Abstract
Background
Enhanced automatic spreading of activation in the semantic network has been suggested to underlie formal thought disorder in patients with schizophrenia, but it is not clear how this relates to the dopaminergic dysfunction implicated in the disorder. Previous studies on dopaminergic modulation of priming in healthy volunteers have focused on controlled rather than automatic processes. The present study aimed to examine the effects of both a dopaminergic agonist and a dopaminergic antagonist on semantic priming while minimizing the contribution of controlled processes.
Methods
We investigated the effects of levodopa (L-Dopa; 100 mg), haloperidol (2 mg) and placebo on priming in healthy participants within a randomized, double-blind, crossover design. We used a pronunciation priming task with word triplets; the middle word was an ambiguous word, whereas the first word of the triplet served to provide either a congruent, incongruent or unbiased context for the target word. Two stimulus onset asynchronies (SOA) were used: 150 ms and 750 ms.
Results
The study involved 34 participants. At an SOA of 150 ms, L-Dopa accelerated responses to incongruent targets and subordinate targets of ambiguous words, whereas haloperidol was associated with faster responses in congruent contexts and dominant targets. At an SOA of 750 ms, haloperidol accelerated responses to subordinate targets.
Limitations
Modulations in the relative magnitude of priming according to substance and condition rather than absolute priming were assessed.
Conclusion
Effects of L-Dopa on automatic priming processes appear to be different than those on controlled processes. Our results are consistent with those of studies on semantic priming and the effects on antipsychotics in patients with schizophrenia.
Introduction
The semantic priming effect refers to the observation that reaction times (i.e., pronunciation or speeded lexical decision) to a target word, such as “dog” are faster when the word is preceded by a related prime (e.g., “cat”) than when it is preceded by an unrelated word (e.g., “pen”).1,2 This effect is attributed to an automatic spreading of activation, initiated by the prime word, to adjacent nodes within the semantic network,3,4 but also to controlled processes not related to the semantic store, such as attention-based expectancy (i.e., generation of predictions) and postlexical matching (i.e., evaluation of the semantic association between prime and target).4,5 These processes can be differentially tapped by manipulating various aspects of priming paradigms, such as the prime–target interval (stimulus onset asynchrony [SOA]), the proportion of related word pairs or the nonword ratio in lexical decision designs. It is generally accepted that the contribution of controlled processes to priming becomes more pronounced with the increase of any of the aforementioned variables and is greater in lexical decision tasks than word pronunciation tasks.5,6
Semantic priming tasks have been widely used in the schizophrenia literature. Several studies have reported increased single-word priming in patients with schizophrenia, especially those with formal thought disorder.7–12 Although not always replicated,13–17 this hyperpriming appears to be highly consistent under conditions favouring automatic processes18 as well as for target words either indirectly (e.g., lemon–sweet)10,18–20 or weakly associated with the prime word.21 Based on these observations, it has been suggested that automatic spreading of lexical activation is accelerated and/or travels abnormally large distances in thought-disordered patients with schizophrenia.18,22 In line with this assumption, a functional neuroimaging study reported in-creased activity during indirect automatic priming in patients with schizophrenia compared with controls in regions associated with semantic processing.23 This excessive spread of activation has been hypothesized to constitute the cognitive correlate of positive formal thought disorder.9,18,21
However, it is not clear how these findings relate to the dopaminergic dysfunction assumed to underlie schizophrenia.24,25 Several studies showing a reduction of indirect semantic priming upon administration of levodopa (L-Dopa) or dopaminergic agonists in healthy individuals26–28 have led to the suggestion that dopamine (DA) increases the signal-to-noise ratio within the semantic network, resulting in a more focused activation pattern.27,29 This assumption is consistent with the results of priming studies using ambiguous words: single-dose administration of L-Dopa in healthy participants has been found to reduce priming for targets related to the subordinate (i.e., less frequent) meaning of ambiguous words — this is hypothesized to reflect an increased semantic focus on the basis of meaning frequency.29,30
However, all of the above findings concerning indirect priming have been obtained under conditions favouring controlled processes, i.e., lexical decision tasks with SOAs greater than 500 ms.26–28 Moreover, one of the studies that combined behavioural with functional neuroimaging data30 implicated attentional/controlled rather than automatic mechanisms in reduced priming for subordinate targets of ambiguous words after administration of L-Dopa. Hence, the findings mentioned previously do not provide a satisfactory account for the priming abnormalities in patients with schizophrenia, which appear to arise from disturbances in automatic rather than controlled spreading of activation. Although it has been argued that increased indirect priming in patients with schizophrenia could result from decreased prefrontal dopaminergic activity and, hence, a dysfunction of controlled processes,27 this assumption is rather implausible, given that patients exhibit hyperpriming mostly at short SOAs; at long SOAs (i.e., similar to those of the previously mentioned studies) and/or controlled conditions, priming tends to be, if anything, reduced in patients with schizophrenia.18,31
Thus, studies investigating priming under automatic access conditions would be very informative with regard to the association between dopaminergic activity and formal thought disorder. So far, few such data are available. In one of the studies reporting decreased indirect priming at the long SOA condition under L-Dopa,26 there was also some evidence of increased indirect priming at the short SOA. Based on this observation, the authors proposed that DA modifies the time course of semantic processing by accelerating both the activation of nodes within the mental lexicon and the decay of such activation;26 however, the effect of L-Dopa at the short SOA was weak. In another similarly designed study, no differences in direct or indirect priming were found between L-Dopa and placebo in the short SOA condition.27 A functional neuroimaging study also found increased activity of the middle temporal gyrus, an area involved in automatic semantic priming, during priming of the dominant meaning of ambiguous words;30 this might suggest an increased activation of the semantic network which, however, was not reflected in the behavioural data. It appears, then, that the effect of L-Dopa on automatic priming processes might be different than, or even opposite to, its effect on controlled processes. Tentative support for this assumption comes from findings in studies of Parkinson disease, a disorder characterized by DA depletion: although patients with Parkinson disease exhibit abnormalities both in automatic and controlled priming, a recent study reported that only the former showed some normalization with dopaminergic agonists.32
The aim of the present study was, therefore, to provide direct evidence for the previous assumption by investigating how dopaminergic modulation affects semantic priming under automatic access conditions. An important consideration was that the failure of previous studies to detect increased automatic semantic priming after administration of L-Dopa could reflect ceiling effects, which have been suggested to limit the extent of priming.27 To enhance observed effects, several methodological modifications were applied in the present study in comparison to previous ones. First, we used a crossover design to increase both the number of observations and statistical power. Second, to increase the contrast of observed effects, priming performance under L-Dopa was not compared only to placebo, but also to the dopaminergic antagonist haloperidol; this should additionally render results more comparable to clinical observations, since dopaminergic antagonists constitute the treatment of choice in patients with schizophrenia. To our knowledge, this is the first time the effects of haloperidol on priming have been investigated in healthy participants. Finally, we used a priming paradigm that takes advantage of a well-known property of ambiguous words: the activation of their different meanings to a various extent of selectivity and strength according to the context.
Methods
Participants and design
The present study was part of a larger project investigating the effects of dopaminergically active drugs on cognitive functions associated with psychotic symptoms. Participants were healthy individuals recruited among students and acquaintances of the research team. Participants were required to be between 18 and 40 years old, right-handed and native speakers of German. Exclusion criteria were any past or current psychiatric or neurologic disorder (including substance use disorders); a family history of schizophrenia or bipolar disorder; history of craniocerebral trauma, arterial hypertension, cardiological or serious medical conditions; pregnancy; or treatment with any psychotropic or other drugs. Eligibility for the study was confirmed by means of an interview conducted by a trained doctoral-level student. The study was approved by the Ethics Committee of the Medical Association Hamburg, and was performed in accordance with the ethical standards laid down in the current version of the Declaration of Helsinki. All participants gave their written informed consent before participating in the study.
We assessed the effects of dopaminergic agents on priming using a randomized, double-blind, 3-way crossover design. In 3 successive visits, participants were administered either 100 mg of L-Dopa and 25 mg of benserazide, 2 mg haloperidol or placebo in randomized order and under double-blind conditions. The dose of L-Dopa was identical to that used in previous behavioural26,27,29 and neuroimaging studies.30,33 The dose of haloperidol was chosen to correspond to a D2-receptor occupancy of around 70%, which is deemed sufficient for a clinical response while minimizing the risk of adverse effects.34,35
The 3 visits were separated by at least 7 days to allow a complete wash-out of the drug with the longer half-life (haloperidol).36 We used a double-dummy design (Table 1) to compensate for the different time to reach maximal serum concentration (Tmax) of haloperidol and L-Dopa.36 The testing session began at the Tmax of each drug; the priming paradigm was the third task administered, approximately 25 minutes after the onset of the testing session. To ensure that the blinded condition was preserved, participants were asked to guess which substance they had received at the end of each session. Moreover, to rule out performance differences due to nonspecific effects of the drugs, we also administered the d2 letter cancelation test, a test of selective attention,37 at each session.
Table 1.
Double-dummy design of study drug administration
| Study drug | t0 | t1 (1.5 h after t0) | t2 (2.5 h after t0) |
|---|---|---|---|
| Haloperidol | Haloperidol | Placebo | Onset of testing session |
| Levodopa | Placebo | Levodopa | Onset of testing session |
| Placebo | Placebo | Placebo | Onset of testing session |
We assessed subjective psychological, somatic and motor (adverse) effects of the drugs through subjective ratings at the time of ingestion of the second capsule (t1) and after the end of the testing session. Moreover, blood pressure and pulse were regularly monitored. Participants were paid a total of 80€ (or 40€ plus course credit for students) or a proportional amount in case of early drop-out from the study.
Priming task and procedure
The priming paradigm was adapted from that used by Schvaneveldt and colleagues.38 Stimuli consisted of 3-word sequences, in which the second word was always an ambiguous word with 2 independent meanings (e.g., “bark”). The third word was always a dominant or subordinate associate of the ambiguous word, whereas the first word was either related to the same meaning of the ambiguous word as the third word (congruent condition; e.g., noise–bark–dog), related to a different meaning of the ambiguous word (incongruent condition; e.g., tree–bark–dog), or completely unrelated to the ambiguous word (unbiased condition; e.g., pen–bark–dog).
Various versions of this paradigm have produced consistent facilitation effects for congruent targets and inhibition effects for incongruent targets under controlled access conditions.38–41 However, under conditions favouring automatic processes (short SOAs, masked priming, pronunciation tasks), similar priming effects have been reported for both congruent and incongruent targets.1,40 In the present study, we implemented the paradigm as a pronunciation task with an SOA of 150 ms to eliminate both expectancy and postlexical matching effects.5 We also included a second SOA of 750 ms to allow a view into the time course of the effects of the 3 substances. We expected that the effect of condition described at the beginning of this paragraph would be observable with placebo only at the long SOA, whereas reduced inhibition of incongruent targets would appear at the short SOA for L-Dopa if it has an enhancing effect on automatic spreading of activation.
We constructed 3 stimulus lists as follows. For 2 sets of 18 ambiguous German words, 2 dominant and 2 subordinate associates were selected from published association norms41,42 such that 2 triplets (1 with a dominant and 1 with a subordinate target) were built for each of the congruent and incongruent conditions. Two unbiased triplets were also built by replacing the first word of the triplets with associates of other ambiguous words on the list. Each stimulus list contained 2 sets of 12 congruent, 12 incongruent and 12 unbiased triplets, with half of them ending in dominant and half in subordinate associates of the ambiguous word. Thus, participants were presented with all possible permutations of triplet conditions over the 3 sessions. In any single session, there was no repetition of end words (targets), whereas each ambiguous word appeared twice in different contexts and with different targets. Filler trials were constructed by combining a further 36 ambiguous words with various permutations of 6 unrelated words according to the same procedure. Thus, at each session, 36 experimental and 36 filler trials were presented at both SOAs. An example set of stimuli is presented in Table 2.
Table 2.
Example stimuli for the priming paradigm*
| Condition | Target type | Prime 1 | Prime 2 | Target |
|---|---|---|---|---|
| Congruent | Play | Ball | Round | |
| Incongruent | Dominant | Gown | Ball | Round |
| Unbiased | Pen | Ball | Round | |
| Congruent | Gown | Ball | Dance | |
| Incongruent | Subordinate | Play | Ball | Dance |
| Unbiased | Pen | Ball | Dance |
Actual stimuli were German words; for the sake of comprehensibility, the example has been translated into English (“ball” = ball; dominant meaning = “round object”; subordinate meaning = “dance”).
There were no differences in mean length, printed frequency43 and subjective familiarity,42 neither in orthographic neighbourhood43 nor in initial phoneme, between dominant and subordinate targets (all p > 0.20). Moreover, there were no differences in length, frequency and familiarity among the first words of congruent, incongruent and unbiased triplets (all p > 0.20), nor between the 2 homonym sets regarding the first, ambiguous or target words (all p > 0.20).
Stimuli were presented on a PC running Microsoft Windows XP. We used the E-prime software44 to present stimuli and record accuracy rates and reaction times (RTs). The order of presentation of stimulus lists was counterbalanced across sessions; the order of presentation of the 2 SOA conditions was also counterbalanced per participant and session. Each trial started with the presentation of a fixation cross for 1000 ms. Then the 3 words of the triplet appeared in succession on the screen (Arial 20 point font, centre position, black on white background); the first and second word appeared each for a duration equal to the SOA (i.e., either 150 ms or 750 ms), and the third word remained on the screen for 2 seconds. We instructed participants to pronounce the third word of each triplet as quickly and accurately as possible. A voice key trigger recorded RTs, and the experimenter recorded response accuracy with a button click before the onset of the next trial. Each session began with a set of 20 practice trials that were not included in the analysis; participants were required to achieve an accuracy of at least 80% before the experimental session started.
Statistical analysis
The dependent variable was the RT for correct responses. Because of the skewed distribution of RT data, outliers (RTs exceeding 2 standard deviations from the participant’s mean per condition) were eliminated, and a logarithmic transformation was applied before conducting the analysis. To avoid unrepresentative means, we determined a priori that participants would be excluded from analyses if their mean RTs in at least 1 experimental condition were based on fewer than 5 valid values after elimination of incorrect responses, failed voice key recordings and outliers.
We investigated group differences using a 3 (substance) × 3 (condition) repeated-measures analysis of variance (ANOVA) for each SOA separately. For the sake of interpretability, significant interactions were followed by simple contrasts. Moreover, in light of previous studies suggesting differences in L-Dopa effects between dominant and subordinate targets,29,30 we conducted 3 (condition) × 2 (target type) repeated-measures ANOVAs for each substance and SOA to more closely inspect the activation profiles for each substance per condition and target type. However, these latter analyses should be considered exploratory because they were based on 5–6 items per condition. In cases of violation of the sphericity assumption, we applied a Greenhouse–Geisser correction. Significant condition effects were followed up with polynomial contrasts; according to previous findings, we expected a linear trend for placebo at an SOA of 750 ms, with increasing RTs from congruent to unbiased to incongruent triplets.
Results
A total of 41 university students (17 women) with a mean age of 24.4 ± 3.8 (range 19–36) years participated in the study. One person was excluded from our analyses owing to technical failure of the priming experiment in the placebo session, and 6 were excluded owing to an insufficient number of valid responses per condition, leading to a final sample of 34 participants. Mean RTs of correct responses per condition are provided in Table 3. The rate of errors (0.7%), outliers (0.6%) and failed voice key recordings (0.4%) was very low, and there was no evidence of a speed–accuracy trade-off (r = 0.001, p = 0.98).
Table 3.
Mean reaction times and standard deviations (in milliseconds) for each substance, condition and target type separately*
| Group; mean RT ± SD | ANOVA results | |||||
|---|---|---|---|---|---|---|
|
|
|
|||||
| Substance; condition | Dominant | Subordinate | Effect/interaction | F | p value | Direction/post hoc |
| SOA of 150 ms | ||||||
| Haloperidol | Target type | 4.96 | 0.033 | Dominant < subordinate linear F = 4.74, p = 0.037 | ||
| Condition | 3.17 | 0.049 | ||||
| Congruent | 464.86 ± 67.4 | 480.39 ± 61.5 | ||||
| Incongruent | 483.68 ± 76.3 | 488.40 ± 65.0 | ||||
| Unbiased | 481.08 ± 56.5 | 494.46 ± 59.5 | ||||
| L-Dopa | Target type | 0.48 | 0.49 | Linear F = 6.48, p = 0.016 | ||
| Condition | 3.04 | 0.06 | ||||
| Congruent | 485.54 ± 71.3 | 486.43 ± 68.8 | ||||
| Incongruent | 463.70 ± 67.6 | 473.37 ± 69.9 | ||||
| Unbiased | 474.93 ± 64.1 | 475.87 ± 82.1 | ||||
| Placebo | Target type | 2.55 | 0.12 | Dominant < subordinate | ||
| Condition | 0.73 | 0.49 | ||||
| Congruent | 478.17 ± 51.5 | 483.88 ± 55.1 | ||||
| Incongruent | 470.28 ± 58.0 | 490.63 ± 70.3 | ||||
| Unbiased | 470.48 ± 61.6 | 474.45 ± 67.7 | ||||
| SOA of 750 ms | ||||||
| Haloperidol | Target type | 3.89 | 0.06 | Subordinate < dominant | ||
| Condition | 0.02 | 0.98 | ||||
| Unbiased | 442.65 ± 99.7 | 428.86 ± 76.2 | ||||
| Congruent | 442.52 ± 105.6 | 431.84 ± 83.4 | ||||
| Incongruent | 437.81 ± 70.0 | 421.36 ± 69.1 | ||||
| L-Dopa | Target type | 1.03 | 0.32 | |||
| Condition | 0.61 | 0.56 | ||||
| Unbiased | 425.19 ± 86.2 | 417.48 ± 76.9 | ||||
| Congruent | 425.75 ± 86.6 | 423.20 ± 63.5 | ||||
| Incongruent | 425.81 ± 82.0 | 422.10 ± 103.9 | ||||
| Placebo | Target type | 0.01 | 0.98 | Linear F = 3.30, p = 0.08 | ||
| Condition | 1.19 | 0.31 | ||||
| Unbiased | 429.13 ± 79.0 | 446.42 ± 116.6 | ||||
| Congruent | 429.86 ± 78.46 | 422.49 ± 62.4 | ||||
| Incongruent | 440.18 ± 86.88 | 440.24 ± 70.2 | ||||
ANOVA = analysis of variance; L-Dopa = levodopa; RT = reaction time; SD = standard deviation; SOA = stimulus onset asynchronies.
Results of 3 (condition) × 2 (target type) ANOVAs on RTs for each substance separately are presented. Target type × condition interactions are not displayed because they were not significant in any case (all p > 0.4).
There were no significant differences among the 3 sub-stances in d2 scores (F2,66 = 1.26, p = 0.3) or adverse effects (main effect of substance: F2,66 = 0.35, p = 0.7; time × substance interaction: F3.2, 107.9 = 1.8, p = 0.2). There were no drop-outs and no pre-mature session terminations owing to adverse effects. There was also no association between ingested and guessed substance (χ26 = 8.5, p = 0.2).
Short SOA (150 ms)
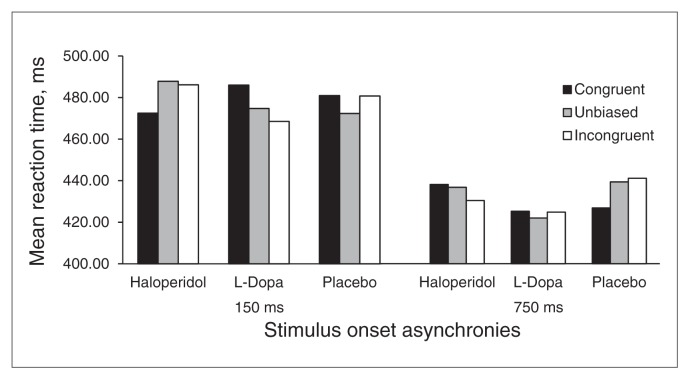
There were no significant main effects of substance (F1.7, 54.6 = 0.30, p = 0.74) or condition (F2,66 = 0.06, p = 0.94). However, there was a significant substance × condition interaction (F4,132 = 3.16, p = 0.016). Simple contrasts indicated that, compared with placebo, haloperidol was associated with significantly faster RTs to targets in the congruent relative to the unbiased condition (F1,33 = 4.71, p = 0.037). Moreover, facilitation of targets in the incongruent relative to the unbiased condition was increased at a trend level with L-Dopa compared with placebo (F1,33 = 2.35, p = 0.13). Follow-up pairwise comparisons showed that there were no differences among substances in the congruent and unbiased condition. However, RTs to incongruent triplets were faster at a trend level after administration of L-Dopa than haloperidol (t = 1.69, p = 0.11) or placebo (t = 1.76, p = 0.09; Fig. 1).
Fig. 1.
Mean response times for each condition, substance and stimulus onset asynchronies. L-Dopa = levodopa.
Analyses by condition and target type are described in Table 3. Condition had no significant effect on RTs in the case of placebo. Its main effect was significant with L-Dopa and haloperidol, but in opposite directions: for haloperidol, RTs decreased from incongruent to congruent triplets, whereas for L-Dopa the reverse effect was observed (Fig. 1). The RTs to dominant targets were significantly faster than those to subordinate targets with haloperidol. This effect was also apparent at a nonsignificant trend level with placebo, but not with L-Dopa.
Long SOA (750 ms)
The main analysis showed no significant main effects for substance (F1.7,54.5 = 0.95, p = 0.39) or condition (F1.7, 55.0 = 0.12, p = 0.88), nor a significant interaction (F4,32 = 1.0, p = 0.41). Analyses by condition and target type for each substance separately (Table 3) showed only a marginally significant effect of target type for haloperidol, with RTs for subordinate targets being faster than for dominant targets. All other main effects and interactions for all substances were nonsignificant. However, a linear trend of condition was observed for placebo, indicating a gradual RT acceleration from incongruent to unbiased to congruent targets (Fig. 1).
Discussion
The present study investigated the effects of L-Dopa and a DA antagonist on semantic priming in healthy individuals; to our knowledge, this is the first time the effects of a DA antagonist have been studied. The task used was designed to maximize the contribution of automatic lexical processes and consisted of a triplet priming paradigm that assessed RTs to pronunciation of ambiguous word associates in various contexts.
An important point to keep in mind when interpreting our results is that the paradigm we used did not include a completely neutral condition, as this would have led to an unacceptable number of condition permutations and/or target repetitions. Priming is expected to have occurred in all conditions, even in the unbiased one, since that too included a related prime–target word pair. Thus, the task did not assess absolute priming, but rather modulations in the relative magnitude of priming according to substance and condition.
Results were in the expected direction after administration of placebo. At the short SOA, there were no RT differences among the 3 conditions, indicating a similar amount of facilitation irrespective of whether the first prime was congruent, incongruent or unbiased to the target. This finding is consistent with those of previous studies that have investigated triplet priming under similar conditions (e.g., masked conditions,40 pronunciation task45). At the long SOA, we observed an RT acceleration trend from the incongruent to the unbiased to the congruent condition. This finding is also in accordance with previous studies investigating ambiguous word priming in conditions favouring controlled processes,38–41 although it was less pronounced in our study, probably because the priming paradigm was not optimal for investigating controlled processes.
At the short SOA, L-Dopa appeared to accelerate responses to targets following incongruent contexts. This finding strongly suggests an increase in automatic spreading of activation in the semantic network under L-Dopa. A contribution of controlled processes to the observed result is highly unlikely, since postlexical matching strategies are not thought to be involved in pronunciation tasks5,45 and expectancy-based priming is unlikely to occur at such short SOAs.5 Thus, this finding can be paralleled to observations of hyperpriming under automatic access conditions and for weak or indirect stimulus dimensions in patients with thought disorder,9–11,18–21 rendering dopaminergic hyperactivity a plausible basis for priming abnormalities in patients with schizophrenia.
On the other hand, acceleration of RTs in the congruent relative to the unbiased condition was more pronounced with haloperidol than with placebo. It is unclear whether these effects constitute a genuine modulation of congruent triplet priming or whether they reflect changes in unbiased triplet priming. For example, inspection of RTs after administration of haloperidol indicates that the significant difference between RTs in the unbiased and congruent condition may be due to decreased priming in the unbiased condition as much as to increased facilitation of targets in the congruent condition. Both of the above interpretations are compatible with the known effects of antipsychotics in normalizing formal thought disorder in patients with schizophrenia (see the 2 studies by Goldberg and colleagues46,47), and they are not mutually exclusive. However, the latter interpretation (i.e., enhanced facilitation of targets in congruent contexts under haloperidol) is more consistent with findings of previous studies in patients with schizophrenia, in whom treatment with antipsychotics increased direct semantic priming47 and led to partial restoration of the normal N400 priming effect,48 especially in automatic access conditions. This would indicate that haloperidol has a “focusing” effect on the automatic spreading of activation within the semantic network. However, since there were no differences between haloperidol and placebo in either the congruent or unbiased condition in the present study, further studies with inclusion of a neutral condition are needed for this claim to be confirmed.
The priming paradigm used in the present study was not optimal for the investigation of dopaminergic effects on controlled priming processes. Although some expectancy effects might be expected at the long SOA, the relatedness proportion was not particularly high; moreover, the inhibition effects observed in lexical decision tasks, which are of central importance in the case of incongruent triplets,39–41 have been suggested to be either nonexistent or weak in pronunciation tasks like the one we used.5 Thus, the absence of a significant condition × substance interaction at the long SOA should probably not be interpreted as evidence for the absence of an effect of L-Dopa or haloperidol on priming under controlled access conditions. However, this finding suggests that the effects of dopaminergic modulation on automatic processes are short-lived. This assumption is compatible with the results of a recent meta-analysis on priming performance in patients with schizophrenia, which showed that findings of hyperpriming apply only to short SOAs.18 It is also consistent with models postulating an accelerated but short-lived automatic spreading of activation in thought-disordered patients with schizophrenia.21
A final interesting point that deserves discussion is the effect of haloperidol and L-Dopa on targets depending on meaning frequency. Previous studies29,30 have reported reduced priming for subordinate targets of ambiguous words with L-Dopa; although this effect was obtained at short SOAs, it appeared to depend on controlled, attention-based processes.30 The present study could not replicate this effect, possibly owing to the very different design of the priming task used. Interestingly, however, there was evidence of exactly the opposite effect (i.e., increased priming for subordinate targets) at the long SOA after administration of haloperidol. Thus, our findings lend support to the view that dopaminergic activity does not modulate only context-driven meaning selection, but also the selection of semantic focus based on meaning frequency,30 with L-Dopa leading to an increased focus on dominant meanings. However, these effects appear to depend on controlled processes and might again be quite different under conditions of automatic access. In contrast to findings at the long SOA, haloperidol was associated with significantly faster RTs for dominant than subordinate targets at the short SOA. This effect was also apparent, although nonsignificant, for placebo (partial η2 = 0.7 for placebo v. 0.13 for haloperidol, both effects in the medium range49). In contrast, the difference between dominant and subordinate targets virtually disappeared in the case of L-Dopa (partial η2 = 0.015). Thus, at conditions of automatic access L-Dopa appeared to decrease and haloperidol appeared to increase frequency-based semantic focus; these effects are compatible with their proposed effects on the automatic spreading of activation we described.
Limitations
Certain limitations of the study need to be acknowledged. First, our sample was younger and had a higher level of education than typical samples of patients with schizophrenia. Second, we used a short SOA of 150 ms, such that even the interval between first prime and target was below the limits, beyond which controlled processes and inhibition occur (400 ms and 300 ms, respectively6). However, in patients with schizophrenia, hyperpriming ceases to exist at such “very short” SOAs,18 possibly because of perceptual processing abnormalities.9 Thus, although the investigation of healthy young participants under such “ideal” conditions might provide more accurate insights into the mechanisms governing priming, the generalizability of our findings to clinical populations should be confirmed in further studies.
Conclusion
Administration of L-Dopa, a dopaminergic agonist, led to increased priming for incongruent contexts and subordinate targets in a priming paradigm that maximized the contribution of automatic lexical processes. In contrast, the dopaminergic antagonist haloperidol was associated with increased priming for dominant targets. These findings are quite different than those obtained with L-Dopa under conditions of controlled access, suggesting that dopaminergic modulation has differential effects on the various processes involved in priming. Moreover, our findings are consistent with those of studies in patients with schizophrenia, lending further support to the view that aberrant automatic spreading of semantic activation underlies formal thought disorder in patients with schizophrenia.
Acknowledgments
We thank Anna Schildt and Aljoscha Rieger for their help with participant recruitment and testing. This work was partly supported by a grant to C. Andreou by the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG, Project-Nr: AN970/1-1).
Footnotes
Competing interests: None declared.
Contributors: C. Andreou, V. Bozikas and S. Moritz designed the study. K. Veith and T.M. Lincoln acquired the data, which C. Andreou analyzed. C. Andreou, K. Veith and S. Moritz wrote the article, which all authors reviewed and approved for publication.
References
- 1.Balota DA. Visual word recognition. In: Gernsbacher M, editor. Handbook of psycholinguistics. San Diego (CA): Academic Press; 1994. pp. 303–58. [Google Scholar]
- 2.Meyer DE, Schvaneveldt RW. Facilitation in recognizing pairs of words: evidence of a dependence between retrieval operations. J Exp Psychol. 1971;90:227–34. doi: 10.1037/h0031564. [DOI] [PubMed] [Google Scholar]
- 3.Collins AM, Loftus EF. A spreading-activation theory of semantic processing. Psychol Rev. 1975;82:407–28. [Google Scholar]
- 4.Neely HJ. Semantic priming and retrieval from lexical memory: role of inhibitionless spreading activation and limited-capacity attention. J Exp Psychol Gen. 1977;106:226–54. [Google Scholar]
- 5.Neely HJ. Semantic priming effects in visual word recognition: a selective review of current findings and theories. In: Besner D, Humphreys G, editors. Basic processes in reading: visual word recognition. Hillsdale (NJ): Erlbaum; 1991. pp. 264–336. [Google Scholar]
- 6.McNamara TP. Semantic priming: perspectives from memory and word recognition. New York (NY): Psychology Press; 2005. [Google Scholar]
- 7.Lecardeur L, Giffard B, Laisney M, et al. Semantic hyperpriming in schizophrenic patients: Increased facilitation or impaired inhibition in semantic association processing? Schizophr Res. 2007;89:243–50. doi: 10.1016/j.schres.2006.08.025. [DOI] [PubMed] [Google Scholar]
- 8.Moritz S, Mersmann K, Kloss M, et al. Enhanced semantic priming in thought-disordered schizophrenic patients using a word pronunciation task. Schizophr Res. 2001;48:301–5. doi: 10.1016/s0920-9964(00)00057-8. [DOI] [PubMed] [Google Scholar]
- 9.Moritz S, Mersmann K, Kloss M, et al. ‘Hyper-priming’ in thought-disordered schizophrenic patients. Psychol Med. 2001;31:221–9. doi: 10.1017/s0033291701003105. [DOI] [PubMed] [Google Scholar]
- 10.Spitzer M, Braun U, Hermle L, et al. Associative semantic network dysfunction in thought-disordered schizophrenic patients: direct evidence from indirect semantic priming. Biol Psychiatry. 1993;34:864–77. doi: 10.1016/0006-3223(93)90054-h. [DOI] [PubMed] [Google Scholar]
- 11.Spitzer M, Weisker I, Winter M, et al. Semantic and phonological priming in schizophrenia. J Abnorm Psychol. 1994;103:485–94. doi: 10.1037//0021-843x.103.3.485. [DOI] [PubMed] [Google Scholar]
- 12.Manschreck TC, Maher BA, Milavetz JJ, et al. Semantic priming in thought disordered schizophrenic patients. Schizophr Res. 1988;1:61–6. doi: 10.1016/0920-9964(88)90041-2. [DOI] [PubMed] [Google Scholar]
- 13.Aloia MS, Gourovitch ML, Missar D, et al. Cognitive substrates of thought disorder, II: specifying a candidate cognitive mechanism. Am J Psychiatry. 1998;155:1677–84. doi: 10.1176/ajp.155.12.1677. [DOI] [PubMed] [Google Scholar]
- 14.Barch DM, Cohen JD, Servan-Schreiber D, et al. Semantic priming in schizophrenia: an examination of spreading activation using word pronunciation and multiple SOAs. J Abnorm Psychol. 1996;105:592–601. doi: 10.1037//0021-843x.105.4.592. [DOI] [PubMed] [Google Scholar]
- 15.Ober BA, Vinogradov S, Shenaut GK. Automatic versus controlled semantic priming in schizophrenia. Neuropsychology. 1997;11:506–13. doi: 10.1037//0894-4105.11.4.506. [DOI] [PubMed] [Google Scholar]
- 16.Passerieux C, Segui J, Besche C, et al. Heterogeneity in cognitive functioning of schizophrenic patients evaluated by a lexical decision task. Psychol Med. 1997;27:1295–302. doi: 10.1017/s003329179700562x. [DOI] [PubMed] [Google Scholar]
- 17.Surguladze S, Rossell S, Rabe-Hesketh S, et al. Cross-modal semantic priming in schizophrenia. J Int Neuropsychol Soc. 2002;8:884–92. doi: 10.1017/s1355617702870023. [DOI] [PubMed] [Google Scholar]
- 18.Pomarol-Clotet E, Oh TM, Laws KR, et al. Semantic priming in schizophrenia: systematic review and meta-analysis. Br J Psychiatry. 2008;192:92–7. doi: 10.1192/bjp.bp.106.032102. [DOI] [PubMed] [Google Scholar]
- 19.Moritz S, Woodward TS, Kuppers D, et al. Increased automatic spreading of activation in thought-disordered schizophrenic patients. Schizophr Res. 2003;59:181–6. doi: 10.1016/s0920-9964(01)00337-1. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer M, Braun U, Maier S, et al. Indirect semantic priming in schizophrenic patients. Schizophr Res. 1993;11:71–80. doi: 10.1016/0920-9964(93)90040-p. [DOI] [PubMed] [Google Scholar]
- 21.Chenery HJ, Copland DA, McGrath J, et al. Maintaining and updating semantic context in schizophrenia: an investigation of the effects of multiple remote primes. Psychiatry Res. 2004;126:241–52. doi: 10.1016/j.psychres.2004.02.011. [DOI] [PubMed] [Google Scholar]
- 22.Kerns JG, Berenbaum H. Cognitive impairments associated with formal thought disorder in people with schizophrenia. J Abnorm Psychol. 2002;111:211–24. [PubMed] [Google Scholar]
- 23.Wilson LB, Rojas DC, Shatti S, et al. Greater neuronal responses during automatic semantic processing in schizophrenia. Neuroreport. 2013;24:212–6. doi: 10.1097/WNR.0b013e32835eb688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crow TJ. Positive and negative schizophrenia symptoms and the role of dopamine. Br J Psychiatry. 1981;139:251–4. doi: 10.1192/bjp.139.3.251. [DOI] [PubMed] [Google Scholar]
- 25.Stahl SM. Psychopharmacology of antipsychotics. London (UK): Martin Duntz; 1999. [Google Scholar]
- 26.Angwin AJ, Chenery HJ, Copland DA, et al. Dopamine and semantic activation: an investigation of masked direct and indirect priming. J Int Neuropsychol Soc. 2004;10:15–25. doi: 10.1017/S1355617704101033. [DOI] [PubMed] [Google Scholar]
- 27.Kischka U, Kammer T, Maier S, et al. Dopaminergic modulation of semantic network activation. Neuropsychologia. 1996;34:1107–13. doi: 10.1016/0028-3932(96)00024-3. [DOI] [PubMed] [Google Scholar]
- 28.Roesch-Ely D, Weiland S, Scheffel H, et al. Dopaminergic modulation of semantic priming in healthy volunteers. Biol Psychiatry. 2006;60:604–11. doi: 10.1016/j.biopsych.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 29.Copland DA, Chenery HJ, Murdoch BE, et al. Dopamine enhances semantic salience: semantic priming evidence from healthy individuals. Brain Lang. 2003;87:103–4. [Google Scholar]
- 30.Copland DA, McMahon KL, Silburn PA, et al. Dopaminergic neuromodulation of semantic processing: a 4-T FMRI study with levodopa. Cereb Cortex. 2009;19:2651–8. doi: 10.1093/cercor/bhp017. [DOI] [PubMed] [Google Scholar]
- 31.Minzenberg MJ, Ober BA, Vinogradov S. Semantic priming in schizophrenia: a review and synthesis. J Int Neuropsychol Soc. 2002;8:699–720. doi: 10.1017/s1355617702801357. [DOI] [PubMed] [Google Scholar]
- 32.Arnott WL, Copland DA, Chenery HJ, et al. The influence of dopamine on automatic and controlled semantic activation in Parkinson’s disease. Parkinson’s Disease. 2011;2011:157072. doi: 10.4061/2011/157072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kelly C, de Zubicaray G, Di Martino A, et al. L-dopa modulates functional connectivity in striatal cognitive and motor networks: a double-blind placebo-controlled study. J Neurosci. 2009;29:7364–78. doi: 10.1523/JNEUROSCI.0810-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kapur S, Zipursky R, Jones C, et al. Relationship between dopamine D(2) occupancy, clinical response, and side effects: a double-blind PET study of first-episode schizophrenia. Am J Psychiatry. 2000;157:514–20. doi: 10.1176/appi.ajp.157.4.514. [DOI] [PubMed] [Google Scholar]
- 35.Kapur S, Zipursky R, Roy P, et al. The relationship between D2 receptor occupancy and plasma levels on low dose oral haloperidol: a PET study. Psychopharmacology (Berl) 1997;131:148–52. doi: 10.1007/s002130050277. [DOI] [PubMed] [Google Scholar]
- 36.Hardman JG, Limbird LE. Goodman & Gilman’s the pharmacological basis of therapeutics. 10th ed. New York: McGraw-Hill; 2001. [Google Scholar]
- 37.Brickenkamp R. Test d2 Aufmerksamkeits-Belastungs-Test. 7th ed. Göttingen, Toronto, and Zürich: Verlag für Psychologie (Hogrefe); 1981. [Google Scholar]
- 38.Schvaneveldt RW, Meyer DE, Becker CA. Lexical ambiguity, semantic context, and visual word recognition. J Exp Psychol Hum Percept Perform. 1976;2:243–56. doi: 10.1037//0096-1523.2.2.243. [DOI] [PubMed] [Google Scholar]
- 39.Bullen JG, Hemsley DR. Schizophrenia: A failure to control the contents of consciousness? Br J Clin Psychol. 1987;26:25–33. doi: 10.1111/j.2044-8260.1987.tb00720.x. [DOI] [PubMed] [Google Scholar]
- 40.Marcel AJ. Conscious and preconscious recognition of polysemous words: locating the selective effects of prior verbal context. In: Nickerson RS, editor. Attention and performance VIII. Hillsdale (NJ): Erlbaum; 1980. [Google Scholar]
- 41.Moritz S, Mersmann K, Quast C, et al. Association norms for 68 German homonyms. Z Exp Psychol. 2001;48:226–38. [PubMed] [Google Scholar]
- 42.Wenke D. Inhibitory processes in the resolution of lexical ambiguity [MSc thesis] Berlin: TU Berlin; 1998. [Google Scholar]
- 43.Max Planck Institute for Psycholinguistics. WebCelex. Nijmegen (NL): The Institute; 2001. [accessed 2013 Sep 26]. Available: http://celex.mpi.nl/ [Google Scholar]
- 44.Schneider W, Eschman A, Zuccolotto A. E-prime user’s guide. Pittsburgh (PA): Psychology Software Tools; 2002. [Google Scholar]
- 45.Balota DA, Paul ST. Summation of activation: evidence from multiple primes that converge and diverge within semantic memory. J Exp Psychol Learn Mem Cogn. 1996;22:827–45. doi: 10.1037//0278-7393.22.4.827. [DOI] [PubMed] [Google Scholar]
- 46.Goldberg TE, Weinberger DR. Thought disorder, working memory and attention: interrelationships and the effects of neuroleptic medications. Int Clin Psychopharmacol. 1995;10(Suppl 3):99–104. doi: 10.1097/00004850-199509000-00013. [DOI] [PubMed] [Google Scholar]
- 47.Goldberg TE, Dodge M, Aloia M, et al. Effects of neuroleptic medications on speech disorganization in schizophrenia: biasing associative networks towards meaning. Psychol Med. 2000;30:1123–30. doi: 10.1017/s0033291799002639. [DOI] [PubMed] [Google Scholar]
- 48.Condray R, Siegle GJ, Cohen JD, et al. Automatic activation of the semantic network in schizophrenia: evidence from event-related brain potentials. Biol Psychiatry. 2003;54:1134–48. doi: 10.1016/s0006-3223(03)00699-1. [DOI] [PubMed] [Google Scholar]
- 49.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale (NJ): Laurence Erlbaum Association; 1988. [Google Scholar]