerance' phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tagaya M, Hata R, Ueda H, Niinobe M, Handa N, Fukunaga R, Kimura K, Mikoshiba K, et al. 'Ischemic tolerance' phenomenon found in the brain. Brain Res. 1990;528:21–24. doi: 10.1016/0006-8993(90)90189-i. [DOI] [PubMed] [Google Scholar]
- Kitano H, Young JM, Cheng J, Wang L, Hurn PD, Murphy SJ. Gender-specific response to isoflurane preconditioning in focal cerebral ischemia. J Cereb Blood Flow Metab. 2007;27:1377–1386. doi: 10.1038/sj.jcbfm.9600444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloner RA, Shook T, Przyklenk K, Davis VG, Junio L, Matthews RV, Burstein S, Gibson M, Poole WK, Cannon CP, et al. Previous angina alters in-hospital outcome in TIMI 4. A clinical correlate to preconditioning? Circulation. 1995;91:37–45. doi: 10.1161/01.cir.91.1.37. [DOI] [PubMed] [Google Scholar]
- Koch S, Katsnelson M, Dong C, Perez-Pinzon M. Remote ischemic limb preconditioning after subarachnoid hemorrhage: a phase Ib study of safety and feasibility. Stroke. 2011;42:1387–1391. doi: 10.1161/STROKEAHA.110.605840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouda K, Iki M. Beneficial effects of mild stress (hormetic effects): dietary restriction and health. J Physiol Anthropol. 2010;29:127–132. doi: 10.2114/jpa2.29.127. [DOI] [PubMed] [Google Scholar]
- Krenz M, Korthuis RJ. Moderate ethanol ingestion and cardiovascular protection: from epidemiologic associations to cellular mechanisms. J Mol Cell Cardiol. 2012;52:93–104. doi: 10.1016/j.yjmcc.2011.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz A, Park L, Abe T, Gallo EF, Anrather J, Zhou P, Iadecola C. Neurovascular protection by ischemic tolerance: role of nitric oxide and reactive oxygen species. J Neurosci. 2007;27:7083–7093. doi: 10.1523/JNEUROSCI.1645-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuroiwa T, Yamada I, Endo S, Hakamata Y, Ito U. 3-Nitropropionic acid preconditioning ameliorates delayed neurological deterioration and infarction after transient focal cerebral ischemia in gerbils. Neurosci Lett. 2000;283:145–148. doi: 10.1016/s0304-3940(00)00937-x. [DOI] [PubMed] [Google Scholar]
- Langdon KD, Maclellan CL, Corbett D. Prolonged, 24-h delayed peripheral inflammation increases short- and long-term functional impairment and histopathological damage after focal ischemia in the rat. J Cereb Blood Flow Metab. 2010;30:1450–1459. doi: 10.1038/jcbfm.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lastres-Becker I, Cartmell T, Molina-Holgado F. Endotoxin preconditioning protects neurones from in vitro ischemia: role of endogenous IL-1beta and TNF-alpha. Journal of neuroimmunology. 2006;173:108–116. doi: 10.1016/j.jneuroim.2005.12.006. [DOI] [PubMed] [Google Scholar]
- Lau YS, Patki G, Das-Panja K, Le WD, Ahmad SO. Neuroprotective effects and mechanisms of exercise in a chronic mouse model of Parkinson's disease with moderate neurodegeneration. European Journal of Neuroscience. 2011;33:1264–1274. doi: 10.1111/j.1460-9568.2011.07626.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leak RK, Liou AK, Zigmond MJ. Effect of sublethal 6-hydroxydopamine on the response to subsequent oxidative stress in dopaminergic cells: evidence for preconditioning. J Neurochem. 2006;99:1151–1163. doi: 10.1111/j.1471-4159.2006.04149.x. [DOI] [PubMed] [Google Scholar]
- Leak RK, Zigmond MJ, Liou AK. Adaptation to chronic MG132 reduces oxidative toxicity by a CuZnSOD-dependent mechanism. J Neurochem. 2008;106:860–874. doi: 10.1111/j.1471-4159.2008.05459.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee HM, Lee DH, Choi JH, Lee SR, Kim YW, Jee DL, Do HS, Park SJ. Sevoflurane-induced post-conditioning has no beneficial effects on neuroprotection after incomplete cerebral ischemia in rats. Acta Anaesthesiol Scand. 2010;54:328–336. doi: 10.1111/j.1399-6576.2009.02129.x. [DOI] [PubMed] [Google Scholar]
- Lee JJ, Li L, Jung HH, Zuo Z. Postconditioning with isoflurane reduced ischemia-induced brain injury in rats. Anesthesiology. 2008;108:1055–1062. doi: 10.1097/ALN.0b013e3181730257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KR, Betz AL, Keep RF, Chenevert TL, Kim S, Hoff JT. Intracerebral infusion of thrombin as a cause of brain edema. J Neurosurg. 1995;83:1045–1050. doi: 10.3171/jns.1995.83.6.1045. [DOI] [PubMed] [Google Scholar]
- Lee KR, Betz AL, Kim S, Keep RF, Hoff JT. The role of the coagulation cascade in brain edema formation after intracerebral hemorrhage. Acta Neurochir (Wien) 1996a;138:396–400. doi: 10.1007/BF01420301. discussion 400–391. [DOI] [PubMed] [Google Scholar]
- Lee KR, Colon GP, Betz AL, Keep RF, Kim S, Hoff JT. Edema from intracerebral hemorrhage: the role of thrombin. J Neurosurg. 1996b;84:91–96. doi: 10.3171/jns.1996.84.1.0091. [DOI] [PubMed] [Google Scholar]
- Lee KR, Drury I, Vitarbo E, Hoff JT. Seizures induced by intracerebral injection of thrombin: a model of intracerebral hemorrhage. J Neurosurg. 1997;87:73–78. doi: 10.3171/jns.1997.87.1.0073. [DOI] [PubMed] [Google Scholar]
- Leger PL, Bonnin P, Nguyen T, Renolleau S, Baud O, Charriaut-Marlangue C. Ischemic postconditioning fails to protect against neonatal cerebral stroke. PLoS One. 2012;7:e49695. doi: 10.1371/journal.pone.0049695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibson CL, Rocca WA, Hanson VA, Cha R, Kokmen E, O'Brien PC, Palumbo PJ. The risk of dementia among persons with diabetes mellitus: a population-based cohort study. Ann N Y Acad Sci. 1997;826:422–427. doi: 10.1111/j.1749-6632.1997.tb48496.x. [DOI] [PubMed] [Google Scholar]
- Lenzser G, Kis B, Bari F, Busija DW. Diazoxide preconditioning attenuates global cerebral ischemia-induced blood-brain barrier permeability. Brain Res. 2005;1051:72–80. doi: 10.1016/j.brainres.2005.05.064. [DOI] [PubMed] [Google Scholar]
- Leung PY, Stevens SL, Packard AE, Lessov NS, Yang T, Conrad VK, van den Dungen NN, Simon RP, Stenzel-Poore MP. Toll-like receptor 7 preconditioning induces robust neuroprotection against stroke by a novel type I interferon-mediated mechanism. Stroke. 2012;43:1383–1389. doi: 10.1161/STROKEAHA.111.641522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Luan X, Clark JC, Rafols JA, Ding Y. Neuroprotection against transient cerebral ischemia by exercise pre-conditioning in rats. Neurol Res. 2004;26:404–408. doi: 10.1179/016164104225016038. [DOI] [PubMed] [Google Scholar]
- Li JS, Zhang W, Kang ZM, Ding SJ, Liu WW, Zhang JH, Guan YT, Sun XJ. Hyperbaric oxygen preconditioning reduces ischemia-reperfusion injury by inhibition of apoptosis via mitochondrial pathway in rat brain. Neuroscience. 2009;159:1309–1315. doi: 10.1016/j.neuroscience.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Li L, Zuo Z. Isoflurane preconditioning improves short-term and long-term neurological outcome after focal brain ischemia in adult rats. Neuroscience. 2009;164:497–506. doi: 10.1016/j.neuroscience.2009.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li QF, Zhu YS, Jiang H. Isoflurane preconditioning activates HIF-1alpha, iNOS and Erk1/2 and protects against oxygen-glucose deprivation neuronal injury. Brain Res. 2008a;1245:26–35. doi: 10.1016/j.brainres.2008.09.069. [DOI] [PubMed] [Google Scholar]
- Li Y, Dong H, Chen M, Liu J, Yang L, Chen S, Xiong L. Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: a prospective, randomized, controlled clinical trial. J Cardiothorac Vasc Anesth. 2011;25:908–916. doi: 10.1053/j.jvca.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Li Y, Xiao D, Dasgupta C, Xiong F, Tong W, Yang S, Zhang L. Perinatal nicotine exposure increases vulnerability of hypoxic-ischemic brain injury in neonatal rats: role of angiotensin II receptors. Stroke. 2012;43:2483–2490. doi: 10.1161/STROKEAHA.112.664698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Liu W, Kang Z, Lv S, Han C, Yun L, Sun X, Zhang JH. Mechanism of hyperbaric oxygen preconditioning in neonatal hypoxia-ischemia rat model. Brain Res. 2008b;1196:151–156. doi: 10.1016/j.brainres.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Liang BT, Gross GJ. Direct preconditioning of cardiac myocytes via opioid receptors and KATP channels. Circ Res. 1999;84:1396–1400. doi: 10.1161/01.res.84.12.1396. [DOI] [PubMed] [Google Scholar]
- Liao SL, Chen WY, Raung SL, Chen CJ. Ethanol attenuates ischemic and hypoxic injury in rat brain and cultured neurons. Neuroreport. 2003;14:2089–2094. doi: 10.1097/00001756-200311140-00016. [DOI] [PubMed] [Google Scholar]
- Liebelt B, Papapetrou P, Ali A, Guo M, Ji X, Peng C, Rogers R, Curry A, Jimenez D, Ding Y. Exercise preconditioning reduces neuronal apoptosis in stroke by up-regulating heat shock protein-70 (heat shock protein-72) and extracellular-signal-regulated-kinase 1/2. Neuroscience. 2010;166:1091–1100. doi: 10.1016/j.neuroscience.2009.12.067. [DOI] [PubMed] [Google Scholar]
- Lim CL, Byrne C, Lee JK. Human thermoregulation and measurement of body temperature in exercise and clinical settings. Ann Acad Med Singapore. 2008;37:347–353. [PubMed] [Google Scholar]
- Lim SY, Yellon DM, Hausenloy DJ. The neural and humoral pathways in remote limb ischemic preconditioning. Basic Res Cardiol. 2010;105:651–655. doi: 10.1007/s00395-010-0099-y. [DOI] [PubMed] [Google Scholar]
- Lin AM, Dung SW, Chen CF, Chen WH, Ho LT. Hypoxic preconditioning prevents cortical infarction by transient focal ischemia-reperfusion. Ann N Y Acad Sci. 2003;993:168–178. doi: 10.1111/j.1749-6632.2003.tb07527.x. discussion 195–166. [DOI] [PubMed] [Google Scholar]
- Lin HY, Huang CC, Chang KF. Lipopolysaccharide preconditioning reduces neuroinflammation against hypoxic ischemia and provides long-term outcome of neuroprotection in neonatal rat. Pediatr Res. 2009a;66:254–259. doi: 10.1203/PDR.0b013e3181b0d336. [DOI] [PubMed] [Google Scholar]
- Lin SJ, Kaeberlein M, Andalis AA, Sturtz LA, Defossez PA, Culotta VC, Fink GR, Guarente L. Calorie restriction extends Saccharomyces cerevisiae lifespan by increasing respiration. Nature. 2002;418:344–348. doi: 10.1038/nature00829. [DOI] [PubMed] [Google Scholar]
- Lin WY, Chang YC, Lee HT, Huang CC. CREB activation in the rapid, intermediate, and delayed ischemic preconditioning against hypoxic-ischemia in neonatal rat. J Neurochem. 2009b;108:847–859. doi: 10.1111/j.1471-4159.2008.05828.x. [DOI] [PubMed] [Google Scholar]
- Ling Z, Chang QA, Tong CW, Leurgans SE, Lipton JW, Carvey PM. Rotenone potentiates dopamine neuron loss in animals exposed to lipopolysaccharide prenatally. Exp Neurol. 2004;190:373–383. doi: 10.1016/j.expneurol.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Ling Z, Gayle DA, Ma SY, Lipton JW, Tong CW, Hong JS, Carvey PM. In utero bacterial endotoxin exposure causes loss of tyrosine hydroxylase neurons in the postnatal rat midbrain. Mov Disord. 2002;17:116–124. doi: 10.1002/mds.10078. [DOI] [PubMed] [Google Scholar]
- Lipsky RH, Xu K, Zhu D, Kelly C, Terhakopian A, Novelli A, Marini AM. Nuclear factor kappaB is a critical determinant in N-methyl-D-aspartate receptor-mediated neuroprotection. J Neurochem. 2001;78:254–264. doi: 10.1046/j.1471-4159.2001.00386.x. [DOI] [PubMed] [Google Scholar]
- Liu C, Weaver J, Liu KJ. Rapid conditioning with oxygen oscillation: neuroprotection by intermittent normobaric hyperoxia after transient focal cerebral ischemia in rats. Stroke. 2012;43:220–226. doi: 10.1161/STROKEAHA.111.625756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M, Alkayed NJ. Hypoxic preconditioning and tolerance via hypoxia inducible factor (HIF) 1alpha-linked induction of P450 2C11 epoxygenase in astrocytes. J Cereb Blood Flow Metab. 2005;25:939–948. doi: 10.1038/sj.jcbfm.9600085. [DOI] [PubMed] [Google Scholar]
- Liu Y, Kato H, Nakata N, Kogure K. Protection of rat hippocampus against ischemic neuronal damage by pretreatment with sublethal ischemia. Brain Res. 1992;586:121–124. doi: 10.1016/0006-8993(92)91380-w. [DOI] [PubMed] [Google Scholar]
- Liu Y, Li J, Yang J, Ji F, Bu X, Zhang N, Zhang B. Inhibition of PKCgamma membrane translocation mediated morphine preconditioning-induced neuroprotection against oxygen-glucose deprivation in the hippocampus slices of mice. Neurosci Lett. 2008a;444:87–91. doi: 10.1016/j.neulet.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Liu Y, Qin L, Wilson B, Wu X, Qian L, Granholm AC, Crews FT, Hong JS. Endotoxin induces a delayed loss of TH-IR neurons in substantia nigra and motor behavioral deficits. Neurotoxicology. 2008b;29:864–870. doi: 10.1016/j.neuro.2008.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lo EH. A new penumbra: transitioning from injury into repair after stroke. Nat Med. 2008;14:497–500. doi: 10.1038/nm1735. [DOI] [PubMed] [Google Scholar]
- Logroscino G, Marder K, Cote L, Tang MX, Shea S, Mayeux R. Dietary lipids and antioxidants in Parkinson's disease: a population-based, case-control study. Ann Neurol. 1996;39:89–94. doi: 10.1002/ana.410390113. [DOI] [PubMed] [Google Scholar]
- Love R. Calorie restriction may be neuroprotective in AD and PD. Lancet Neurol. 2005;4:84. doi: 10.1016/s1474-4422(05)00985-3. [DOI] [PubMed] [Google Scholar]
- Luo Y, Ma D, Ieong E, Sanders RD, Yu B, Hossain M, Maze M. Xenon and sevoflurane protect against brain injury in a neonatal asphyxia model. Anesthesiology. 2008;109:782–789. doi: 10.1097/ALN.0b013e3181895f88. [DOI] [PubMed] [Google Scholar]
- Malhotra S, Naggar I, Stewart M, Rosenbaum DM. Neurogenic pathway mediated remote preconditioning protects the brain from transient focal ischemic injury. Brain Res. 2011 doi: 10.1016/j.brainres.2011.02.032. [DOI] [PubMed] [Google Scholar]
- Mandavilli BS, Boldogh I, Van Houten B. 3-nitropropionic acid-induced hydrogen peroxide, mitochondrial DNA damage, and cell death are attenuated by Bcl-2 overexpression in PC12 cells. Brain Res Mol Brain Res. 2005;133:215–223. doi: 10.1016/j.molbrainres.2004.10.033. [DOI] [PubMed] [Google Scholar]
- Mandir AS, Poitras MF, Berliner AR, Herring WJ, Guastella DB, Feldman A, Poirier GG, Wang ZQ, Dawson TM, Dawson VL. NMDA but not non-NMDA excitotoxicity is mediated by Poly(ADP-ribose) polymerase. J Neurosci. 2000;20:8005–8011. doi: 10.1523/JNEUROSCI.20-21-08005.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini A, Novelli A. DL-threo-3-hydroxyaspartate reduces NMDA receptor activation by glutamate in cultured neurons. Eur J Pharmacol. 1991;194:131–132. doi: 10.1016/0014-2999(91)90136-e. [DOI] [PubMed] [Google Scholar]
- Marini AM, Paul SM. N-methyl-D-aspartate receptor-mediated neuroprotection in cerebellar granule cells requires new RNA and protein synthesis. Proc Natl Acad Sci U S A. 1992;89:6555–6559. doi: 10.1073/pnas.89.14.6555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marini AM, Popolo M, Pan H, Blondeau N, Lipsky RH. Brain adaptation to stressful stimuli: a new perspective on potential therapeutic approaches based on BDNF and NMDA receptors. CNS Neurol Disord Drug Targets. 2008;7:382–390. doi: 10.2174/187152708786441849. [DOI] [PubMed] [Google Scholar]
- Marini AM, Rabin SJ, Lipsky RH, Mocchetti I. Activity-dependent release of brain-derived neurotrophic factor underlies the neuroprotective effect of N-methyl-D-aspartate. J Biol Chem. 1998;273:29394–29399. doi: 10.1074/jbc.273.45.29394. [DOI] [PubMed] [Google Scholar]
- Marsh B, Stevens SL, Packard AE, Gopalan B, Hunter B, Leung PY, Harrington CA, Stenzel-Poore MP. Systemic lipopolysaccharide protects the brain from ischemic injury by reprogramming the response of the brain to stroke: a critical role for IRF3. J Neurosci. 2009;29:9839–9849. doi: 10.1523/JNEUROSCI.2496-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maswood N, Young J, Tilmont E, Zhang Z, Gash DM, Gerhardt GA, Grondin R, Roth GS, Mattison J, Lane MA, Carson RE, Cohen RM, Mouton PR, Quigley C, Mattson MP, Ingram DK. Caloric restriction increases neurotrophic factor levels and attenuates neurochemical and behavioral deficits in a primate model of Parkinson's disease. Proc Natl Acad Sci U S A. 2004;101:18171–18176. doi: 10.1073/pnas.0405831102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushima K, Hakim AM. Transient forebrain ischemia protects against subsequent focal cerebral ischemia without changing cerebral perfusion. Stroke. 1995;26:1047–1052. doi: 10.1161/01.str.26.6.1047. [DOI] [PubMed] [Google Scholar]
- Matsushima K, Hogan MJ, Hakim AM. Cortical spreading depression protects against subsequent focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 1996;16:221–226. doi: 10.1097/00004647-199603000-00006. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Chan SL, Duan W. Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol Rev. 2002;82:637–672. doi: 10.1152/physrev.00004.2002. [DOI] [PubMed] [Google Scholar]
- Mattson MP, Duan W, Wan R, Guo Z. Prophylactic activation of neuroprotective stress response pathways by dietary and behavioral manipulations. NeuroRx. 2004;1:111–116. doi: 10.1602/neurorx.1.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayanagi K, Gaspar T, Katakam PV, Kis B, Busija DW. The mitochondrial K(ATP) channel opener BMS-191095 reduces neuronal damage after transient focal cerebral ischemia in rats. J Cereb Blood Flow Metab. 2007;27:348–355. doi: 10.1038/sj.jcbfm.9600345. [DOI] [PubMed] [Google Scholar]
- McAuliffe JJ, Joseph B, Vorhees CV. Isoflurane-delayed preconditioning reduces immediate mortality and improves striatal function in adult mice after neonatal hypoxia-ischemia. Anesth Analg. 2007;104:1066–1077. doi: 10.1213/01.ane.0000260321.62377.74. tables of contents. [DOI] [PubMed] [Google Scholar]
- McAuliffe JJ, Loepke AW, Miles L, Joseph B, Hughes E, Vorhees CV. Desflurane, isoflurane, and sevoflurane provide limited neuroprotection against neonatal hypoxia-ischemia in a delayed preconditioning paradigm. Anesthesiology. 2009;111:533–546. doi: 10.1097/ALN.0b013e3181b060d3. [DOI] [PubMed] [Google Scholar]
- McCullough JN, Zhang N, Reich DL, Juvonen TS, Klein JJ, Spielvogel D, Ergin MA, Griepp RB. Cerebral metabolic suppression during hypothermic circulatory arrest in humans. Ann Thorac Surg. 1999;67:1895–1899. doi: 10.1016/s0003-4975(99)00441-5. discussion 1919–1821. [DOI] [PubMed] [Google Scholar]
- McKee AC, Stein TD, Nowinski CJ, Stern RA, Daneshvar DH, Alvarez VE, Lee HS, Hall G, Wojtowicz SM, Baugh CM, Riley DO, Kubilus CA, Cormier KA, Jacobs MA, Martin BR, Abraham CR, Ikezu T, Reichard RR, Wolozin BL, Budson AE, Goldstein LE, Kowall NW, Cantu RC. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNaught KS, Belizaire R, Isacson O, Jenner P, Olanow CW. Altered proteasomal function in sporadic Parkinson's disease. Exp Neurol. 2003;179:38–46. doi: 10.1006/exnr.2002.8050. [DOI] [PubMed] [Google Scholar]
- Mehlig K, Skoog I, Guo X, Schutze M, Gustafson D, Waern M, Ostling S, Bjorkelund C, Lissner L. Alcoholic beverages and incidence of dementia: 34-year follow-up of the prospective population study of women in Goteborg. Am J Epidemiol. 2008;167:684–691. doi: 10.1093/aje/kwm366. [DOI] [PubMed] [Google Scholar]
- Meng R, Asmaro K, Meng L, Liu Y, Ma C, Xi C, Li G, Ren C, Luo Y, Ling F, Jia J, Hua Y, Wang X, Ding Y, Lo EH, Ji X. Upper limb ischemic preconditioning prevents recurrent stroke in intracranial arterial stenosis. Neurology. 2012;79:1853–1861. doi: 10.1212/WNL.0b013e318271f76a. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing Res Rev. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meybohm P, Gruenewald M, Zacharowski KD, Albrecht M, Lucius R, Fosel N, Hensler J, Zitta K, Bein B. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit Care. 2010;14:R21. doi: 10.1186/cc8879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meybohm P, Renner J, Broch O, Caliebe D, Albrecht M, Cremer J, Haake N, Scholz J, Zacharowski K, Bein B. Postoperative neurocognitive dysfunction in patients undergoing cardiac surgery after remote ischemic preconditioning: a double-blind randomized controlled pilot study. PLoS One. 2013;8:e64743. doi: 10.1371/journal.pone.0064743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller BA, Perez RS, Shah AR, Gonzales ER, Park TS, Gidday JM. Cerebral protection by hypoxic preconditioning in a murine model of focal ischemia-reperfusion. Neuroreport. 2001;12:1663–1669. doi: 10.1097/00001756-200106130-00030. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Neafsey EJ, Collins MA. Essential involvement of the NMDA receptor in ethanol preconditioning-dependent neuroprotection from amyloid-betain vitro. J Neurochem. 2009;111:580–588. doi: 10.1111/j.1471-4159.2009.06351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncayo J, de Freitas GR, Bogousslavsky J, Altieri M, van Melle G. Do transient ischemic attacks have a neuroprotective effect? Neurology. 2000;54:2089–2094. doi: 10.1212/wnl.54.11.2089. [DOI] [PubMed] [Google Scholar]
- Moroz IA, Pecina S, Schallert T, Stewart J. Sparing of behavior and basal extracellular dopamine after 6-hydroxydopamine lesions of the nigrostriatal pathway in rats exposed to a prelesion sensitizing regimen of amphetamine. Exp Neurol. 2004;189:78–93. doi: 10.1016/j.expneurol.2004.05.012. [DOI] [PubMed] [Google Scholar]
- Morton JP, Kayani AC, McArdle A, Drust B. The exercise-induced stress response of skeletal muscle, with specific emphasis on humans. Sports Med. 2009;39:643–662. doi: 10.2165/00007256-200939080-00003. [DOI] [PubMed] [Google Scholar]
- Mouton PR, Chachich ME, Quigley C, Spangler E, Ingram DK. Caloric restriction attenuates amyloid deposition in middle-aged dtg APP/PS1 mice. Neurosci Lett. 2009;464:184–187. doi: 10.1016/j.neulet.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muir KW. Glutamate-based therapeutic approaches: clinical trials with NMDA antagonists. Curr Opin Pharmacol. 2006;6:53–60. doi: 10.1016/j.coph.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Mukamal KJ, Kuller LH, Fitzpatrick AL, Longstreth WT, Jr, Mittleman MA, Siscovick DS. Prospective study of alcohol consumption and risk of dementia in older adults. JAMA. 2003;289:1405–1413. doi: 10.1001/jama.289.11.1405. [DOI] [PubMed] [Google Scholar]
- Muller DW, Topol EJ, Califf RM, Sigmon KN, Gorman L, George BS, Kereiakes DJ, Lee KL, Ellis SG. Relationship between antecedent angina pectoris and short-term prognosis after thrombolytic therapy for acute myocardial infarction. Thrombolysis and Angioplasty in Myocardial Infarction (TAMI) Study Group. Am Heart J. 1990;119:224–231. doi: 10.1016/s0002-8703(05)80008-0. [DOI] [PubMed] [Google Scholar]
- Nagy K, Kis B, Rajapakse NC, Bari F, Busija DW. Diazoxide preconditioning protects against neuronal cell death by attenuation of oxidative stress upon glutamate stimulation. J Neurosci Res. 2004;76:697–704. doi: 10.1002/jnr.20120. [DOI] [PubMed] [Google Scholar]
- Nakagawa I, Alessandri B, Heimann A, Kempski O. MitoKATP-channel opener protects against neuronal death in rat venous ischemia. Neurosurgery. 2005;57:334–340. doi: 10.1227/01.neu.0000166681.88736.86. discussion 334–340. [DOI] [PubMed] [Google Scholar]
- Nakagawa I, Wajima D, Tamura K, Nishimura F, Park YS, Nakase H. The neuroprotective effect of diazoxide is mediated by mitochondrial ATP-dependent potassium channels in a rat model of acute subdural hematoma. J Clin Neurosci. 2013;20:144–147. doi: 10.1016/j.jocn.2012.03.027. [DOI] [PubMed] [Google Scholar]
- Nielsen SS, Franklin GM, Longstreth WT, Swanson PD, Checkoway H. Nicotine from edible Solanaceae and risk of Parkinson disease. Ann Neurol. 2013 doi: 10.1002/ana.23884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishino A, Suzuki M, Ohtani H, Motohashi O, Umezawa K, Nagura H, Yoshimoto T. Thrombin may contribute to the pathophysiology of central nervous system injury. J Neurotrauma. 1993;10:167–179. doi: 10.1089/neu.1993.10.167. [DOI] [PubMed] [Google Scholar]
- Nishio S, Chen ZF, Yunoki M, Toyoda T, Anzivino M, Lee KS. Hypothermia-induced ischemic tolerance. Ann N Y Acad Sci. 1999;890:26–41. doi: 10.1111/j.1749-6632.1999.tb07978.x. [DOI] [PubMed] [Google Scholar]
- Nishio S, Yunoki M, Chen ZF, Anzivino MJ, Lee KS. Ischemic tolerance in the rat neocortex following hypothermic preconditioning. J Neurosurg. 2000;93:845–851. doi: 10.3171/jns.2000.93.5.0845. [DOI] [PubMed] [Google Scholar]
- Nocera J, Horvat M, Ray CT. Effects of home-based exercise on postural control and sensory organization in individuals with Parkinson disease. Parkinsonism Relat Disord. 2009;15:742–745. doi: 10.1016/j.parkreldis.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiseux N, Borie M, Desnoyers A, Menaouar A, Stevens LM, Mansour S, Danalache BA, Roy DC, Jankowski M, Gutkowska J. Preconditioning of stem cells by oxytocin to improve their therapeutic potential. Endocrinology. 2012;153:5361–5372. doi: 10.1210/en.2012-1402. [DOI] [PubMed] [Google Scholar]
- Notkola IL, Sulkava R, Pekkanen J, Erkinjuntti T, Ehnholm C, Kivinen P, Tuomilehto J, Nissinen A. Serum total cholesterol, apolipoprotein E epsilon 4 allele, and Alzheimer's disease. Neuroepidemiology. 1998;17:14–20. doi: 10.1159/000026149. [DOI] [PubMed] [Google Scholar]
- Ogita K, Okuda H, Yamamoto Y, Nishiyama N, Yoneda Y. In vivo neuroprotective role of NMDA receptors against kainate-induced excitotoxicity in murine hippocampal pyramidal neurons. J Neurochem. 2003;85:1336–1346. doi: 10.1046/j.1471-4159.2003.01778.x. [DOI] [PubMed] [Google Scholar]
- Oldenburg O, Cohen MV, Yellon DM, Downey JM. Mitochondrial K(ATP) channels: role in cardioprotection. Cardiovasc Res. 2002;55:429–437. doi: 10.1016/s0008-6363(02)00439-x. [DOI] [PubMed] [Google Scholar]
- Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J Immunol. 2004;173:3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- Orgogozo JM, Dartigues JF, Lafont S, Letenneur L, Commenges D, Salamon R, Renaud S, Breteler MB. Wine consumption and dementia in the elderly: a prospective community study in the Bordeaux area. Rev Neurol (Paris) 1997;153:185–192. [PubMed] [Google Scholar]
- Ostrowski RP, Graupner G, Titova E, Zhang J, Chiu J, Dach N, Corleone D, Tang J, Zhang JH. The hyperbaric oxygen preconditioning-induced brain protection is mediated by a reduction of early apoptosis after transient global cerebral ischemia. Neurobiol Dis. 2008;29:1–13. doi: 10.1016/j.nbd.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota A, Ikeda T, Abe K, Sameshima H, Xia XY, Xia YX, Ikenoue T. Hypoxic-ischemic tolerance phenomenon observed in neonatal rat brain. Am J Obstet Gynecol. 1998;179:1075–1078. doi: 10.1016/s0002-9378(98)70218-2. [DOI] [PubMed] [Google Scholar]
- Paradis S, Ruvkun G. Caenorhabditis elegans Akt/PKB transduces insulin receptor-like signals from AGE-1 PI3 kinase to the DAF-16 transcription factor. Genes Dev. 1998;12:2488–2498. doi: 10.1101/gad.12.16.2488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SU, Ferrer JV, Javitch JA, Kuhn DM. Peroxynitrite inactivates the human dopamine transporter by modification of cysteine 342: potential mechanism of neurotoxicity in dopamine neurons. J Neurosci. 2002;22:4399–4405. doi: 10.1523/JNEUROSCI.22-11-04399.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paulson JR, Yang T, Selvaraj PK, Mdzinarishvili A, Van der Schyf CJ, Klein J, Bickel U, Abbruscato TJ. Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. J Pharmacol Exp Ther. 2010;332:371–379. doi: 10.1124/jpet.109.157776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne RS, Akca O, Roewer N, Schurr A, Kehl F. Sevoflurane-induced preconditioning protects against cerebral ischemic neuronal damage in rats. Brain Res. 2005;1034:147–152. doi: 10.1016/j.brainres.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Pepping JK, Freeman LR, Gupta S, Keller JN, Bruce-Keller AJ. NOX2 deficiency attenuates markers of adiposopathy and brain injury induced by high-fat diet. Am J Physiol Endocrinol Metab. 2013;304:E392–404. doi: 10.1152/ajpendo.00398.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera J, Zawadzka M, Kaminska B, Szczudlik A. Influence of chemical and ischemic preconditioning on cytokine expression after focal brain ischemia. J Neurosci Res. 2004;78:132–140. doi: 10.1002/jnr.20232. [DOI] [PubMed] [Google Scholar]
- Perez-Pinzon MA, Xu GP, Dietrich WD, Rosenthal M, Sick TJ. Rapid preconditioning protects rats against ischemic neuronal damage after 3 but not 7 days of reperfusion following global cerebral ischemia. J Cereb Blood Flow Metab. 1997;17:175–182. doi: 10.1097/00004647-199702000-00007. [DOI] [PubMed] [Google Scholar]
- Persky RW, Turtzo LC, McCullough LD. Stroke in women: disparities and outcomes. Curr Cardiol Rep. 2010;12:6–13. doi: 10.1007/s11886-009-0080-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters R, Peters J, Warner J, Beckett N, Bulpitt C. Alcohol, dementia and cognitive decline in the elderly: a systematic review. Age Ageing. 2008;37:505–512. doi: 10.1093/ageing/afn095. [DOI] [PubMed] [Google Scholar]
- Petrovski G, Das DK. Does autophagy take a front seat in lifespan extension? J Cell Mol Med. 2010;14:2543–2551. doi: 10.1111/j.1582-4934.2010.01196.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzinger GM, Fisher BE, Van Leeuwen JE, Vukovic M, Akopian G, Meshul CK, Holschneider DP, Nacca A, Walsh JP, Jakowec MW. Enhancing neuroplasticity in the basal ganglia: the role of exercise in Parkinson's disease. Mov Disord. 2010;25(Suppl 1):S141–145. doi: 10.1002/mds.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinder RM, Sandler M. Alcohol, wine and mental health: focus on dementia and stroke. J Psychopharmacol. 2004;18:449–456. doi: 10.1177/0269881104047272. [DOI] [PubMed] [Google Scholar]
- Poehlman ET, Turturro A, Bodkin N, Cefalu W, Heymsfield S, Holloszy J, Kemnitz J. Caloric restriction mimetics: physical activity and body composition changes. J Gerontol A Biol Sci Med Sci. 2001;56(Spec No 1):45–54. doi: 10.1093/gerona/56.suppl_1.45. [DOI] [PubMed] [Google Scholar]
- Prass K, Scharff A, Ruscher K, Lowl D, Muselmann C, Victorov I, Kapinya K, Dirnagl U, Meisel A. Hypoxia-induced stroke tolerance in the mouse is mediated by erythropoietin. Stroke. 2003;34:1981–1986. doi: 10.1161/01.STR.0000080381.76409.B2. [DOI] [PubMed] [Google Scholar]
- Prins ML, Giza CC. Repeat traumatic brain injury in the developing brain. Int J Dev Neurosci. 2011 doi: 10.1016/j.ijdevneu.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Purcell JE, Lenhard SC, White RF, Schaeffer T, Barone FC, Chandra S. Strain-dependent response to cerebral ischemic preconditioning: differences between spontaneously hypertensive and stroke prone spontaneously hypertensive rats. Neurosci Lett. 2003;339:151–155. doi: 10.1016/s0304-3940(02)01476-3. [DOI] [PubMed] [Google Scholar]
- Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson's disease and a prime target for therapy. J Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin W, Chachich M, Lane M, Roth G, Bryant M, de Cabo R, Ottinger MA, Mattison J, Ingram D, Gandy S, Pasinetti GM. Calorie restriction attenuates Alzheimer's disease type brain amyloidosis in Squirrel monkeys (Saimiri sciureus) J Alzheimers Dis. 2006;10:417–422. doi: 10.3233/jad-2006-10411. [DOI] [PubMed] [Google Scholar]
- Quigney DJ, Gorman AM, Samali A. Heat shock protects PC12 cells against MPP+ toxicity. Brain Res. 2003;993:133–139. doi: 10.1016/j.brainres.2003.09.004. [DOI] [PubMed] [Google Scholar]
- Quik M, Campos C, Grady SR. Multiple CNS nicotinic receptors mediate L-dopa-induced dyskinesias; studies with parkinsonian nicotinic receptor knockout mice. Biochem Pharmacol. 2013 doi: 10.1016/j.bcp.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quik M, Huang LZ, Parameswaran N, Bordia T, Campos C, Perez XA. Multiple roles for nicotine in Parkinson's disease. Biochem Pharmacol. 2009;78:677–685. doi: 10.1016/j.bcp.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Radak Z, Taylor AW, Ohno H, Goto S. Adaptation to exercise-induced oxidative stress: from muscle to brain. Exerc Immunol Rev. 2001;7:90–107. [PubMed] [Google Scholar]
- Rajapakse N, Kis B, Horiguchi T, Snipes J, Busija D. Diazoxide pretreatment induces delayed preconditioning in astrocytes against oxygen glucose deprivation and hydrogen peroxide-induced toxicity. J Neurosci Res. 2003;73:206–214. doi: 10.1002/jnr.10657. [DOI] [PubMed] [Google Scholar]
- Rao VL, Dogan A, Todd KG, Bowen KK, Dempsey RJ. Neuroprotection by memantine, a non-competitive NMDA receptor antagonist after traumatic brain injury in rats. Brain Res. 2001;911:96–100. doi: 10.1016/s0006-8993(01)02617-8. [DOI] [PubMed] [Google Scholar]
- Raval AP, Dave KR, Perez-Pinzon MA. Resveratrol mimics ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2006;26:1141–1147. doi: 10.1038/sj.jcbfm.9600262. [DOI] [PubMed] [Google Scholar]
- Raval AP, Hirsch N, Dave KR, Yavagal DR, Bramlett H, Saul I. Nicotine and estrogen synergistically exacerbate cerebral ischemic injury. Neuroscience. 2011;181:216–225. doi: 10.1016/j.neuroscience.2011.02.036. [DOI] [PubMed] [Google Scholar]
- Rehni AK, Singh TG, Jaggi AS, Singh N. Pharmacological preconditioning of the brain: a possible interplay between opioid and calcitonin gene related peptide transduction systems. Pharmacol Rep. 2008;60:904–913. [PubMed] [Google Scholar]
- Ren C, Gao M, Dornbos D, 3rd, Ding Y, Zeng X, Luo Y, Ji X. Remote ischemic post-conditioning reduced brain damage in experimental ischemia/reperfusion injury. Neurol Res. 2011;33:514–519. doi: 10.1179/016164111X13007856084241. [DOI] [PubMed] [Google Scholar]
- Ren C, Gao X, Steinberg GK, Zhao H. Limb remote-preconditioning protects against focal ischemia in rats and contradicts the dogma of therapeutic time windows for preconditioning. Neuroscience. 2008;151:1099–1103. doi: 10.1016/j.neuroscience.2007.11.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberge MC, Hotte-Bernard J, Messier C, Plamondon H. Food restriction attenuates ischemia-induced spatial learning and memory deficits despite extensive CA1 ischemic injury. Behav Brain Res. 2008;187:123–132. doi: 10.1016/j.bbr.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Lessov NS, Henshall DC, Minami M, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning prevents cellular inflammatory response during ischemic neuroprotection in mice. Stroke. 2004;35:2576–2581. doi: 10.1161/01.STR.0000143450.04438.ae. [DOI] [PubMed] [Google Scholar]
- Rosenzweig HL, Minami M, Lessov NS, Coste SC, Stevens SL, Henshall DC, Meller R, Simon RP, Stenzel-Poore MP. Endotoxin preconditioning protects against the cytotoxic effects of TNFalpha after stroke: a novel role for TNFalpha in LPS-ischemic tolerance. J Cereb Blood Flow Metab. 2007;27:1663–1674. doi: 10.1038/sj.jcbfm.9600464. [DOI] [PubMed] [Google Scholar]
- Ross GW, Petrovitch H. Current evidence for neuroprotective effects of nicotine and caffeine against Parkinson's disease. Drugs Aging. 2001;18:797–806. doi: 10.2165/00002512-200118110-00001. [DOI] [PubMed] [Google Scholar]
- Sakata H, Narasimhan P, Niizuma K, Maier CM, Wakai T, Chan PH. Interleukin 6-preconditioned neural stem cells reduce ischaemic injury in stroke mice. Brain. 2012a;135:3298–3310. doi: 10.1093/brain/aws259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakata H, Niizuma K, Yoshioka H, Kim GS, Jung JE, Katsu M, Narasimhan P, Maier CM, Nishiyama Y, Chan PH. Minocycline-preconditioned neural stem cells enhance neuroprotection after ischemic stroke in rats. J Neurosci. 2012b;32:3462–3473. doi: 10.1523/JNEUROSCI.5686-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saleh MC, Connell BJ, Saleh TM. Ischemic tolerance following low dose NMDA involves modulation of cellular stress proteins. Brain Res. 2009;1247:212–220. doi: 10.1016/j.brainres.2008.10.014. [DOI] [PubMed] [Google Scholar]
- Sasaoka N, Kawaguchi M, Kawaraguchi Y, Nakamura M, Konishi N, Patel H, Patel PM, Furuya H. Isoflurane exerts a short-term but not a long-term preconditioning effect in neonatal rats exposed to a hypoxic-ischaemic neuronal injury. Acta Anaesthesiol Scand. 2009;53:46–54. doi: 10.1111/j.1399-6576.2008.01822.x. [DOI] [PubMed] [Google Scholar]
- Schroeder JE, Richardson JC, Virley DJ. Dietary manipulation and caloric restriction in the development of mouse models relevant to neurological diseases. Biochim Biophys Acta. 2010;1802:840–846. doi: 10.1016/j.bbadis.2010.04.007. [DOI] [PubMed] [Google Scholar]
- Shankaran M, Gudelsky GA. Effect of 3,4-methylenedioxymethamphetamine (MDMA) on hippocampal dopamine and serotonin. Pharmacol Biochem Behav. 1998;61:361–366. doi: 10.1016/s0091-3057(98)00103-8. [DOI] [PubMed] [Google Scholar]
- Shein NA, Horowitz M, Shohami E. Heat acclimation: a unique model of physiologically mediated global preconditioning against traumatic brain injury. Prog Brain Res. 2007;161:353–363. doi: 10.1016/S0079-6123(06)61025-X. [DOI] [PubMed] [Google Scholar]
- Sigaut S, Jannier V, Rouelle D, Gressens P, Mantz J, Dahmani S. The preconditioning effect of sevoflurane on the oxygen glucose-deprived hippocampal slice: the role of tyrosine kinases and duration of ischemia. Anesth Analg. 2009;108:601–608. doi: 10.1213/ane.0b013e31818e2018. [DOI] [PubMed] [Google Scholar]
- Silachev DN, Isaev NK, Pevzner IB, Zorova LD, Stelmashook EV, Novikova SV, Plotnikov EY, Skulachev VP, Zorov DB. The mitochondria-targeted antioxidants and remote kidney preconditioning ameliorate brain damage through kidney-to-brain cross-talk. PLoS One. 2012;7:e51553. doi: 10.1371/journal.pone.0051553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh AK, Jiang Y, Gupta S, Benlhabib E. Effects of chronic ethanol drinking on the blood brain barrier and ensuing neuronal toxicity in alcohol-preferring rats subjected to intraperitoneal LPS injection. Alcohol Alcohol. 2007;42:385–399. doi: 10.1093/alcalc/agl120. [DOI] [PubMed] [Google Scholar]
- Singh N, Agrawal M, Dore S. Neuroprotective Properties and Mechanisms of Resveratrol in in Vitro and in Vivo Experimental Cerebral Stroke Models. ACS Chem Neurosci. 2013 doi: 10.1021/cn400094w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima Y, Ostrowski RP, Manaenko A, Fujii M, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hyperglycemia enhanced hemorrhagic transformation after transient MCAO in rats. Med Gas Res. 2012;2:9. doi: 10.1186/2045-9912-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sommer C. Ischemic preconditioning: postischemic structural changes in the brain. J Neuropathol Exp Neurol. 2008;67:85–92. doi: 10.1097/nen.0b013e3181630ba6. [DOI] [PubMed] [Google Scholar]
- Speelman AD, van de Warrenburg BP, van Nimwegen M, Petzinger GM, Munneke M, Bloem BR. How might physical activity benefit patients with Parkinson disease? Nat Rev Neurol. 2011;7:528–534. doi: 10.1038/nrneurol.2011.107. [DOI] [PubMed] [Google Scholar]
- Speetzen LJ, Endres M, Kunz A. Bilateral common carotid artery occlusion as an adequate preconditioning stimulus to induce early ischemic tolerance to focal cerebral ischemia. J Vis Exp. 2013 doi: 10.3791/4387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stagliano NE, Perez-Pinzon MA, Moskowitz MA, Huang PL. Focal ischemic preconditioning induces rapid tolerance to middle cerebral artery occlusion in mice. J Cereb Blood Flow Metab. 1999;19:757–761. doi: 10.1097/00004647-199907000-00005. [DOI] [PubMed] [Google Scholar]
- Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med. 1988;319:267–273. doi: 10.1056/NEJM198808043190503. [DOI] [PubMed] [Google Scholar]
- Stevens SL, Ciesielski TM, Marsh BJ, Yang T, Homen DS, Boule JL, Lessov NS, Simon RP, Stenzel-Poore MP. Toll-like receptor 9: a new target of ischemic preconditioning in the brain. J Cereb Blood Flow Metab. 2008;28:1040–1047. doi: 10.1038/sj.jcbfm.9600606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su Z, Han D, Sun B, Qiu J, Li Y, Li M, Zhang T, Yang Z. Heat stress preconditioning improves cognitive outcome after diffuse axonal injury in rats. J Neurotrauma. 2009;26:1695–1706. doi: 10.1089/neu.2008.0519. [DOI] [PubMed] [Google Scholar]
- Sugino T, Nozaki K, Takagi Y, Hashimoto N. 3-Nitropropionic acid induces ischemic tolerance in gerbil hippocampus in vivo. Neurosci Lett. 1999;259:9–12. doi: 10.1016/s0304-3940(98)00875-1. [DOI] [PubMed] [Google Scholar]
- Sun L, Zhou W, Heiland S, Marti HH, Veltkamp R. A translationally relevant thromboembolic stroke model for the study of secondary hemorrhage after thrombolysis in rats. Brain Res. 2011;1368:346–354. doi: 10.1016/j.brainres.2010.10.067. [DOI] [PubMed] [Google Scholar]
- Suzuki Y, Kim HW, Ashraf M, Haider H. Diazoxide potentiates mesenchymal stem cell survival via NF-kappaB-dependent miR-146a expression by targeting Fas. Am J Physiol Heart Circ Physiol. 2010;299:H1077–1082. doi: 10.1152/ajpheart.00212.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taga K, Patel PM, Drummond JC, Cole DJ, Kelly PJ. Transient neuronal depolarization induces tolerance to subsequent forebrain ischemia in rats. Anesthesiology. 1997;87:918–925. doi: 10.1097/00000542-199710000-00027. [DOI] [PubMed] [Google Scholar]
- Tai KK, Truong DD. Activation of adenosine triphosphate-sensitive potassium channels confers protection against rotenone-induced cell death: therapeutic implications for Parkinson's disease. J Neurosci Res. 2002;69:559–566. doi: 10.1002/jnr.10309. [DOI] [PubMed] [Google Scholar]
- Takagi H, Manabe H, Kawai N, Goto SN, Umemoto T. Review and meta-analysis of randomized controlled clinical trials of remote ischemic preconditioning in cardiovascular surgery. Am J Cardiol. 2008;102:1487–1488. doi: 10.1016/j.amjcard.2008.07.036. [DOI] [PubMed] [Google Scholar]
- Tanay E, Mundel P, Sommer C. Short-term ischemia usually used for ischemic preconditioning causes loss of dendritic integrity after long-term survival in the gerbil hippocampus. Brain Res. 2006;1112:222–226. doi: 10.1016/j.brainres.2006.07.004. [DOI] [PubMed] [Google Scholar]
- Tang JX, Mardini F, Caltagarone BM, Garrity ST, Li RQ, Bianchi SL, Gomes O, Laferla FM, Eckenhoff RG, Eckenhoff MF. Anesthesia in presymptomatic Alzheimer's disease: a study using the triple-transgenic mouse model. Alzheimers Dement. 2011;7:521–531. doi: 10.1016/j.jalz.2010.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang SC, Arumugam TV, Xu X, Cheng A, Mughal MR, Jo DG, Lathia JD, Siler DA, Chigurupati S, Ouyang X, Magnus T, Camandola S, Mattson MP. Pivotal role for neuronal Toll-like receptors in ischemic brain injury and functional deficits. Proc Natl Acad Sci U S A. 2007;104:13798–13803. doi: 10.1073/pnas.0702553104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang XQ, Feng JQ, Chen J, Chen PX, Zhi JL, Cui Y, Guo RX, Yu HM. Protection of oxidative preconditioning against apoptosis induced by H2O2 in PC12 cells: mechanisms via MMP, ROS, and Bcl-2. Brain Res. 2005;1057:57–64. doi: 10.1016/j.brainres.2005.07.072. [DOI] [PubMed] [Google Scholar]
- Tang Y, Pacary E, Freret T, Divoux D, Petit E, Schumann-Bard P, Bernaudin M. Effect of hypoxic preconditioning on brain genomic response before and following ischemia in the adult mouse: identification of potential neuroprotective candidates for stroke. Neurobiol Dis. 2006;21:18–28. doi: 10.1016/j.nbd.2005.06.002. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain Res. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Texel SJ, Mattson MP. Impaired adaptive cellular responses to oxidative stress and the pathogenesis of Alzheimer's disease. Antioxid Redox Signal. 2011;14:1519–1534. doi: 10.1089/ars.2010.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Caudle WM, Zigmond MJ, Schallert T, Miller GW. Forced nonuse in unilateral parkinsonian rats exacerbates injury. J Neurosci. 2002;22:6790–6799. doi: 10.1523/JNEUROSCI.22-15-06790.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Cohen AD, Philhower J, Miller GW, Zigmond MJ, Schallert T. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. doi: 10.1523/JNEUROSCI.21-12-04427.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tissenbaum HA, Ruvkun G. An insulin-like signaling pathway affects both longevity and reproduction in Caenorhabditis elegans. Genetics. 1998;148:703–717. doi: 10.1093/genetics/148.2.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu YF, Lu PJ, Huang CC, Ho CJ, Chou YP. Moderate dietary restriction reduces p53-mediated neurovascular damage and microglia activation after hypoxic ischemia in neonatal brain. Stroke. 2012;43:491–498. doi: 10.1161/STROKEAHA.111.629931. [DOI] [PubMed] [Google Scholar]
- Turrens JF. Mitochondrial formation of reactive oxygen species. J Physiol. 2003;552:335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umschweif G, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Hypoxia-inducible factor 1 is essential for spontaneous recovery from traumatic brain injury and is a key mediator of heat acclimation induced neuroprotection. J Cereb Blood Flow Metab. 2013;33:524–531. doi: 10.1038/jcbfm.2012.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umschwief G, Shein NA, Alexandrovich AG, Trembovler V, Horowitz M, Shohami E. Heat acclimation provides sustained improvement in functional recovery and attenuates apoptosis after traumatic brain injury. J Cereb Blood Flow Metab. 2010;30:616–627. doi: 10.1038/jcbfm.2009.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hamersvelt HW, Kloke HJ, de Jong DJ, Koene RA, Huysmans FT. Oedema formation with the vasodilators nifedipine and diazoxide: direct local effect or sodium retention? J Hypertens. 1996;14:1041–1045. [PubMed] [Google Scholar]
- Vance RE, Isberg RR, Portnoy DA. Patterns of pathogenesis: discrimination of pathogenic and nonpathogenic microbes by the innate immune system. Cell host & microbe. 2009;6:10–21. doi: 10.1016/j.chom.2009.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vannucci RC, Vannucci SJ. A model of perinatal hypoxic-ischemic brain damage. Ann N Y Acad Sci. 1997;835:234–249. doi: 10.1111/j.1749-6632.1997.tb48634.x. [DOI] [PubMed] [Google Scholar]
- Vannucci SJ, Hagberg H. Hypoxia-ischemia in the immature brain. J Exp Biol. 2004;207:3149–3154. doi: 10.1242/jeb.01064. [DOI] [PubMed] [Google Scholar]
- Vaughan PJ, Pike CJ, Cotman CW, Cunningham DD. Thrombin receptor activation protects neurons and astrocytes from cell death produced by environmental insults. J Neurosci. 1995;15:5389–5401. doi: 10.1523/JNEUROSCI.15-07-05389.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velly LJ, Canas PT, Guillet BA, Labrande CN, Masmejean FM, Nieoullon AL, Gouin FM, Bruder NJ, Pisano PS. Early anesthetic preconditioning in mixed cortical neuronal-glial cell cultures subjected to oxygen-glucose deprivation: the role of adenosine triphosphate dependent potassium channels and reactive oxygen species in sevoflurane-induced neuroprotection. Anesth Analg. 2009;108:955–963. doi: 10.1213/ane.0b013e318193fee7. [DOI] [PubMed] [Google Scholar]
- Wacker BK, Park TS, Gidday JM. Hypoxic preconditioning-induced cerebral ischemic tolerance: role of microvascular sphingosine kinase 2. Stroke. 2009;40:3342–3348. doi: 10.1161/STROKEAHA.109.560714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Ito M, Miyazawa T, Katoh H, Nawashiro H, Shima K, Chigasaki H. Repeated hyperbaric oxygen induces ischemic tolerance in gerbil hippocampus. Brain Res. 1996;740:15–20. doi: 10.1016/s0006-8993(96)00831-1. [DOI] [PubMed] [Google Scholar]
- Wada K, Miyazawa T, Nomura N, Tsuzuki N, Nawashiro H, Shima K. Preferential conditions for and possible mechanisms of induction of ischemic tolerance by repeated hyperbaric oxygenation in gerbil hippocampus. Neurosurgery. 2001;49:160–166. doi: 10.1097/00006123-200107000-00025. discussion 166–167. [DOI] [PubMed] [Google Scholar]
- Walsh SR, Nouraei SA, Tang TY, Sadat U, Carpenter RH, Gaunt ME. Remote ischemic preconditioning for cerebral and cardiac protection during carotid endarterectomy: results from a pilot randomized clinical trial. Vasc Endovascular Surg. 2010;44:434–439. doi: 10.1177/1538574410369709. [DOI] [PubMed] [Google Scholar]
- Walsh SR, Tang TY, Kullar P, Jenkins DP, Dutka DP, Gaunt ME. Ischaemic preconditioning during cardiac surgery: systematic review and meta-analysis of perioperative outcomes in randomised clinical trials. Eur J Cardiothorac Surg. 2008;34:985–994. doi: 10.1016/j.ejcts.2008.07.062. [DOI] [PubMed] [Google Scholar]
- Wang H, Luo M, Li C, Wang G. Propofol post-conditioning induced long-term neuroprotection and reduced internalization of AMPAR GluR2 subunit in a rat model of focal cerebral ischemia/reperfusion. J Neurochem. 2011a;119:210–219. doi: 10.1111/j.1471-4159.2011.07400.x. [DOI] [PubMed] [Google Scholar]
- Wang J, Ho L, Qin W, Rocher AB, Seror I, Humala N, Maniar K, Dolios G, Wang R, Hof PR, Pasinetti GM. Caloric restriction attenuates beta-amyloid neuropathology in a mouse model of Alzheimer's disease. FASEB J. 2005;19:659–661. doi: 10.1096/fj.04-3182fje. [DOI] [PubMed] [Google Scholar]
- Wang J, Lei B, Popp S, Meng F, Cottrell JE, Kass IS. Sevoflurane immediate preconditioning alters hypoxic membrane potential changes in rat hippocampal slices and improves recovery of CA1 pyramidal cells after hypoxia and global cerebral ischemia. Neuroscience. 2007a;145:1097–1107. doi: 10.1016/j.neuroscience.2006.12.047. [DOI] [PubMed] [Google Scholar]
- Wang JK, Yu LN, Zhang FJ, Yang MJ, Yu J, Yan M, Chen G. Postconditioning with sevoflurane protects against focal cerebral ischemia and reperfusion injury via PI3K/Akt pathway. Brain Res. 2010a;1357:142–151. doi: 10.1016/j.brainres.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhu QL, Wang GZ, Deng TZ, Chen R, Liu MH, Wang SW. The protective roles of mitochondrial ATP-sensitive potassium channels during hypoxia-ischemia-reperfusion in brain. Neurosci Lett. 2011b;491:63–67. doi: 10.1016/j.neulet.2010.12.065. [DOI] [PubMed] [Google Scholar]
- Wang Q, Kalogeris TJ, Wang M, Jones AW, Korthuis RJ. Antecedent ethanol attenuates cerebral ischemia/reperfusion-induced leukocyte-endothelial adhesive interactions and delayed neuronal death: role of large conductance, Ca2+-activated K+ channels. Microcirculation. 2010b;17:427–438. doi: 10.1111/j.1549-8719.2010.00041.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Sun AY, Simonyi A, Kalogeris TJ, Miller DK, Sun GY, Korthuis RJ. Ethanol preconditioning protects against ischemia/reperfusion-induced brain damage: role of NADPH oxidase-derived ROS. Free Radic Biol Med. 2007b;43:1048–1060. doi: 10.1016/j.freeradbiomed.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Yan JY, Lo YK, Carvey PM, Ling Z. Dopaminergic and serotoninergic deficiencies in young adult rats prenatally exposed to the bacterial lipopolysaccharide. Brain Res. 2009;1265:196–204. doi: 10.1016/j.brainres.2009.02.022. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Jones S, Stepheson LL, Khiabani KT, Zamboni WA. Microvascular protection induced by late preconditioning was abolished in STZ-induced acute diabetic rats. J Reconstr Microsurg. 2002;18:689–696. doi: 10.1055/s-2002-36501. [DOI] [PubMed] [Google Scholar]
- Warner DS, McFarlane C, Todd MM, Ludwig P, McAllister AM. Sevoflurane and halothane reduce focal ischemic brain damage in the rat. Possible influence on thermoregulation. Anesthesiology. 1993;79:985–992. doi: 10.1097/00000542-199311000-00017. [DOI] [PubMed] [Google Scholar]
- Wegelius K, Korpi ER. Ethanol inhibits NMDA-induced toxicity and trophism in cultured cerebellar granule cells. Acta Physiol Scand. 1995;154:25–34. doi: 10.1111/j.1748-1716.1995.tb09882.x. [DOI] [PubMed] [Google Scholar]
- Wegener S, Gottschalk B, Jovanovic V, Knab R, Fiebach JB, Schellinger PD, Kucinski T, Jungehulsing GJ, Brunecker P, Muller B, Banasik A, Amberger N, Wernecke KD, Siebler M, Rother J, Villringer A, Weih M. Transient ischemic attacks before ischemic stroke: preconditioning the human brain? A multicenter magnetic resonance imaging study. Stroke. 2004;35:616–621. doi: 10.1161/01.STR.0000115767.17923.6A. [DOI] [PubMed] [Google Scholar]
- Wei D, Ren C, Chen X, Zhao H. The chronic protective effects of limb remote preconditioning and the underlying mechanisms involved in inflammatory factors in rat stroke. PLoS One. 2012a;7:e30892. doi: 10.1371/journal.pone.0030892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei L, Fraser JL, Lu ZY, Hu X, Yu SP. Transplantation of hypoxia preconditioned bone marrow mesenchymal stem cells enhances angiogenesis and neurogenesis after cerebral ischemia in rats. Neurobiol Dis. 2012b;46:635–645. doi: 10.1016/j.nbd.2012.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei N, Yu SP, Gu X, Taylor TM, Song D, Liu XF, Wei L. Delayed intranasal delivery of hypoxic-preconditioned bone marrow mesenchymal stem cells enhanced cell homing and therapeutic benefits after ischemic stroke in mice. Cell Transplant. 2013;22:977–991. doi: 10.3727/096368912X657251. [DOI] [PubMed] [Google Scholar]
- Weih M, Kallenberg K, Bergk A, Dirnagl U, Harms L, Wernecke KD, Einhaupl KM. Attenuated stroke severity after prodromal TIA: a role for ischemic tolerance in the brain? Stroke. 1999;30:1851–1854. doi: 10.1161/01.str.30.9.1851. [DOI] [PubMed] [Google Scholar]
- Werner C, Mollenberg O, Kochs E, Schulte JaE. Sevoflurane improves neurological outcome after incomplete cerebral ischaemia in rats. Br J Anaesth. 1995;75:756–760. doi: 10.1093/bja/75.6.756. [DOI] [PubMed] [Google Scholar]
- Whitham M, Fortes MB. Heat shock protein 72: release and biological significance during exercise. Front Biosci. 2008;13:1328–1339. doi: 10.2741/2765. [DOI] [PubMed] [Google Scholar]
- Wu C, Zhan RZ, Qi S, Fujihara H, Taga K, Shimoji K. A forebrain ischemic preconditioning model established in C57Black/Crj6 mice. J Neurosci Methods. 2001;107:101–106. doi: 10.1016/s0165-0270(01)00356-9. [DOI] [PubMed] [Google Scholar]
- Wu P, Jiang C, Shen Q, Hu Y. Systematic gene expression profile of hypothalamus in calorie-restricted mice implicates the involvement of mTOR signaling in neuroprotective activity. Mech Ageing Dev. 2009;130:602–610. doi: 10.1016/j.mad.2009.07.005. [DOI] [PubMed] [Google Scholar]
- Wu P, Shen Q, Dong S, Xu Z, Tsien JZ, Hu Y. Calorie restriction ameliorates neurodegenerative phenotypes in forebrain-specific presenilin-1 and presenilin-2 double knockout mice. Neurobiol Aging. 2008;29:1502–1511. doi: 10.1016/j.neurobiolaging.2007.03.028. [DOI] [PubMed] [Google Scholar]
- Wu SY, Wang TF, Yu L, Jen CJ, Chuang JI, Wu FS, Wu CW, Kuo YM. Running exercise protects the substantia nigra dopaminergic neurons against inflammation-induced degeneration via the activation of BDNF signaling pathway. Brain Behav Immun. 2011;25:135–146. doi: 10.1016/j.bbi.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Xi G, Keep RF, Hua Y, Xiang J, Hoff JT. Attenuation of thrombin-induced brain edema by cerebral thrombin preconditioning. Stroke. 1999;30:1247–1255. doi: 10.1161/01.str.30.6.1247. [DOI] [PubMed] [Google Scholar]
- Xiao F, Fratkin JD, Rhodes PG, Cai Z. Reduced nitric oxide is involved in prenatal ischemia-induced tolerance to neonatal hypoxic-ischemic brain injury in rats. Neurosci Lett. 2000;285:5–8. doi: 10.1016/s0304-3940(00)00997-6. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zheng Y, Wu M, Hou L, Zhu Z, Zhang X, Lu Z. Preconditioning with isoflurane produces dose-dependent neuroprotection via activation of adenosine triphosphate-regulated potassium channels after focal cerebral ischemia in rats. Anesth Analg. 2003;96:233–237. doi: 10.1097/00000539-200301000-00047. table of contents. [DOI] [PubMed] [Google Scholar]
- Xu H, Aibiki M, Nagoya J. Neuroprotective effects of hyperthermic preconditioning on infarcted volume after middle cerebral artery occlusion in rats: role of adenosine receptors. Crit Care Med. 2002;30:1126–1130. doi: 10.1097/00003246-200205000-00028. [DOI] [PubMed] [Google Scholar]
- Xu K, Sun X, Puchowicz MA, LaManna JC. Increased sensitivity to transient global ischemia in aging rat brain. Adv Exp Med Biol. 2007;599:199–206. doi: 10.1007/978-0-387-71764-7_26. [DOI] [PubMed] [Google Scholar]
- Xu Q, Park Y, Huang X, Hollenbeck A, Blair A, Schatzkin A, Chen H. Physical activities and future risk of Parkinson disease. Neurology. 2010;75:341–348. doi: 10.1212/WNL.0b013e3181ea1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang H, Hreggvidsdottir HS, Palmblad K, Wang H, Ochani M, Li J, Lu B, Chavan S, Rosas-Ballina M, Al-Abed Y, Akira S, Bierhaus A, Erlandsson-Harris H, Andersson U, Tracey KJ. A critical cysteine is required for HMGB1 binding to Toll-like receptor 4 and activation of macrophage cytokine release. Proc Natl Acad Sci U S A. 2010a;107:11942–11947. doi: 10.1073/pnas.1003893107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang QW, Xiang J, Zhou Y, Zhong Q, Li JC. Targeting HMGB1/TLR4 signaling as a novel approach to treatment of cerebral ischemia. Frontiers in bioscience (Scholar edition) 2010b;2:1081–1091. doi: 10.2741/s119. [DOI] [PubMed] [Google Scholar]
- Yannopoulos FS, Makela T, Niemela E, Tuominen H, Lepola P, Alestalo K, Kaakinen H, Kiviluoma K, Anttila V, Juvonen T. Improved cerebral recovery from hypothermic circulatory arrest after remote ischemic preconditioning. Ann Thorac Surg. 2010;90:182–188. doi: 10.1016/j.athoracsur.2010.03.058. [DOI] [PubMed] [Google Scholar]
- Yao QL, Zhang MF, Wang CH, Hu F, Lan AP, Guo RX, Chen PX, Feng JQ. Protective effects of early hypoxic post-conditioning in cultured cortical neurons. Brain Inj. 2011;25:604–613. doi: 10.3109/02699052.2011.568035. [DOI] [PubMed] [Google Scholar]
- Ye R, Yang Q, Kong X, Li N, Zhang Y, Han J, Xiong L, Liu X, Zhao G. Sevoflurane preconditioning improves mitochondrial function and long-term neurologic sequelae after transient cerebral ischemia: role of mitochondrial permeability transition. Crit Care Med. 2012a;40:2685–2693. doi: 10.1097/CCM.0b013e318258fb90. [DOI] [PubMed] [Google Scholar]
- Ye Z, Guo Q, Wang N, Xia P, Yuan Y, Wang E. Delayed neuroprotection induced by sevoflurane via opening mitochondrial ATP-sensitive potassium channels and p38 MAPK phosphorylation. Neurol Sci. 2012b;33:239–249. doi: 10.1007/s10072-011-0665-6. [DOI] [PubMed] [Google Scholar]
- Yu JT, Lee CH, Yoo KY, Choi JH, Li H, Park OK, Yan B, Hwang IK, Kwon YG, Kim YM, Won MH. Maintenance of anti-inflammatory cytokines and reduction of glial activation in the ischemic hippocampal CA1 region preconditioned with lipopolysaccharide. J Neurol Sci. 2010;296:69–78. doi: 10.1016/j.jns.2010.06.004. [DOI] [PubMed] [Google Scholar]
- Yu ZF, Mattson MP. Dietary restriction and 2-deoxyglucose administration reduce focal ischemic brain damage and improve behavioral outcome: evidence for a preconditioning mechanism. J Neurosci Res. 1999;57:830–839. [PubMed] [Google Scholar]
- Yuan HB, Huang Y, Zheng S, Zuo Z. Hypothermic preconditioning increases survival of purkinje neurons in rat cerebellar slices after an in vitro simulated ischemia. Anesthesiology. 2004;100:331–337. doi: 10.1097/00000542-200402000-00023. [DOI] [PubMed] [Google Scholar]
- Yuan HB, Huang Y, Zheng S, Zuo Z. Hypothermic preconditioning reduces Purkinje cell death possibly by preventing the over-expression of inducible nitric oxide synthase in rat cerebellar slices after an in vitro simulated ischemia. Neuroscience. 2006;142:381–389. doi: 10.1016/j.neuroscience.2006.06.053. [DOI] [PubMed] [Google Scholar]
- Yunoki M, Nishio S, Ukita N, Anzivino MJ, Lee KS. Characteristics of hypothermic preconditioning influencing the induction of delayed ischemic tolerance. J Neurosurg. 2002;97:650–657. doi: 10.3171/jns.2002.97.3.0650. [DOI] [PubMed] [Google Scholar]
- Yurkewicz L, Weaver J, Bullock MR, Marshall LF. The effect of the selective NMDA receptor antagonist traxoprodil in the treatment of traumatic brain injury. J Neurotrauma. 2005;22:1428–1443. doi: 10.1089/neu.2005.22.1428. [DOI] [PubMed] [Google Scholar]
- Zhan L, Wang T, Li W, Xu ZC, Sun W, Xu E. Activation of Akt/FoxO signaling pathway contributes to induction of neuroprotection against transient global cerebral ischemia by hypoxic pre-conditioning in adult rats. J Neurochem. 2010;114:897–908. doi: 10.1111/j.1471-4159.2010.06816.x. [DOI] [PubMed] [Google Scholar]
- Zhang HP, Yuan LB, Zhao RN, Tong L, Ma R, Dong HL, Xiong L. Isoflurane preconditioning induces neuroprotection by attenuating ubiquitin-conjugated protein aggregation in a mouse model of transient global cerebral ischemia. Anesth Analg. 2010;111:506–514. doi: 10.1213/ANE.0b013e3181e45519. [DOI] [PubMed] [Google Scholar]
- Zhang J, Qian H, Zhao P, Hong SS, Xia Y. Rapid hypoxia preconditioning protects cortical neurons from glutamate toxicity through delta-opioid receptor. Stroke. 2006;37:1094–1099. doi: 10.1161/01.STR.0000206444.29930.18. [DOI] [PubMed] [Google Scholar]
- Zhang W, Wang B, Zhou S, Qiu Y. The effect of ischemic post-conditioning on hippocampal cell apoptosis following global brain ischemia in rats. J Clin Neurosci. 2012;19:570–573. doi: 10.1016/j.jocn.2011.07.051. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Park TS, Gidday JM. Hypoxic preconditioning protects human brain endothelium from ischemic apoptosis by Akt-dependent survivin activation. Am J Physiol Heart Circ Physiol. 2007;292:H2573–2581. doi: 10.1152/ajpheart.01098.2006. [DOI] [PubMed] [Google Scholar]
- Zhao H. Ischemic postconditioning as a novel avenue to protect against brain injury after stroke. J Cereb Blood Flow Metab. 2009;29:873–885. doi: 10.1038/jcbfm.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Ren C, Chen X, Shen J. From rapid to delayed and remote postconditioning: the evolving concept of ischemic postconditioning in brain ischemia. Curr Drug Targets. 2012;13:173–187. doi: 10.2174/138945012799201621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H, Sapolsky RM, Steinberg GK. Interrupting reperfusion as a stroke therapy: ischemic postconditioning reduces infarct size after focal ischemia in rats. J Cereb Blood Flow Metab. 2006a;26:1114–1121. doi: 10.1038/sj.jcbfm.9600348. [DOI] [PubMed] [Google Scholar]
- Zhao P, Huang Y, Zuo Z. Opioid preconditioning induces opioid receptor-dependent delayed neuroprotection against ischemia in rats. J Neuropathol Exp Neurol. 2006b;65:945–952. doi: 10.1097/01.jnen.0000235123.05677.4b. [DOI] [PubMed] [Google Scholar]
- Zhao P, Peng L, Li L, Xu X, Zuo Z. Isoflurane preconditioning improves long-term neurologic outcome after hypoxic-ischemic brain injury in neonatal rats. Anesthesiology. 2007;107:963–970. doi: 10.1097/01.anes.0000291447.21046.4d. [DOI] [PubMed] [Google Scholar]
- Zhao P, Zuo Z. Isoflurane preconditioning induces neuroprotection that is inducible nitric oxide synthase-dependent in neonatal rats. Anesthesiology. 2004;101:695–703. doi: 10.1097/00000542-200409000-00018. [DOI] [PubMed] [Google Scholar]
- Zheng S, Zuo Z. Isoflurane preconditioning induces neuroprotection against ischemia via activation of P38 mitogen-activated protein kinases. Mol Pharmacol. 2004;65:1172–1180. doi: 10.1124/mol.65.5.1172. [DOI] [PubMed] [Google Scholar]
- Zhou AM, Li WB, Li QJ, Liu HQ, Feng RF, Zhao HG. A short cerebral ischemic preconditioning up-regulates adenosine receptors in the hippocampal CA1 region of rats. Neurosci Res. 2004;48:397–404. doi: 10.1016/j.neures.2003.12.010. [DOI] [PubMed] [Google Scholar]
- Zhou C, Tu J, Zhang Q, Lu D, Zhu Y, Zhang W, Yang F, Brann DW, Wang R. Delayed ischemic postconditioning protects hippocampal CA1 neurons by preserving mitochondrial integrity via Akt/GSK3beta signaling. Neurochem Int. 2011a;59:749–758. doi: 10.1016/j.neuint.2011.08.008. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Fathali N, Lekic T, Ostrowski RP, Chen C, Martin RD, Tang J, Zhang JH. Remote limb ischemic postconditioning protects against neonatal hypoxic-ischemic brain injury in rat pups by the opioid receptor/Akt pathway. Stroke. 2011b;42:439–444. doi: 10.1161/STROKEAHA.110.592162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu D, Wu X, Strauss KI, Lipsky RH, Qureshi Z, Terhakopian A, Novelli A, Banaudha K, Marini AM. N-methyl-D-aspartate and TrkB receptors protect neurons against glutamate excitotoxicity through an extracellular signal-regulated kinase pathway. J Neurosci Res. 2005;80:104–113. doi: 10.1002/jnr.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H, Guo Q, Mattson MP. Dietary restriction protects hippocampal neurons against the death-promoting action of a presenilin-1 mutation. Brain Res. 1999;842:224–229. doi: 10.1016/s0006-8993(99)01827-2. [DOI] [PubMed] [Google Scholar]
- Zhu HC, Gao XQ, Xing Y, Sun SG, Li HG, Wang YF. Inhibition of caspase-3 activation and apoptosis is involved in 3-nitropropionic acid-induced ischemic tolerance to transient focal cerebral ischemia in rats. J Mol Neurosci. 2004;24:299–305. doi: 10.1385/JMN:24:2:299. [DOI] [PubMed] [Google Scholar]
- Zhu W, Wang L, Zhang L, Palmateer JM, Libal NL, Hurn PD, Herson PS, Murphy SJ. Isoflurane preconditioning neuroprotection in experimental focal stroke is androgen-dependent in male mice. Neuroscience. 2010;169:758–769. doi: 10.1016/j.neuroscience.2010.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziegler G, Harhausen D, Schepers C, Hoffmann O, Rohr C, Prinz V, Konig J, Lehrach H, Nietfeld W, Trendelenburg G. TLR2 has a detrimental role in mouse transient focal cerebral ischemia. Biochemical and biophysical research communications. 2007;359:574–579. doi: 10.1016/j.bbrc.2007.05.157. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Hoffer BJ, Smeyne RJ. Neurorestoration by physical exercise: moving forward. Parkinsonism Relat Disord. 2012;18(Suppl 1):S147–150. doi: 10.1016/S1353-8020(11)70046-3. [DOI] [PubMed] [Google Scholar]
- Zigmond MJ, Cameron JL, Leak RK, Mirnics K, Russell VA, Smeyne RJ, Smith AD. Triggering endogenous neuroprotective processes through exercise in models of dopamine deficiency. Parkinsonism Relat Disord. 2009;15(Suppl 3):S42–45. doi: 10.1016/S1353-8020(09)70778-3. [DOI] [PubMed] [Google Scholar]