Abstract
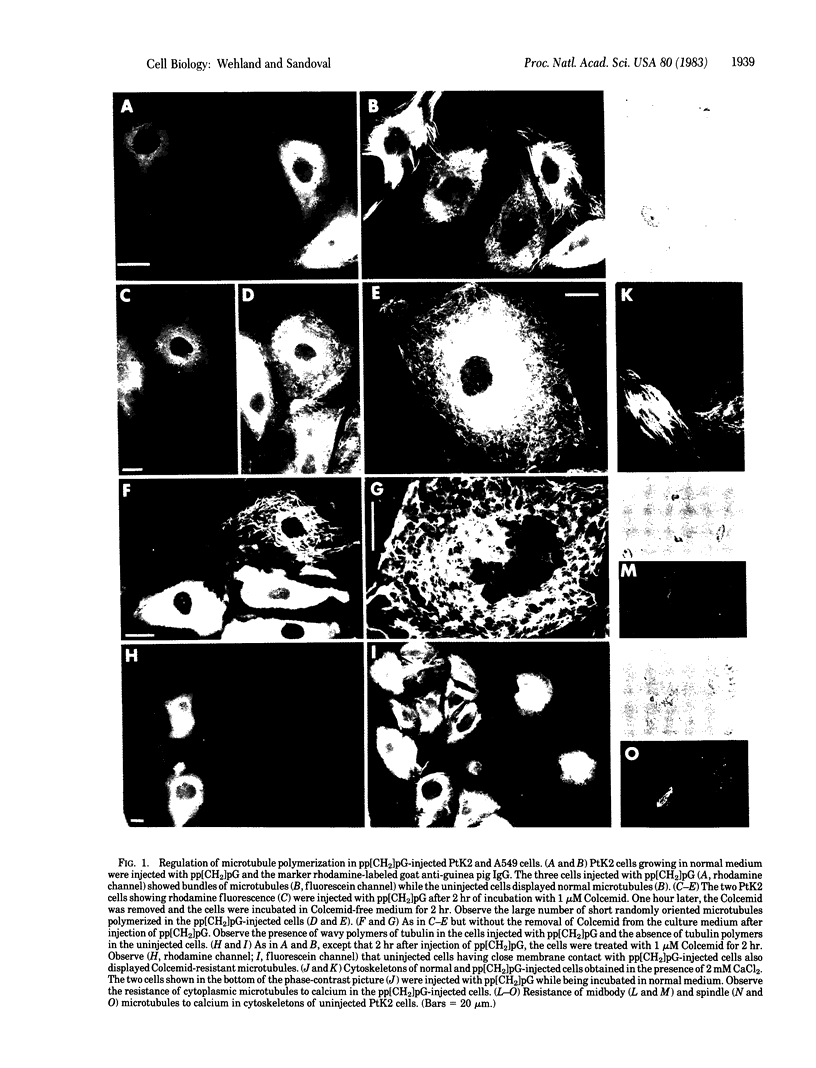
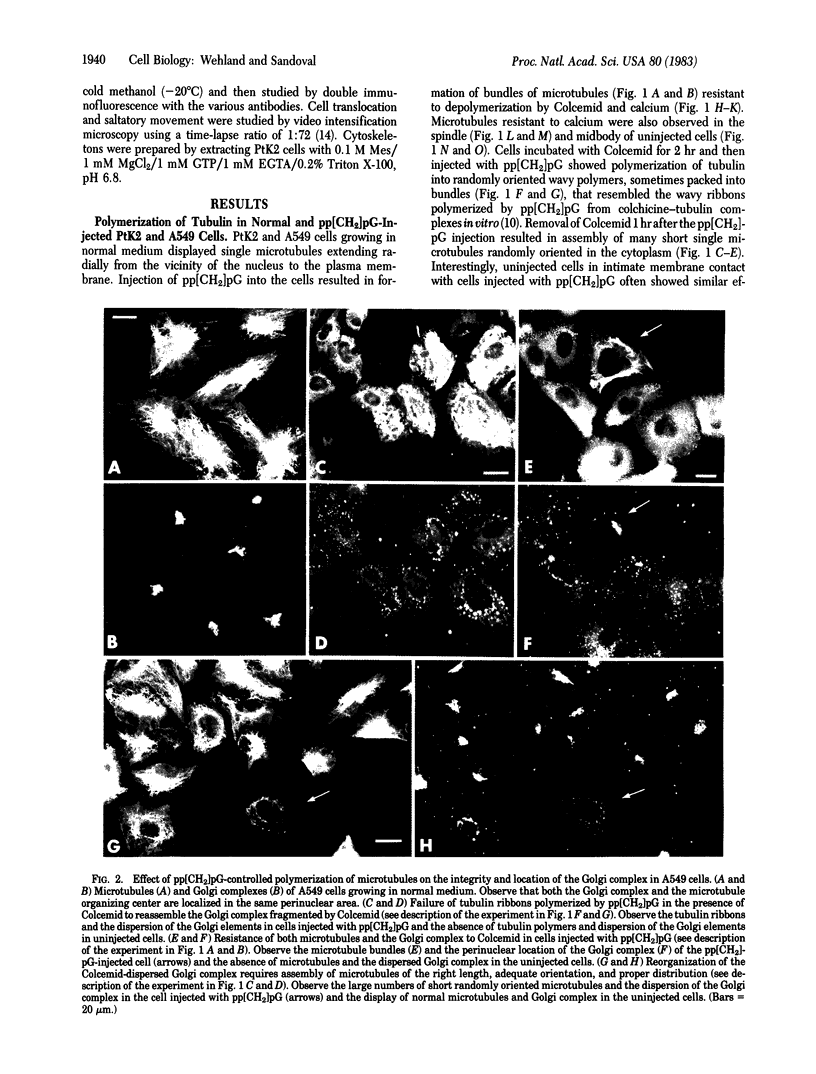
Injection of the alpha, beta-nonhydrolyzable GTP analog, guanosine 5'-[alpha, beta-methylene]triphosphate (pp[CH2]pG) into PtK2, A549, and Swiss 3T3 cells produced dramatic changes in the normal pattern of long radiating microtubules displayed by the cells before injection. Injection of pp[CH2]pG into cells growing in normal medium resulted in the formation of microtubule bundles resistant to depolymerization by Colcemid and calcium. Cells injected with pp[CH2]pG after incubation with Colcemid for 2 hr showed polymerization of tubulin into long wavy ribbons within 2 hr after injection. Removal of Colcemid 1 hr after the injection of pp[CH2]pG resulted in assembly of tubulin into short single randomly oriented microtubules. All cells injected with pp[CH2]pG showed impeded translocation and restriction or absence of intracellular movement. pp[CH2]pG also prevented the fragmentation of Golgi elements in A549 cells treated with Colcemid. Cells first treated with Colcemid and then injected with pp[CH2]pG failed to reassemble the Golgi elements after the removal of Colcemid. Cells in intimate membrane contact with cells injected with pp[CH2]pG showed similar changes in microtubule polymerization, cell movement, and organization of Golgi elements.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berger E. G., Mandel T., Schilt U. Immunohistochemical localization of galactosyltransferase in human fibroblasts and HeLa cells. J Histochem Cytochem. 1981 Mar;29(3):364–370. doi: 10.1177/29.3.6787115. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Fuller E. M., Highfield D. P. Cytoplasmic microtubules in normal and transformed cells in culture: analysis by tubulin antibody immunofluorescence. Proc Natl Acad Sci U S A. 1975 Dec;72(12):4981–4985. doi: 10.1073/pnas.72.12.4981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs M., Smith H., Taylor E. W. Tublin: nucleotide binding and enzymic activity. J Mol Biol. 1974 Nov 5;89(3):455–468. doi: 10.1016/0022-2836(74)90475-6. [DOI] [PubMed] [Google Scholar]
- Kilmartin J. V., Wright B., Milstein C. Rat monoclonal antitubulin antibodies derived by using a new nonsecreting rat cell line. J Cell Biol. 1982 Jun;93(3):576–582. doi: 10.1083/jcb.93.3.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirschner M. W. Implications of treadmilling for the stability and polarity of actin and tubulin polymers in vivo. J Cell Biol. 1980 Jul;86(1):330–334. doi: 10.1083/jcb.86.1.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margolis R. L., Wilson L. Opposite end assembly and disassembly of microtubules at steady state in vitro. Cell. 1978 Jan;13(1):1–8. doi: 10.1016/0092-8674(78)90132-0. [DOI] [PubMed] [Google Scholar]
- OOSAWA F., KASAI M. A theory of linear and helical aggregations of macromolecules. J Mol Biol. 1962 Jan;4:10–21. doi: 10.1016/s0022-2836(62)80112-0. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., MacDonald E., Jameson J. L., Cuatrecasas P. Role of nucleotides in tubulin polymerization: effect of guanylyl 5'-methylenediphosphonate. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4881–4885. doi: 10.1073/pnas.74.11.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandoval I. V., Weber K. Guanasone 5'-(alpha,beta-methylene)triphosphate enhances specifically microtubule nucleation and stops the treadmill of tubulin protomers. J Biol Chem. 1980 Jul 25;255(14):6966–6974. [PubMed] [Google Scholar]
- Sandoval I. V., Weber K. Polymerization of tubulin in the presence of colchicine or podophyllotoxin. Formation of a ribbon structure induced by guanylyl-5'-methylene diphosphonate. J Mol Biol. 1979 Oct 15;134(1):159–172. doi: 10.1016/0022-2836(79)90418-2. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Piasek A., Moskalewski S. Effects of colchicine on the Golgi complex and GERL of cultured rat peritoneal macrophages and epiphyseal chondrocytes. J Cell Sci. 1980 Oct;45:41–58. doi: 10.1242/jcs.45.1.41. [DOI] [PubMed] [Google Scholar]
- Wehland J., Osborn M., Weber K. Phalloidin-induced actin polymerization in the cytoplasm of cultured cells interferes with cell locomotion and growth. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5613–5617. doi: 10.1073/pnas.74.12.5613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisenberg R. C., Borisy G. G., Taylor E. W. The colchicine-binding protein of mammalian brain and its relation to microtubules. Biochemistry. 1968 Dec;7(12):4466–4479. doi: 10.1021/bi00852a043. [DOI] [PubMed] [Google Scholar]
- Weisenberg R. C. Microtubule formation in vitro in solutions containing low calcium concentrations. Science. 1972 Sep 22;177(4054):1104–1105. doi: 10.1126/science.177.4054.1104. [DOI] [PubMed] [Google Scholar]
- Willingham M. C., Pastan I. The visualization of fluorescent proteins in living cells by video intensification microscopy (VIM). Cell. 1978 Mar;13(3):501–507. doi: 10.1016/0092-8674(78)90323-9. [DOI] [PubMed] [Google Scholar]