Abstract
Heparin plays an important role in many biological processes, via its interaction with various proteins, and hydrogels and nanoparticles comprising heparin exhibit attractive properties such as anticoagulant activity, growth factor binding, as well as antiangiogenic and apoptotic effects, making them great candidates for emerging applications. Accordingly, this review summarizes recent efforts in the preparation of heparin-based hydrogels and formation of nanoparticles, as well as the characterization of their properties and applications. The challenges and future perspectives for heparin-based materials are also discussed. Prospects are promising for heparin-containing polymeric biomaterials in diverse applications ranging from cell carriers for promoting cell differentiation to nanoparticle therapeutics for cancer treatment.
Keywords: Heparin, Hydrogels, Nanoparticles, Tissue engineering, Drug delivery
1. Introduction
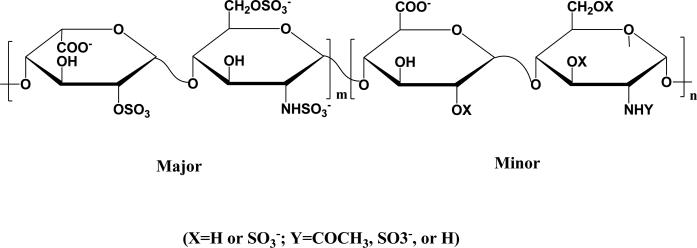
Heparin is a linear polysaccharide which consists of 1→4 linked disaccharide repeating units of uronic acid and glucosamine residues (Fig. 1). Discovered nearly 100 years ago, heparin has been clinically employed as blood anticoagulant since 1935; this activity is a result of its ability to bind to the serine protease inhibitor antithrombin, causing the inhibitor to inactivate thrombin [1, 2]. Unfractionated heparin is usually isolated from natural tissues such as porcine intestine or bovine lung, with an average molecular weight of about 15 kDa, and is highly heterogeneous in chemical structure and molecular weight.
Fig. 1.
Major (ca. 85%) and minor (ca. 15%) disaccharide sequences of heparin.
The prevalence of sulfate and carboxylate groups endows heparin with a high negative charge (approximately −75), which mediates its electrostatic interactions with many proteins, such as growth factors, proteases and chemokines [3]. These interactions in many cases serve to stabilize proteins or increase their affinity for cell receptors [4]; such stabilization by heparin of the growth factors fibroblast growth factor (FGF) and vascular endothelial growth factor (VEGF) has thus been employed in the design of materials as engineered scaffolds for tissue regeneration and controlled release platforms for growth factor delivery [5].
Given its function as an anticoagulant, heparin can exhibit undesirable side effects such as hemorrhagic complications, heparin-induced thrombocytopenia (HIT) and/or low bioavailability when administered non-intravenously [6]. As a result, low molecular weight heparin (LMWH), which has a better defined chemical composition, has been developed to provide more predictable anticoagulant dose, longer half-lives, and reduced side effects [7]. Many studies have indicated that LMWH is more effective in the inhibition of tumor growth via its regulation of the binding of many angiogenic growth factors (e.g. FGF and VEGF), when compared with unfractionated heparin. LMWH can also interfere with tumor metastasis by reducing the activity of heparanase and thus reducing tumor metastatic potential and growth, or by competing with P-selectin binding, thereby inhibiting the adhesion of tumor cells [8, 9]. More recent research has suggested that heparin can also interact with transcription factors to induce apoptotic cell death [10]. Therefore, LMWH has been widely explored as a component in tumor-targeted delivery systems, and a few chemically modified LMWH derivatives, such as LMWH-deoxycholic acid (DOCA), have been developed with reduced anticoagulant activity and high antiangiogenic efficacy [11, 12]. Recent research has also suggested that LMWH may be potentially used as an anti-fibrotic agent in patients with chronic hepatitis B [13].
Given the pharmacokinetics difficulties posed by heparin's chemical heterogeneity and diversity of biological activities, heparin-mimetic compounds such as negatively-charged polymers and sulfonate-modified peptides have been exploited [14-19]. It has been demonstrated that sulfonated polymers such as poly(styrenesulfonate-co-PEG methacrylate) and peptide amphiphile nanofibers (composed of heparin-mimetic peptides decorated with bioactive chemical groups such as sulfonate, carboxylic acid, and hydroxyl groups) have better-defined structures, more specific bioactivity, and are capable of stabilizing growth factors and promoting angiogenesis. Additionally, some polysulfonated heparin mimics such as poly(2-acrylamido-2-methyl-1-propane sulfonic acid), poly(anetholesulfonic acid), poly(4-styrenesulfonic acid) and polymethacrylic derivatives of 5-amino-2-naphthalenesulfonic acid are found to be potent inhibitors of angiogenesis. They can potentially modulate angiogenic processes with low toxicity based on the interaction between the sulfonic acid groups and FGF, preventing FGF-induced endothelial cell migration and proliferation [20-22]. The use of these heparin mimics may reduce the risk associated with batch-to-batch variation and impurities of heparin.
Due to its advantageous biological activities, the incorporation of heparin in biomaterials has been highly attractive. Heparin is often physically encapsulated or covalently conjugated to hydrogels to provide sustained release of the anticoagulant [23]. Also, heparin-containing hydrogels have been widely used for the sequestration and controlled release of growth factors to promote angiogenesis and bone regeneration [24, 25]. Heparin-functionalized, naturally derived hydrogels have been developed by taking advantage of their biocompatibility, low toxicity, relatively low cost, and benign gelation conditions while heparin-synthetic polymer biohybrid hydrogels have been studied to provide increased control over their mechanical and chemical properties [26]. Additionally, surfaces and scaffolds modified with heparin have been explored to suppress non-specific protein absorption and localize growth factors to promote cell attachment and proliferation [27-29].
In clinical application, most of the scaffolds and hydrogels either need to be implanted through a surgical intervention or require injection into the pathological site. The former mode of administration is inconvenient and poses risks to patients while materials administered via the latter route can be limited in scope of use owing to the fact that many pathological sites are not easily accessible via injection. To address this concern, nanoparticle-based approaches, which render all administration routes possible, have been developed. Nanoparticles pass through capillary vessels with prolonged circulation half-lives in the blood stream and can also penetrate cells and tissue barriers to reach target organs [30]. Heparin-functionalized nanocarriers can both stabilize the growth factors against denaturation or proteolysis and provide controlled release behaviors, but also promote therapeutic efficacy by increasing the cellular uptake of the delivered molecules [31-34].
Nanoparticulate drug delivery systems have demonstrated great promise in cancer nanotechnology due to their ability to accumulate and reside in tumor tissues via the enhanced permeation and retention (EPR) effect. Their surface versatility permits conjugation of a broad range of biomolecules to enhance targeting and therapeutic efficacy [35, 36]. Given the increasing appreciation of the antiangiogenic and apoptotic effects of heparin, heparin-based nanoparticles have been widely explored for cancer therapy as well.
In this review, recent progress in the development of heparin-based hydrogels and nanoparticles is systematically summarized, with an emphasis on those aiming at tissue engineering and drug delivery applications. Types of polymeric networks (crosslinking strategies and stimuli-responsiveness) and the formation of the nanoparticles (physical and chemical synthesis methods) are introduced and some of the important applications for these types of materials are highlighted. Perspectives on the challenges and promise of heparin-based materials are also discussed.
2. Heparin Based Hydrogels
Heparin-based hydrogels have been increasingly employed as antithrombogenic materials, growth factor carriers and scaffolds for tissue regeneration via covalent or non-covalent strategies. In addition, several chemically labile crosslinks have been explored to temporally regulate hydrogel degradation in response to environmental stimuli, to encourage the development of tissue-like structures and achieve on-demand or targeted delivery.
2.1 Physically crosslinked heparin hydrogels
Physical crosslinking occurs as a result of entanglements between dynamic macromolecular species or due to specific non-covalent interactions between polymer chains. Typically, the motifs employed in the formation of physically crosslinked hydrogels include hydrogen bonding, multivalent ionic, metal-ligand, and host-guest interactions, and stereocomplexation [37]. Physical hydrogels avoid the addition of toxic crosslinking reagents, which might potentially affect the integrity and bioactivity of encapsulated therapeutic agents. In addition, physically crosslinked hydrogels permit injection due to their shear-thinning capability and the facile re-establishment of the noncovalent bonds [38].
Among the various physical crosslinking mechanisms, biomimetic interactions, including the heparin-protein interaction, have been used widely in the formation of physically crosslinked heparin hydrogels. Several heparin-binding peptides (HBPs) such as those derived from antithrombin III (ATIII), heparin interacting protein (HIP) and human platelet factor 4 (PF4ZIP) have been exploited for the non-covalent assembly of hydrogel networks. Based on the fact that growth factors including bFGF (basic fibroblast growth factor), VEGF (vascular endothelial growth factor), and HGF (hepatocyte growth factor), can bind to short sequences in heparin, multivalent species that incorporate the heparin-binding domains of growth factors could also be potentially employed as multifunctional crosslinkers in the formation of physical networks [39].
In the earliest studies of physically crosslinked, heparin-containing hydrogels, Panitch and co-workers employed the non-covalent interaction between heparin and multi-arm poly(ethylene glycol) (PEG) star polymers functionalized with heparin-binding peptides (HBPs) [40-43]. Similarly, our group later employed star PEGs functionalized with low molecular weight heparin (LMWH) for interaction with various HBP-functionalized PEGs to form non-covalent assembled hydrogels [44-46]. Since then, multiple other approaches have been developed toward the production of noncovalently crosslinked, heparin-containing materials.
2.1.1 Protein- or peptide-heparin crosslinked hydrogels
Recently, Tan and co-workers have prepared nano-fibrous hydrogels via the non-covalent assembly of LMWH-modified hyaluronic acid (HA) derivatives and HIP-functionalized star PEG copolymers [47]. The hydrogels have a sponge-like morphology and display a continuous, porous and nano-fibrous structure with fiber sizes of 50~70 nm, as observed via SEM. The HA-LMWH/PEG-HIP hydrogels were non-cytotoxic and capable of promoting proliferation and differentiation of adipose-derived stem cells (ASCs) over 14 days of culture. This work introduces a novel idea of applying extracellular matrix (ECM)-derived polysaccharide in the preparation of protein or peptide mediated assembly of heparin hydrogels.
Although proteins and peptides have been widely exploited in the assembly of non-covalent heparin-containing matrices, the mechanism and prerequisite of hydrogel formation still remain unclear. Recently, Werner and co-workers have studied the key properties of a minimal heparin-interacting motif required for the non-covalent peptide-mediated assembly of physical hydrogels [48]. Various peptides were synthesized based the repetition of the (BA)n and (BG)n motifs (where B is a basic residue, either arginine or lysine, A is alanine and G is glycine). (BA)n peptides tend to form α-helical structures while (BG)n peptides, which have the same charge density as (BA)n, do not form helices owing to the disruption of the helix by the flexible glycine residues. The PEG-peptide conjugates were subsequently synthesized via Michael-type addition reactions between cysteine-terminated peptides and maleimide-terminated 4-arm PEG. Hydrogel formation was tested by the mixing of heparin with a variety of star PEG-peptide conjugates, in which the amino acid sequences on the peptides were altered. Particularly, star PEGs modified with the peptides CWGGRARARARARARARA (RA7), CWGGKAKAKAKAKA, (KA5), or CWGGKAKAKAKAKAKAKA (KA7) formed stable hydrogels with heparin. The gels exhibited a wide range of shear storage moduli which could be further tuned by changing the concentrations of heparin and the peptide-star PEG conjugate. These studies suggest that the formation of non-covalent peptide-heparin hydrogels is dependent not only on the presence of positive charges on the peptides, but also on the ability of the peptide to adopt an α-helix peptide conformation; this assertion was supported by the observation that the gels disassembled upon treatment with TFE (trifluoroethanol). The studies also suggested that while charge densities do not necessarily influence the mechanical strength of the non-covalent network, that the mechanical strength of the hydrogels is proportional to the number of (BA)n repeats on the peptide motif. Interestingly, hydrogels formed by KA7-PEG hydrogels in which the L-lysines and the L-alanines were replaced by corresponding the D-amino acids still showed mechanical properties similar to those of the L-hydrogels, providing potential opportunities for producing protease-resistant gels [49]. The screening of peptide−polymer conjugates for their ability to form hydrogels with heparin provides insight into the structure-function relationships of the assembled matrix and allows production of novel, non-covalent hydrogel systems with a broad range of tunability.
2.1.2 Growth factor crosslinked hydrogels
Although heparin-peptide interactions have been used for the assembly of non-covalent hydrogels and for growth factor sequestration and delivery, we have employed growth factors directly as crosslinks to yield cell surface receptor-responsive hydrogels via the crosslinking of PEG–LMWH with the dimeric heparin-binding growth factor VEGF [50]. The hydrogels were formed immediately in situ by the mixing of low-viscosity solutions of PEG-LMWH and dimeric VEGF, as confirmed via optical tweezer microrheology by an increase in elastic modulus upon the addition of VEGF. The hydrogels demonstrated receptor-mediated erosion and sustained release of VEGF in the presence of VEGF receptors (VEGFR-2) with a total release of approximately 80% over a 10-day time period. The selective cell-mediated erosion of the hydrogel networks and the activation of the target receptor by the release of VEGF were further studied using VEGFR-2 overexpressing PAE/KDR cells (porcine aortic endothelial cells (PAE)) equipped with the transcript for the kinase insert domain receptor (KDR)) [51]. The release of VEGF and hydrogel erosion reached 100% only in the presence of PAE/KDR, which again illustrates the receptor responsiveness of the hydrogels. (Fig. 2) These studies offer a novel potential mechanism for targeted delivery and erosion via the release of protein therapeutic crosslinkers in response to cell surface receptors.
Fig 2.
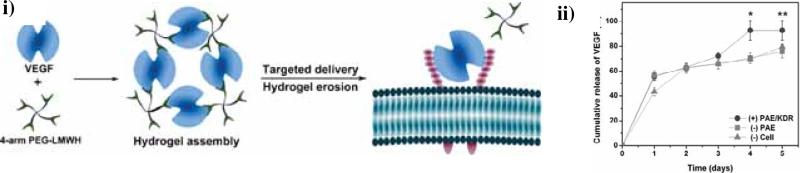
Cell-mediated delivery and targeted erosion of growth factor-crosslinked hydrogels. i) Schematic of non-covalently assembled hydrogel formed by the crosslinking of polysaccharide-derivatized star copolymers by dimeric, heparin-binding growth factors, followed by receptor-mediated erosion. ii) The release profile of VEGF from non-covalently assembled [PEG-LMWH/VEGF] hydrogels. Release profiles of VEGF in the presence of PAE/KDR (●) or PAE (■) cells, and in the absence of cells (▲), respectively. *p<0.002; **p<0.004. Reprinted with permission from [48], copyright (2010) John Wiley and Sons.
2.2 Chemically crosslinked heparin hydrogels
Although physically crosslinked hydrogels can provide improved cytocompatibility due to the absence of toxic initiators, they often suffer from poor mechanical properties. The covalent bonds in chemically crosslinked hydrogels yield hydrogel networks that are generally more resistant to mechanical forces and can be easily tuned to adequately alter the mechanical properties of the final materials for specific biomedical applications. Among the numerous coupling reactions used for the formation of covalent networks, photo-induced radical polymerization, Michael-type addition, amide coupling reactions, and enzyme-catalyzed reactions have been widely exploited in the synthesis of chemically crosslinked heparin hydrogels [52].
2.2.1 Photo-crosslinked hydrogels
Photo-induced polymerization allows in situ hydrogel formation with spatial and temporal control. Anseth and co-workers modified heparin with methacrylate groups and copolymerized it with dimethacrylated poly-(ethylene glycol) (PEG) to obtain heparin-functionalized PEG hydrogels [53]. The heparin--functionalized PEG hydrogels were capable of sequestering bFGF and releasing it in a controlled manner over a 5-week time period, with zero-order release kinetics after an initial burst. The bFGF-loaded hydrogels demonstrated enhanced stimulation of the growth of human mesenchymal stem cell (hMSC) compared to the controls. The potential of heparin-modified gels as a scaffold for osteogenic differentiation of hMSCs was further investigated by utilizing the materials as a three-dimensional culture system for human mesenchymal stem cells (hMSCs) [54]. It is indicated that the heparin-functionalized hydrogels support hMSC viability as quantified through live/dead imaging and induce osteogenic differentiation which is measured by increased alkaline phosphatase (ALP) production and osteopontin (OPN) and collagen I (COL I) gene expression over 5 weeks. Similarly, methacrylated heparin has been incorporated into various networks such as poly-(vinyl alcohol) (PVA) and alginate via photo-induced radical popolymerization to allow affinity-based growth factor delivery [55, 56].
Recently, Netti and co-workers have developed porous PEG-heparin hydrogels as a 3-D matrix with sustained release of VEGF to promote angiogenesis [57]. The hydrogel precursors were modified with methacrylate groups and photo crosslinked along with a foaming process to cause the formation of a porous network. The VEGF was released from the hydrogels in a controlled manner, with only 34% released over 13 days. The released VEGF was demonstrated to induce the proliferation of human umbilical vein endothelial cells (HUVECs), as well as capillary network formation in vitro. The hydrogels loaded with VEGF showed a more than 2-fold increase in the induction of angiogenesis in vivo, in comparison to PEG hydrogels loaded with fresh VEGF, after implantation on a chorioallantoic membrane model. Together, these works demonstrate the utility of incorporating heparin into covalent networks to promote cell adhesion, proliferation and differentiation as well as sustained local delivery of growth factors.
2.2.2 Hydrogels formed via Michael-type addition reactions
Michael-type addition is extensively used as a crosslinking strategy for injectable hydrogels, because the reaction occurs in aqueous medium, at room temperature, and at physiological pH. Accordingly, our group has designed covalently crosslinked PEG-heparin hydrogels via the Michael-type addition between thiolated PEGs and maleimide-functionalized heparin [58]. These materials showed a broad range of tunability in the rate of formation and mechanical properties by varying polymer concentration, composition and heparin functionality. The hydrogels also demonstrated the potential for controlled release of bFGF over various timescales for different wound-healing and vascular therapies. The material's abilities to modulate cell responses were further studied using human aortic adventitial fibroblasts (AoAF) in 2-D cell culture experiments [59]. Hydrogels with different mechanical properties (13.7 kPa, 5.2 kPa and 0.3kPa) were subsequently employed to study cellular responses of three human vascular cell types (human aortic adventitial fibroblasts (AoAF), human umbilical endothelial cells (HUVEC) and human aortic smooth muscle cells (T/G HA-VSMC)) [60], showing that the different cell types behave differently on the surfaces of PEG-LMWH hydrogels with different mechanical properties. Particularly, AoAF and T/G HA-VSMC demonstrated preferential growth and attachment respectively on the highest-modulus hydrogel while HUVEC demonstrated preferential growth on the lowest-modulus hydrogel. These types of materials are suggested to provide opportunities not only in controlled release, but also offer insight in tailoring and optimizing the biochemical and mechanical properties of matrices for improved responses of multiple cell types in cardiovascular tissue engineering.
In another example, Tae and co-workers have developed an injectable heparin-based hydrogel system via the Michael-type addition between thiol-derivatized heparin and PEG diacrylates under physiologically relevant buffer conditions [61, 62]. The hydrogels showed tunable gelation kinetics and mechanical properties by controlling crosslinking density; sustained release of human growth hormone (hGH) was observed over two weeks. Primary rat hepatocytes were encapsulated in the heparin-based hydrogels by mixing the cells with polymer solutions [63]. Immunochemical analysis of the cell culture medium revealed higher levels of albumin and urea synthesis after three weeks in culture when compared to PEG-only control gels. Recently, the potential of these heparin-based hydrogels to serve as vehicles for cell transplantation and matrices for induced cell differentiation and tissue regeneration was also evaluated using de-differentiated chondrocytes [64]. Chondrocytes cultured in the heparin-based hydrogels without addition of any exogenous growth factors or chondrogenic components exhibited effective re-differentiation and production of expected glycosaminoglycans (GAGs) and extracellular matrices (ECMs) proteins within a week. Similarly, efficient re-differentiation of cells and cartilage formation in the de-differentiated cell/hydrogel construct were also observed in vivo after subcutaneous implantation on the back of nude mice, confirmed by the up-regulated expressions of Type II collagen and aggrecan and the completely suppressed expression of Type I collagen (as assessed through analysis of real-time PCR and immunohistological staining). Additionally, excellent cartilage regeneration, in partial-thickness defects in the rabbit knee, was observed after the delivery of de-differentiated chondrocytes using the heparin-based hydrogel. The chondrogenic nature of these heparin-based hydrogels may be attributed to their ability to sequester endogenous growth factors secreted from the chondrocytes. These studies suggest the promise of utilizing heparin-based hydrogels as matrices for cell encapsulation and cultivation of some difficult-to-culture cells, as well as injectable carriers for cell differentiation and induced cartilage regeneration.
In addition to the synthetic polymer materials comprising poly-(ethylene glycol) (PEG) and poly-(vinyl alcohol) (PVA), hydrogel networks composed of naturally derived polymers have also been modified with heparin [65, 66]. Heparin, along with other GAGs or natural polymers such as hyaluronic acid (HA), chondroitin sulfate (CS) and gelatin (Gtn), were modified with thiol groups and therefore formed various covalent networks via the Michael-type reaction with PEG-diacrylate crosslinkers. These hydrogels were further demonstrated to stimulate localized angiogenic responses in vivo in a mouse model, which suggests their potential in specific diseased tissue/organ angiogenesis [67].
2.2.3 Amide coupling for crosslinking of hydrogels
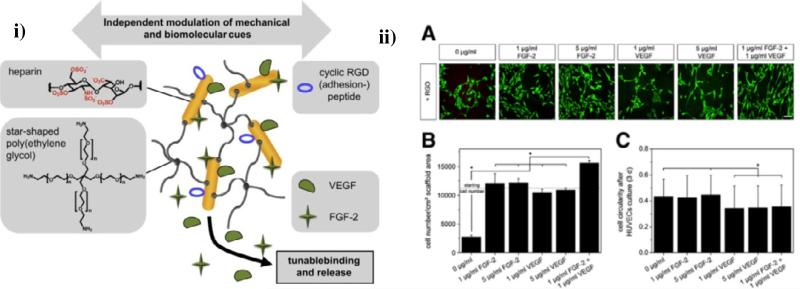
A 3-D heparin-containing network was recently developed by Werner and co-workers via the crosslinking of amino end-functionalized star-PEG with EDC/sulfo-NHS-activated carboxylic acid groups of heparin [68-70]. The hydrogels were modified with cell-adhesion-mediating RGD peptides via the EDC/sulfo-NHS activation of the carboxylic acid groups of heparin (Fig. 3i). Pro-angiogenic growth factors such as FGF-2 and VEGF were non-covalently sequestered in the star PEG-heparin networks to permit combined delivery of the two factors, based on the observed improvement of the formation of stable blood vessels with the dual GF delivery [70], due to the fact that different factors are known to act at distinct stages of vascular development [71]. In experiments with human umbilical vein endothelial cells (HUVECs), the combination of both growth factors delivered by the hydrogels significantly increased cell survival, proliferation, differentiation and migration compared to experiments in which single cytokines were delivered, (Fig. 3ii) and vascularization was also improved, as confirmed in a chicken embryo chorioallantoic membrane (CAM) model. These star PEG-heparin hydrogels were also employed for sustained release of stromal cell-derived factor-1α (SDF-1α) to promote the localized accumulation of early endothelial progenitor cells (eEPCs) [72, 73]. More recently, the Werner group has designed macroporous scaffolds with adaptable mechanical and biomolecular properties using the EDC/sulfo-NHS crosslinking between amine-terminated star-PEG and heparin, combined with cryogenic treatment of the gel-forming reaction mixture, followed by lyophilization [74]. These materials showed sponge-like morphology and high permeability due to their interconnected pore structure, allowing HUVECs in culture to migrate into the cryogels. These works demonstrate the advantageous structural characteristic as well as tunable physical and biomolecular properties of the star PEG-heparin hydrogels, suggesting their use as multi-factor delivery vehicles and cell carriers for clinical applications in tissue regeneration. It would be valuable to study the temporally staged delivery of multiple growth factors and their influence on blood vessel formation compared to simultaneous delivery.
Fig. 3.
Dual growth factor delivery from star PEG-heparin hydrogels. i) Design of star poly(ethylene glycol)–heparin hydrogels showing decoupled mechanical and modular biomolecular characteristics. ii)Interactions of differently modified hydrogels on the different substrates. (A): representative fluorescence microscopy images after live/dead staining of HUVECs (scale bar 130 μm); (B): HUVECs proliferation/survival as accessed via cell numbers quantified by an MTT assay; (C): HUVECs morphology as accessed via cell circularity by the circularity calculation within ImageJ. Reprinted with permission from [66], copyright (2010) Elsevier and [67], copyright (2011) Elsevier.
2.2.4 Enzyme-mediated crosslinked hydrogels
Dijkstra and co-workers prepared heparin-containing injectable hydrogels by horseradish peroxidase (HRP)-mediated crosslinking of dextran-tyramine (Dex-TA) and heparin-tyramine (Hep-TA) conjugates [75]. These materials revealed tunable gelation kinetics (ca. 30-350s) that were achieved by varying the HRP concentration, and adjustable mechanical properties (G’, 3.6 to 48 kPa) that were achieved by changing the composition of the hydrogels (heparin content, 100 to 0 wt%, respectively). The incorporation of heparin into the hydrogels greatly increased the hydrogel swelling, facilitating nutrient transport for cell culture. The encapsulation of bovine chondrocytes into these gels promoted enhanced chondrocyte viability, proliferation, and matrix production, when compared to control hydrogels composed of Dex-TA conjugates alone, demonstrating the potential of Dex–TA/Hep–TA hydrogels as matrices for cartilage tissue engineering. Horseradish peroxidase (HRP)-mediated chemical crosslinking has also been used to prepare in situ forming heparin hydrogel surfaces for blood-compatible coating of metallic biomaterials [76], with significantly enhanced blood compatibility and reduced fibrinogen absorption and platelet adhesion.
2.3 Stimuli-responsive heparin hydrogels
Stimuli-responsive hydrogels undergo significant physical and/or chemical changes, such as swelling, shrinkage, degradation or sol-gel phase transition, in response to environmental stimuli. In recent years, a wide variety of chemically labile bonds have been exploited to regulate hydrogel degradation in response to environmental stimuli such as pH, enzyme and light, in order to provide a mechanism for triggered release of therapeutic molecules. Although the field of stimuli-responsive heparin hydrogels is still in early stages, several strategies have been developed. Some of the first environmentally sensitive heparin-based hydrogels showed reversibly temperature-responsive rheological behavior by the application of a combination of covalent and non-covalent crosslinking strategies [41-43]. Since then, multiple other strategies have been developed, as detailed below.
2.3.1 Enzymatically responsive hydrogels
Heparin, as a naturally derived glycosaminoglycan (GAG), is degraded by heparanase, which is a hydrolase found in human platelets at high levels. Other sources of heparanase include endothelial cells (EC) and smooth muscle cells (SMC). Heparanase cleaves heparin chains at the 3-O-sulfated glucosamine, diminishing heparin's anticoagulation properties while increasing its FGF-2 signaling activity. Additionally, other enzymes such as heparinase and heparitinase, are also found to be capable of degrading heparin by cleaving it into di- or tri-saccharide units [77].
Whitelock and co-workers have observed the enzymatic degradation of heparin/PVA hydrogels by incubating the hydrogels with platelet extract (PE) [78]. The PE, which contains heparanase, was found to be capable of releasing covalently crosslinked heparin fragments from the hydrogels. Through analysis of proliferation of BaF3 cells and plasma clotting time, the release of the heparin was confirmed by increased in proliferation index and plasma clotting time in PE-treated hydrogels supernatant compared with those of non-PE treated hydrogels. This heparin/PVA hydrogel system provides insight on the potential effects of heparinized hydrogel scaffolds when they are applied to a site of injury. Owing to potential degradation by enzymes, the stability of heparin in vivo can be a challenge for heparin-containing materials, which can be addressed by employing heparin-mimetic materials that are inactive to enzymatic degradation [16-19, 21, 22].
A commonly employed alternative mechanism to design enzymatically degradable hydrogels is to incorporate peptides, which may be cleaved by cell-produced proteases, into the hydrogel networks. A wide range of peptide motifs are degraded by matrix metalloproteinases (MMPs) and can be readily incorporated into hydrogels [79-83]. Michael-type addition reactions are perhaps the most commonly used strategy for the conjugation of enzyme-cleavable peptide sequences, via the reaction of a multifunctional polymer (e.g. PEG-acrylates or PEG-vinyl sulfones) with thiols from the cysteine residues of the peptides [84]. Such approaches can also be easily employed with heparin-containing materials, as well [42, 43, 85-87].
Likewise, Werner and co-workers have prepared enzymatically degradable PEG-heparin gels by including a matrix metalloproteinase (MMP)-cleavable peptide sequence into the hydrogel crosslinks [85]. The MMP-cleavable peptide NH2–GPQGIWGQC–CONH2 was conjugated to star-PEG polymers via Michael-type addition and then a 3-D network was formed via the coupling of the carboxylate groups of heparin (EDC/sNHS chemistry) to the N-terminal amine groups of the PEG-peptide conjugates. Degradation of the hydrogel network was triggered by the action of bacterial collagenase IV, as confirmed via UV-Vis spectroscopy by the characteristic absorption of the tryptophan residue of the cleaved peptides in the supernatant of the gels. Bifunctional peptide crosslinkers for the PEG-heparin hydrogels were designed by combining MMP-sensitive and RGD cell adhesive modules [86] into a single peptide (GGRGDGPQGIWGQGGCG–CONH2 (MMP-RGD)) and then incorporated into the hydrogel networks as covalent crosslinkers. The resulting hydrogels were demonstrated to stimulate cellular remodeling and three-dimensional migration of human endothelial cells. Recently, PEG-heparin hydrogels with two-tier degradation profiles were developed by combining MMP-sensitive motifs and hydrolytically labile ester linkages via the Michael-type addition between the acrylate groups of star PEG-polymers and cysteine residues in the peptide [87]. With better control over the rate of hydrogel degradation by these two mechanisms, it was shown that gels with accelerated degradation strongly enhanced the invasion of human endothelial cells and exhibited increased proangiogenic response both in in vitro culture (endothelial cells (ECs)) and in a chicken embryo chorioallantoic membrane (CAM) model. The incorporation of matrix metalloproteinase (MMP)-sensitive peptides into hydrogel networks not only permits cell-mediated degradation and matrix remodeling for engineering tissues such as bone and vascular structures, but also offers opportunities for controlled delivery of molecules in diseases where protease activity is altered, such as cancer, rheumatoid arthritis and after myocardial infarction [84]. Furthermore, MMP-sensitive matrices can also serve as cell carriers that permit cellular morphogenesis, and therefore encourage the formation of a variety of tissue structures.
2.3.2 Glutathione (GSH)-responsive hydrogels
Glutathione (GSH) is a thiol-containing tripeptide localized in cellular compartments or tumor microenvironments, which has motivated the use of reduction-sensitive bonds, such as disulfide linkages, in the production of hydrogels. Given the greatly increased intracellular concentration of GSH (ca. 2–10mM in cells versus 2–20μM in plasma), GSH-sensitive hydrogels remain stable outside of cells, while they are rapidly degraded in intracellular compartments; targeted intracellular delivery of drugs is thus possible [88-92]. The reduction of disulfide bonds can suffer from limited control of cleavage kinetics (half-lives ranging from 8 to 45 min), which can temporally limit the therapeutic effects of the biomolecules encapsulated in these materials.
Although thiol-maleimide Michael-type reactions have long been employed for the in situ formation of hydrogels owing to the stability of the adducts, our group has exploited the little-appreciated reversibility of these Michael-type reactions in the presence of GSH [93]. Select maleimide-thiol adducts synthesized from the Michael-type addition were shown to undergo retro and exchange reactions in the presence of GSH at physiologically relevant pH and temperature. In initial investigations, small-molecule adducts were formed by the Michael-type addition, to N-ethylmaleimide (NEM), of various thiols (4-mercaptophenylacetic acid (MPA), N-acetylcysteine, or 3-mercaptopropionic acid (MP)). The adducts incubated with glutathione showed half-lives of the retro reaction ranging from 20 to 80 h with extents of conversion varied from 20% to 90%. Adducts formed with the arylthiol showed the greatest rate and extent of conversion of the retro reaction, with the MPA-NEM adduct showing conversion of nearly 85% after 70 h. The N-acetylcysteine-NEM conjugate showed much slower kinetics and substantially lower conversion, while the MP-NEM adduct exhibited almost no activity in the retro reaction (Fig. 4i). The rate of the exchange reaction was suggested, on the basis of these experiments, to be dependent on the pKa of the Michael donors (thiols), with a higher thiol pKa correlating with a decreased rate, likely owing to the poorer Michael-donor properties of the thiol.
Fig. 4.
GSH-responsive PEG-LMWH hydrogels via reversible maleimide–thiol Michael-type addition. i)Conversion of retro-Michael adducts formed with Michael donors of different reactivity. ii) Schematic representation of the formation and degradation of GSH-responsive PEG-LMWH hydrogels. iii) Comparison of storage moduli for select degrading hydrogels: PEG–SH hydrogel (★) LMWH–PEG–MPP (●) and –DMMPP (■) under high reducing conditions (10mM GSH) and LMWH–PEG–MPP (○) under standard reducing conditions (10μM GSH). Reprinted with permission from [90], copyright (2011) American Chemical Society and [91], copyright (2013) The Royal Society of Chemistry.
The observed differences in the rate of the retro-reaction offer opportunities to tune degradation rates to achieve long term delivery of drugs in reducing environments, with rates of degradation that are intermediate between those of disulfide- and ester-based strategies. The potential use of these labile chemical linkages in biomedical applications was thus further explored in hydrogels comprising maleimide-functionalized low molecular weight heparin (LMWH) and various thiol-functionalized poly(ethylene glycol) (PEG) star polymers [94] (Fig. 4ii). Specifically, mercaptopropionic acid (MP), mercaptoisobutyric acid (MIB), 4-mercaptophenylpropionic acid (MPP) and 2,2-dimethyl-3-(4-mercaptophenyl)propionic acid (DMMPP) were used to modify four-arm, hydroxyl-functionalized PEG to yield thiolated four-arm PEGs of various Michael-donor activity. The arylthiol derivatives have lower pKa values than those of the alkylthiols (6.6 vs. 10.3) and should thus form hydrogels that exhibit greater rates of degradation via the retro reaction.
Characterization of the degradation of and LMWH release from the hydrogels further confirmed the GSH sensitivity of the arylthiol adducts in these materials. Gels prepared with arylthiol-modified PEGs (PEG–MPP and PEG–DMMPP) showed significantly more rapid degradation under conditions similar to those in intracellular compartments and tumor microenvironments (10mM GSH) when compared to the rate of degradation under reducing conditions in blood circulation (10mM GSH) (Fig. 4iii). The PEG-LMWH hydrogels produced by the thiol-maleimide Michael-type reactions exhibited increased stability against reducing agents than disulfide-crosslinked hydrogels, with 10-fold slower rates of degradation; these results suggesting the potential of the Michael adducts in providing alternative options for drug delivery over longer timescales than those afforded by disulfide-linked hydrogels. These strategies could be applied to a broad range of biomaterials for matrix remodeling in tissue engineering applications and targeted/controlled delivery of therapeutics to pathological sites where GSH is overproduced (e.g. up to 10-fold greater levels of GSH in tumors than that in adjacent normal tissues) [95].
3. Heparin Based/Modified Nanoparticles
Over the past few decades, nanotechnology has made significant impact on the development of drug delivery systems. Many types of nanoparticles have been developed such as lipid-based carriers, polymeric nanostructures including polymer-drug conjugates, block copolymer micelles and nanogels, and inorganic nanoparticles. Compared to conventional drug delivery vehicles, nanoparticulate systems can enhance therapeutic efficacy by improving the solubility of hydrophobic drugs, prolonging circulation half-life, reducing potential immunogenicity, and releasing drugs in a sustained or stimuli-triggered fashion [96-98]. Particularly, nanoscale particles can actively and passively target specific tissues (e.g. tumors), on the basis of their surface functionalization with specific ligands and the enhanced permeability and retention (EPR) effect, respectively. Furthermore, metallic nanoparticles provide opportunities for magnetic resonance imaging (MRI) or photothermal therapy in addition to drug delivery applications, while nanogels introduce better biocompatibility and enhanced cellular uptake due to their gel-like structures [99-102].
With the appreciation and exploitation of the biological properties of heparin, heparin based/modified nanoparticles have become of increased interest owing to the advantages resulting from their nanoscale dimension and biological activities as described above. Heparin has been conjugated to the surface of metallic nanoparticles such as those iron oxide and gold nanoparticles to prevent self-aggregation, improve stability and increase cellular uptake, as well as to provide targeted detection and induce cellular apoptosis [103-105]. For heparin-functionalized polymeric nanoparticles, heparin has been used to coat nanoparticle surfaces and also as a building block of the nanoparticle. As heparin-protein interactions and anti-tumor effects continue to be of interest, heparin based/modified polymeric nanoparticles have been widely studied as therapeutic delivery vehicles in the fields of tissue engineering and cancer therapy.
3.1 Nanoparticles for growth factor delivery
A major challenge in both hydrogel and nanoparticulate systems for growth factor delivery is protein stability. Encapsulation of growth factors in nanoparticles (e.g. PLGA) synthesized by emulsion methods usually results in disruption of the secondary and tertiary structures of the protein due to protein-organic solvent and protein-polymer hydrophobic interactions. Compared to simple physical entrapment of growth factors, the protein affinity offered by heparin-based/modified nanoparticles may yield increased protein stability and provide more controllable sustained release behaviors. This improvement may obviate the need to load the protein during nanoparticle synthesis, which could eliminate concerns regarding the potential denaturation and aggregation of protein in the presence of organic solvent.
3.1.1 Heparin-coated nanoparticles
Strategies commonly used in surface functionalization of nanoparticles are covalent coupling, noncovalent attachment (including electrostatic interaction), and physical encapsulation [106]. Tae and co-workers have reported heparin-coated nanoparticles that are composed of biodegradable hydrophobic cores (PLGA) and hydrophilic surface layers (Pluronic® F-127), with heparin physically entrapped near the surface of the particle, via a solvent-diffusion emulsion method and high-speed centrifugation [32]. These nanoparticles showed an average diameter ranging from 156nm to188nm, depending on the amount of heparin used, as indicated by DLS (dynamic light scattering). The presence of heparin on the particle surface was indicated by the increased negative zeta potential value and the presence of heparin activity in an anti-factor Xa assay. The heparin content at the surface layer of the resulting nanoparticles varied from 1.9% to 4.0% depending on the amount of heparin used, as determined via characterization of the heparin on the surface of the particles versus that recovered after disruption of the nanoparticles. These nanoparticles exhibited controlled release of VEGF, without initial burst, for over a month. Subsequently, the heparin-functionalized PLGA nanoparticles were combined with fibrin gels to form composite structures that enhanced the sustained release of the growth factors, as well as improved cell proliferation and migration [107].
3.1.2 Heparin-containing nanoparticles
The high negative charge density of heparin has been exploited to form nanoparticles based on electrostatic interaction. Kipper and co-workers have designed polyelectrolyte complex nanoparticles (PCN) consisting of chitosan-heparin and chitosan-hyaluronan polyelectrolyte pairs [108, 109]. The PCNs were prepared by the addition of polycation/polyanion solutions to the oppositely charged polymer solution. These heparin-containing polyelectrolyte complex nanoparticles were then combined with nanostructured chitosan-based electrospun fibers with high porosity for the binding, stabilization and controlled release of FGF-2 [110]. The heparin-containing PCN/fiber complex released FGF-2 in a sustained manner with zero-order kinetics over a 30-day time period, and exhibited mitogenic activity on ovine bone marrow-derived mesenchymal stem cells, suggesting opportunities for such growth factor sequestration and controlled delivery based on polysaccharide-containing, matrix-mimetic nanomaterials.
Similarly, Tan and co-workers have prepared polysaccharide nanoparticles that are non-covalently assembled via the electrostatic interaction between N,N,N–trimethyl chitosan chloride (TMC) and LMWH [33]. These LMWH/TMC nanoparticles displayed spherical morphology with a number-average size of 146.5 nm. Controlled release of VEGF from these polysaccharide nanoparticles was observed with reduced burst release and a total cumulative release of about 49% after 14-day incubation in a zero-order fashion. The VEGF release rate was further decreased by integrating the VEGF-loaded polysaccharide nanoparticles into a nanofibrous HA–PEG hydrogel network formed via the interaction of streptavidin-functionalized HA and biotin-terminated four-arm PEG; these composite materials exhibited a total cumulative release VEGF of approximately 34% over two weeks.
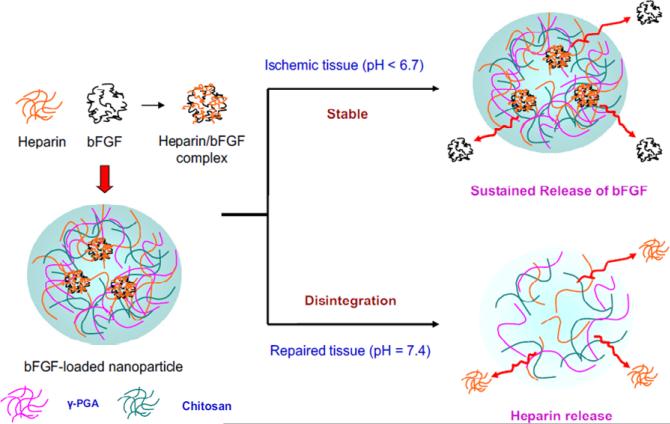
In another example, Mi, Sung and co-workers developed heparin-functionalized chitosan (CS)/poly(γ-glutamic acid) (γ-PGA) nanoparticles (Heparin-CS/g-PGA nanoparticles) for multi-functional delivery of fibroblast growth factor (bFGF) and heparin [111]. The nanoparticles self-assembled from electrostatic interaction between the anionic γ-PGA and heparin, with the cationic chitosan, and thus were sensitive to variations in pH (Fig. 5). The nanoparticles were stable at pH=6.0 and gradually swelled without disintegration at pH 6.6, but quickly disintegrated at pH=7.4, as was confirmed via TEM and an increase in light transmission. The disintegration of the particles was attributed to the loss of electrostatic interaction, which was suggested by measurements of zeta potential. The heparinized nanoparticles sequestered bFGF and controlled its release at physiological pH values, at which the nanoparticles were intact, but released heparin upon disintegration of the nanoparticles at the pH of repaired tissue (pH=7.4); these properties prevent blood vessel rethrombosis. The controlled release of bFGF from the nanoparticles significantly enhanced the proliferation of human foreskin fibroblasts (HFF) and effectively stimulated tube formation of human umbilical vein endothelial cells (HUVEC) (1.5 fold increase at 24h when compared to effects of bFGF alone). The heparin released as a result of the pH-induced disintegration of the nanoparticles was demonstrated to maintain its anticoagulant property, as indicated by the presence of anti-factor Xa activity in blood plasma. This nanoparticulate system provides a novel example of heparin-based nanoparticles for multi-functional controlled delivery of therapeutic molecules for ischemic tissue regeneration and prevention of harmful clots. The stability of the system needs to be further studied under normal circulation in vivo, to ensure that they do not degrade significantly at physiological pH during transport of the nanoparticles from their site of introduction to their molecular site of action.
Fig. 5.
Schematic of polyelectrolyte self-assembly of heparinized CS/γ-PGA nanoparticles (HP-CS/γ-PGA NPs) and release of bFGF or heparin from the nanoparticles, depending on the environmental pH. Reprinted with permission from [108], copyright (2010) Elsevier.
In addition to natural polymers such as chitosan, well-defined cationic block copolymers synthesized via reversible addition-fragmentation chain-transfer (RAFT) polymerization have also been recently developed as building blocks for heparin-containing nanoparticles based on electrostatic interaction [112, 113]. Cationic block copolymers such as poly(methyl methacrylate-b-trimethyl aminoethyl methacrylate) and poly[(oligo(ethylene glycol) methyl ether methacrylate-b- 2-(methacryloyloxy)ethyl trimethyl ammonium chloride] were utilized to complex with negatively-charged heparin molecules, demonstrating tunable properties depending on the block copolymer composition. Such well-defined synthetic cationic polymers prepared by controlled/living radical polymerization offer a novel strategy in formulating heparin-based nanoparticles based on electrostatic interactions.
3.1.3 Heparin-containing hydrogel particles
In addition to the physical incorporation of heparin into nanomaterials, Jia and co-workers have synthesized heparin (HP)-decorated, hyaluronic acid (HA)-based hydrogel particles [34]. The HA was modified with heparin and then formed into nanoparticles via an inverse emulsion polymerization technique; these particles were employed as vehicles for growth factor delivery in hydrogel matrices. The particles exhibited spherical morphology and showed a bimodal size distribution (~1μm and 70nm). Due to the affinity of growth factors for the heparin, these particles demonstrated a high loading capacity for bone morphogenetic protein-2 (BMP-2) and released the protein in a controlled manner over 13 days with tunable release kinetics; variation of the heparin composition of the particles permitted variation in the release rates. The BMP-2 released from the particles, combined with the bioactivity of HA molecules, induced robust and consistent chondrogenic differentiation of murine mesenchymal stem cells, as indicated by up-regulation of the mRNA levels of chondrogenic markers and the production of cartilage-specific extracellular matrix components; such hydrogel particle approaches may thus be promising for cartilage repair and regeneration.
3.2 Nanoparticles for cancer therapy
In recent years, there has been increasing interest in the antitumor effects of heparin and in harnessing these activities in nanomaterials as a chemotherapeutic approach. Heparin itself could serve as a chemotherapeutic owing to its antianiogenic and apoptotic effects. Heparin can also function as a targeting moiety in such approaches, by taking advantage of its high binding affinity to angiogenic growth factors, which are often overexpressed in tumor tissues, and its internalization in dividing endothelial cells [114, 115].
3.2.1 Heparin-coated nanoparticles
Tae and co-workers have reported heparin-functionalized poly (lactide-co-glycolide) (PLGA)-based nanoparticles for improved tumor-targeting efficacy and cellular internalization via the interaction of heparin with cell-surface receptors [116]. Heparin was introduced to the nanoparticles surface via its covalent conjugation to Pluronic® F127; the inclusion of heparin was intended for the targeting of dividing vascular endothelial cells, which are abundant in tumor tissues. The nanoparticles were synthesized via nanoprecipitation/solvent diffusion methods. Although the morphology of these nanoparticles was not reported, the heparin-coated PLGA nanoparticles showed a diameter of 144nm and zeta potential value of −50mV (in deionized water). They were also shown to effectively enhance in vitro cellular uptake when incubated with normal fibroblast (NIH/3T3) and tumor cells (SCC7). In an in vivo tumor model study on SCC7 tumor-bearing athymic mice, a 2.2-fold increase in the accumulation of heparin-functionalized PLGA nanoparticles in the tumor was observed compared to that of a non-heparin-functionalized control particle, suggesting a limited, but positive effect of heparin surface functionalization on tumor targeting.
3.2.2 Heparin-based nanogels
Park and co-workers have also developed a novel self-assembled heparin-Pluronic® nanogels for the intracellular delivery of RNase A [117]. Heparin was covalently conjugated to carboxylated Pluronic® via EDC/NHS coupling chemistry and the polymer thus formed self-assembled nanostructures (~30-90 nm in diameter), via hydrophobic interactions, in aqueous solution. Heparin-protein interactions were also exploited in these studies to improve cellular internalization of the nanoparticles. RNase A was incorporated into the heparin-Pluronic® nanogels via electrostatic interactions during nanogel formation. The mean diameter of the nanogels decreased significantly (from ca. 90 to 30 nm) and the surface of the nanogels became more negative as the ratio of RNase A was increased. Since the pKa values of the sulfonate group of heparin and of RNase A are 1.9 and 9.0 respectively, the electrostatic interaction between the nanogel and RNase were stronger under acidic conditions, which led to slower release kinetics at pH 5.0 compared to those at pH 7.4. These nanogels exhibited high loading efficiency of RNase A (>78%) and demonstrated effective cellular uptake and nucleus penetration when incubated with HeLa cells, although they exhibited an irregular morphology. Subsequently, the heparin-Pluronic® nanogels were loaded with paclitaxel and DNase for combined chemotherapy [118]. The combined delivery of paclitaxel and DNase by the nanogels, over a 24 hour period, exhibited a dose-dependence and synergistic cytotoxicity when compared to the effects of single drug and free-drug treatment, as determined by the MTT assay.
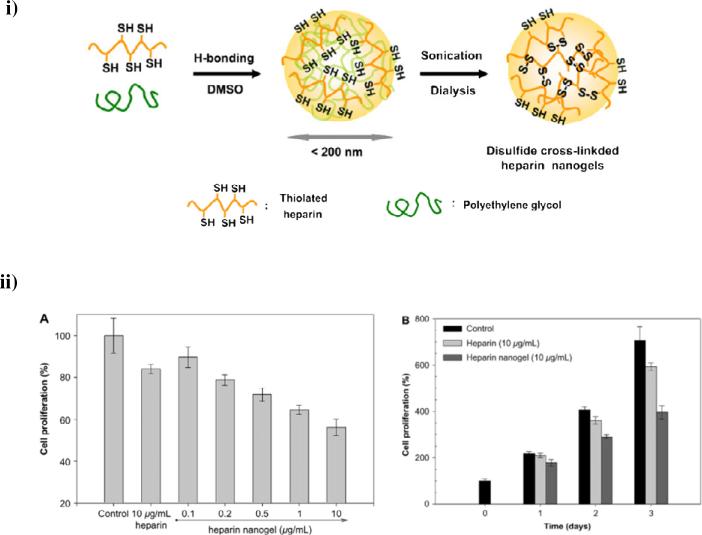
In addition to self-assembled heparin-based nanogels, Park and co-workers have synthesized covalently crosslinked heparin nanogels in order to induce apoptotic cell death [119]. The heparin was chemically modified with thiol groups and then crosslinked via disulfide linkages in organic solvent under sonication to form reduction-sensitive heparin nanogels (Fig. 6). These materials showed a spherical morphology with an average diameter of ca. 191nm and exhibited sustained release of free heparin in a GSH-concentration-dependent fashion, due to degradation of the disulfide crosslinked nanogels, with almost 65% of the heparin released at 5mM GSH while less than 3% of heparin was released in the absence of GSH over 150 hrs. The heparin nanogels significantly inhibited the proliferation of mouse melanoma B16F10 cells by inducing caspase-mediated apoptotic cell death, with a ca. 44% reduction in the cell growth for nanogels at a concentration of 10 μg/mL compared to ca. 12% for free heparin alone (Fig. 6ii). The apoptotic effect of these heparin nanogels was further determined via confocal laser scanning microscopy and flow cytometry analysis. The cells incubated with the heparin-containing nanogels displayed strong purple fluorescence when incubated with a fluorogenic substrate for caspases 3 and 7 (proteases essential for apoptosis in mammalian cells). Quantitative analysis of apoptosis was performed via flow cytometry after propidium iodide staining of the cell nuclei; apoptotic cells have lower DNA content within their nuclei. These studies indicated a remarkable increase of the sub-G1 cell population (with DNA content of less than diploid) was observed in cells treated with heparin nanogels (31.5%) vs. that of untreated cells (6.1%). Such heparin-based nanocarrier systems could induce effective apoptotic activity under a reducing cellular microenvironment and potentially be employed as tumor-targeted delivery vehicles for cancer therapeutics and diagnostics.
Fig. 6.
Intracellular delivery of reducible heparin nanogels for apoptotic cell death. i) Synthetic scheme of disulfide crosslinked heparin nanogels. ii) (A) Dose-dependent anti-proliferative effect of heparin nanogels against mouse melanoma B16F10 cells after 3 days; (B) Growth profiles of B16F10 cells cultured in the presence of heparin or heparin nanogels. Reprinted with permission from [114], copyright (2008) Elsevier.
3.2.3 Self-assembled heparin-based nanoparticles
With abundant carboxyl (COO−) and sulfate (OSO3−) groups along the heparin chain, heparin is highly hydrophilic and thus could be potentially used as hydrophilic segments to form self-assembled core/shell nanoparticle structure in aqueous solution when conjugated with hydrophobic molecules. Xiang and co-workers have synthesized amphiphilic heparin-paclitaxel conjugates as anti-cancer drug delivery systems by using different amino acids as spacers [120]. The heparin was succinylated via an O-acylation reaction with succinic anhydride to increase the number of carboxyl groups before its conjugation to paclitaxel via amino acid linkers. The self-assembled nanoparticles, consisting of a hydrophobic paclitaxel core and a hydrophilic heparin shell, displayed a spherical shape with mean diameters of 140-180 nm. Paclitaxel released from these heparin-paclitaxel conjugates exhibited increased cytotoxicity after 48h, compared to free paclitaxel, for MCF-7 human breast cancer cells. The anticoagulant activity of these prodrugs was decreased significantly compared to that of heparin, likely through a reduction of binding to antithrombin III, therefore reducing the potential side effects with the use of heparin such as hemorrhagic complications. Subsequently, Yao and co-workers have developed taxol-loaded heparin-PEG-folate nanoparticles for improved cellular uptake efficiency [121]. The succinylated heparin was conjugated with PEGylated folate via EDC/NHS coupling to endow tumor-targeting capability to the self-assembled nanoparticles. These nanoparticles showed a spherical shape with mean diameters of ca. 165 and 190 nm for nanoparticles with PEG1000 and PEG3000 spacers, respectively. These materials revealed significantly increased cellular uptake via folate-receptor-mediated endocytosis, which was suggested by the increased uptake of the nanoparticles by KB-3-1 cells (a cell line that overexpresses folate receptor) compared to those incubated with A549 cells (a folate receptor-deficient cell line), suggesting their potential as targeted delivery vehicles.
In another example, heparin was conjugated with poly(β-benzyl-L-aspartate) (PBLA) to afford amphiphilic copolymers that were further modified with folate–poly(ethylene glycol) to allow targeted delivery of doxorubicin [122]. These materials self-assembled into well-defined nanoparticles with a spherical shape and average diameters of ca. 105 and 135 nm, with the larger particle size arising from copolymers with a higher PBLA content. The zeta potentials of these nanoparticles were negative owing to the presence of heparin in the outer shells, with values of −24 and −22 mV, suggesting their long-term stability in the physiological environment; nanoparticles incubated in PBS showed no significant changes in size over 14 days. The DOX sequestered in the nanoparticles by its hydrophobic interaction with the PLBA cores at pH 7.4 was selectively released under acidic conditions, likely owing to the pH-dependent solubility of DOX. The DOX-loaded heparin-PLBA nanoparticles with folate-targeting ligands, compared with folate-free nanoparticles, showed enhanced cellular uptake and increased targeting capacity, as indicated via confocal laser scanning microscopy (CLSM) of treated KB cells; assessment of proliferation via MTT assays also indicated the increased cytotoxicity of the targeted nanoparticles for folate-receptor-expressing KB cells. In contrast, both types of nanoparticles exhibited similar cytotoxic effects when incubated with a folate-receptor deficient A549 cell line.
In a related approach, Gu and co-workers have synthesized heparin-doxorubicin conjugates as pH-sensitive drug delivery vehicles for cancer therapy [123]. Azido-functionalized heparin was dendronized with a low-generation dendron via Cu(I)-catalyzed azide-alkyne cycloaddition (CuAAC) and then covalently modified with doxorubicin (DOX) via pH-sensitive hydrazine linkages. The dendronized heparin-doxorubicin (heparin-DOX) conjugates self-assembled into well-defined nanoparticles with an average hydrodynamic diameter of ~90nm and zeta potential of −35 mV; they also showed increased release of DOX at pH=5.0 compared to that at pH=7.4. These nanoparticles demonstrated significantly increased antitumor efficacy and antiangiogenesis effects in a mouse 4T1 breast cancer tumor model in vivo, as assessed through measurements of tumor weights, tumor growth curves and immunohistochemical assessment, compared to results for free DOX and saline controls. Taken together, these studies illustrate the potential for exploiting the hydrophilicity and high negative charge density of heparin in preparing self-assembled core-shell nanoparticles with improved colloidal stability and antitumor activities in vivo.
4. Conclusions and Perspectives
Owing to the biological and chemical versatility of heparin, heparin-based hydrogels and nanoparticles have significant potential in a variety of biomedical applications; indeed, the use of heparin in hydrogels and nanoparticles has demonstrated advantageous biocompatibility and therapeutic efficacy, which could have significant implications in various applications including growth factor delivery, cell carriers, and cancer therapeutics. The abundant functional groups in heparin offer opportunities for conjugation of a wide range of biomolecules to improve biocompatibility and therapeutic efficacy, promoting cell adhesion, enabling cell-mediated proteolytic degradation, and permitting sequestration and controlled delivery of growth factors. The benefit of attaching targeting ligands to heparin-based nanoparticles has been demonstrated in the improved cellular uptake and therapeutic efficacy of functionalized nanoparticulate systems.
However, many challenges remain. Only few studies have been reported with an aim of developing heparin-based materials with more specific bioactivities and reduced side effects; integration of the extensive work on the biological activity of specific heparin isoforms [124-126] could significantly benefit biomaterials-related investigations, as the targeting and antitumor roles of heparin could be more extensively exploited in materials design. The use of other stimuli such as light and mechanical force, for triggered action of heparin-based hydrogels, would permit improved spatial and temporal control of hydrogel degradation, which could, for growth-factor-sequestering gels, also yield concomitant benefits of manipulating local release of cytokines, stimulating matrix production by cells, and encouraging tissue regeneration [127, 128]. Recent advances in mechanical measurements, such as microrheology, could also be brought to bear on these materials, providing a rapid and straightforward method for generating rheological libraries of hydrogelation conditions over a large composition parameter space. Such approaches would be of great value in the identification of hydrogel assembly and efficient optimization of heparin-containing materials for therapeutic applications [129, 130]. The development of a broader range of heparin-based biomaterials, including nanopatterned materials and particle-hydrogel complexes, would also further expand potential applications and impact of these materials. As such approaches are developed for clinical use, materials must be designed to maintain desired stability in vivo while being effectively eliminated from the body without formation of undesired metabolites. As these challenges are addressed, heparin-based biomaterials are likely to have an increasingly significant impact on the treatment of various diseases and damaged tissues.
Acknowledgments
Related work in the author's laboratories has been supported in part by the Nemours Foundation, the National Science Foundation (DGE-0221651), and the National Institutes of Health (P20-RR017716, P20-RR016472). The contents of this manuscript are the sole responsibility of the authors and do not necessarily reflect the views of the Nemours Foundation, NSF, or NIH.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Capila I, Linhardt RJ. Heparin - protein interactions. Angew Chem Int Ed. 2002;41:391–412. doi: 10.1002/1521-3773(20020201)41:3<390::aid-anie390>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 2.Linhardt RJ. 2003 Claude S. Hudson Award address in carbohydrate chemistry. Heparin: structure and activity. J Med Chem. 2003;46:2551–64. doi: 10.1021/jm030176m. [DOI] [PubMed] [Google Scholar]
- 3.Rabenstein DL. Heparin and heparan sulfate: structure and function. Nat Prod Rep. 2002;19:312–31. doi: 10.1039/b100916h. [DOI] [PubMed] [Google Scholar]
- 4.Gospodarowicz D, Cheng J. Heparin protects basic and acidic FGF from inactivation. J Cell Physiol. 1986;128:475–84. doi: 10.1002/jcp.1041280317. [DOI] [PubMed] [Google Scholar]
- 5.Kharkar PM, Kiick KL, Kloxin AM. Designing degradable hydrogels for orthogonal control of cell microenvironments. Chem Soc Rev. Advance Article. 2013 doi: 10.1039/c3cs60040h. DOI: 10.1039/C3CS60040H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Freedman MD. Pharmacodynamics, clinical indications, and adverse-effects of heparin. J Clin Pharmacol. 1992;32:584–96. doi: 10.1002/j.1552-4604.1992.tb05765.x. [DOI] [PubMed] [Google Scholar]
- 7.Xu YM, Masuko S, Takieddin M, Xu HM, Liu RP, Jing J, et al. Chemoenzymatic synthesis of homogeneous ultralow molecular weight heparins. Science. 2011;334:498–501. doi: 10.1126/science.1207478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Norrby K, Ostergaard P. Basic-fibroblast-growth-factor-mediated de novo angiogenesis is more effectively suppressed by low-molecular-weight than by high-molecular-weight heparin. Int J Microcirc. 1996;16:8–15. doi: 10.1159/000179145. [DOI] [PubMed] [Google Scholar]
- 9.Sasisekharan R, Shriver Z, Venkataraman G, Narayanasami U. Roles of heparan-sulphate glycosaminoglycans in cancer. Nat Rev Cancer. 2002;2:521–8. doi: 10.1038/nrc842. [DOI] [PubMed] [Google Scholar]
- 10.Linhardt RJ. Heparin-induced cancer cell death. Chem Biol. 2004;11:420–2. doi: 10.1016/j.chembiol.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 11.Cho KJ, Moon HT, Park GE, Jeon OC, Byun Y, Lee YK. Preparation of sodium deoxycholate (DOC) conjugated heparin derivatives for inhibition of angiogenesis and cancer cell growth. Bioconjugate Chem. 2008;19:1346–51. doi: 10.1021/bc800173m. [DOI] [PubMed] [Google Scholar]
- 12.Park JW, Jeon OC, Kim SK, Al-Hilal TA, Jin SJ, Moon HT, et al. High antiangiogenic and low anticoagulant efficacy of orally active low molecular weight heparin derivatives. J Control Release. 2010;148:317–26. doi: 10.1016/j.jconrel.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Lee JH, Lee H, Joung YK, Jung KH, Choi JH, Lee DH, et al. The use of low molecular weight heparin-pluronic nanogels to impede liver fibrosis by inhibition the TGF-beta/Smad signaling pathway. Biomaterials. 2011;32:1438–45. doi: 10.1016/j.biomaterials.2010.10.023. [DOI] [PubMed] [Google Scholar]
- 14.Christman KL, Vazquez-Dorbatt V, Schopf E, Kolodziej CM, Li RC, Broyer RM, et al. Nanoscale growth factor patterns by immobilization on a heparin-mimicking polymer. J Am Chem Soc. 2008;130:16585–91. doi: 10.1021/ja803676r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kolodziej CM, Kim SH, Broyer RM, Saxer SS, Decker CG, Maynard HD. Combination of integrin-binding peptide and growth factor promotes cell adhesion on electron-beam-fabricated patterns. J Am Chem Soc. 2012;134:247–55. doi: 10.1021/ja205524x. [DOI] [PubMed] [Google Scholar]
- 16.Nguyen TH, Kim S-H, Decker CG, Wong DY, Loo JA, Maynard HD. A heparin-mimicking polymer conjugate stabilizes basic fibroblast growth factor. Nat Chem. 2013;5:221–7. doi: 10.1038/nchem.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sangaj N, Kyriakakis P, Yang D, Chang CW, Arya G, Varghese S. Heparin mimicking polymer promotes myogenic differentiation of muscle progenitor cells. Biomacromolecules. 2010;11:3294–300. doi: 10.1021/bm101041f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mammadov R, Mammadov B, Toksoz S, Aydin B, Yagci R, Tekinay AB, et al. Heparin mimetic peptide nanofibers promote angiogenesis. Biomacromolecules. 2011;12:3508–19. doi: 10.1021/bm200957s. [DOI] [PubMed] [Google Scholar]
- 19.Mammadov R, Mammadov B, Guler MO, Tekinay AB. Growth factor binding on heparin mimetic peptide nanofibers. Biomacromolecules. 2012;13:3311–9. doi: 10.1021/bm3010897. [DOI] [PubMed] [Google Scholar]
- 20.Presta M, Leali D, Stabile H, Ronca R, Camozzi M, Coco L, et al. Heparin derivatives as angiogenesis inhibitors. Curr Pharm Design. 2003;9:553–66. doi: 10.2174/1381612033391379. [DOI] [PubMed] [Google Scholar]
- 21.García-Fernández L, Aguilar MaR, Fernández MaM, Lozano RM, Giménez G, Román JS. Antimitogenic polymer drugs based on AMPS: monomer distribution–bioactivity relationship of water-soluble macromolecules. Biomacromolecules. 2010;11:626–34. doi: 10.1021/bm901194e. [DOI] [PubMed] [Google Scholar]
- 22.García-Fernández L, Halstenberg S, Unger RE, Aguilar MR, Kirkpatrick CJ, San Román J. Anti-angiogenic activity of heparin-like polysulfonated polymeric drugs in 3D human cell culture. Biomaterials. 2010;31:7863–72. doi: 10.1016/j.biomaterials.2010.07.022. [DOI] [PubMed] [Google Scholar]
- 23.Baldwin AD, Robinson KG, Militar JL, Derby CD, Kiick KL, Akins RE. In situ crosslinkable heparin-containing poly(ethylene glycol) hydrogels for sustained anticoagulant release. J Biomed Mater Res A. 2012;100A:2106–18. doi: 10.1002/jbm.a.34050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fujita M, Ishihara M, Simizu M, Obara K, Ishizuka T, Saito Y, et al. Vascularization in vivo caused by the controlled release of fibroblast growth factor-2 from an injectable chitosan/non-anticoagulant heparin hydrogel. Biomaterials. 2004;25:699–706. doi: 10.1016/s0142-9612(03)00557-x. [DOI] [PubMed] [Google Scholar]
- 25.Nillesen STM, Geutjes PJ, Wismans R, Schalkwijk J, Daamen WF, van Kuppevelt TH. Increased angiogenesis and blood vessel maturation in acellular collagen-heparin scaffolds containing both FGF2 and VEGF. Biomaterials. 2007;28:1123–31. doi: 10.1016/j.biomaterials.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 26.Li YL, Rodrigues J, Tomas H. Injectable and biodegradable hydrogels: gelation, biodegradation and biomedical applications. Chem Soc Rev. 2012;41:2193–221. doi: 10.1039/c1cs15203c. [DOI] [PubMed] [Google Scholar]
- 27.Rohman G, Baker SC, Southgate J, Cameron NR. Heparin functionalisation of porous PLGA scaffolds for controlled, biologically relevant delivery of growth factors for soft tissue engineering. J Mater Chem. 2009;19:9265–73. [Google Scholar]
- 28.Hudalla GA, Koepsel JT, Murphy WL. Surfaces that sequester serum-borne heparin amplify growth factor activity. Adv Mater. 2011;23:5415. doi: 10.1002/adma.201103046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li J, Lin F, Li LD, Li J, Liu S. Surface engineering of poly(ethylene terephthalate) for durable hemocompatibility via a surface interpenetrating network technique. Macromol Chem Phys. 2012;213:2120–9. [Google Scholar]
- 30.Hubbell JA, Chilkoti A. Nanomaterials for drug delivery. Science. 2012;337:303–5. doi: 10.1126/science.1219657. [DOI] [PubMed] [Google Scholar]
- 31.Kemp MM, Linhardt RJ. Heparin-based nanoparticles. Wires Nanomed Nanobi. 2010;2:77–87. doi: 10.1002/wnan.68. [DOI] [PubMed] [Google Scholar]
- 32.Chung YI, Tae G, Yuk SH. A facile method to prepare heparin-functionalized nanoparticles for controlled release of growth factors. Biomaterials. 2006;27:2621–6. doi: 10.1016/j.biomaterials.2005.11.043. [DOI] [PubMed] [Google Scholar]
- 33.Tan HP, Shen Q, Jia XJ, Yuan ZP, Xiong DS. Injectable nanohybrid scaffold for biopharmaceuticals delivery and soft tissue engineering. Macromol Rapid Comm. 2012;33:2015–22. doi: 10.1002/marc.201200360. [DOI] [PubMed] [Google Scholar]
- 34.Xu X, Jha AK, Duncan RL, Jia XQ. Heparin-decorated, hyaluronic acid-based hydrogel particles for the controlled release of bone morphogenetic protein 2. Acta Biomater. 2011;7:3050–9. doi: 10.1016/j.actbio.2011.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peer D, Karp JM, Hong S, FaroKHzad OC, Margalit R, Langer R. Nanocarriers as an emerging platform for cancer therapy. Nat Nanotechnol. 2007;2:751–60. doi: 10.1038/nnano.2007.387. [DOI] [PubMed] [Google Scholar]
- 36.Davis ME, Chen Z, Shin DM. Nanoparticle therapeutics: an emerging treatment modality for cancer. Nat Rev Drug Discov. 2008;7:771–82. doi: 10.1038/nrd2614. [DOI] [PubMed] [Google Scholar]
- 37.Appel EA, del Barrio J, Loh XJ, Scherman OA. Supramolecular polymeric hydrogels. Chem Soc Rev. 2012;41:6195–214. doi: 10.1039/c2cs35264h. [DOI] [PubMed] [Google Scholar]
- 38.Guvendiren M, Lu HD, Burdick JA. Shear-thinning hydrogels for biomedical applications. Soft Matter. 2012;8:260–72. [Google Scholar]
- 39.Kiick KL. Peptide- and protein-mediated assembly of heparinized hydrogels. Soft Matter. 2008;4:29–37. doi: 10.1039/b711319f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Seal BL, Panitch A. Physical polymer matrices based on affinity interactions between peptides and polysaccharides. Biomacromolecules. 2003;4:1572–82. doi: 10.1021/bm0342032. [DOI] [PubMed] [Google Scholar]
- 41.Seal BL, Panitch A. Viscoelastic behavior of environmentally sensitive biomimetic polymer matrices. Macromolecules. 2006;39:2268–74. [Google Scholar]
- 42.Seal BL, Panitch A. Physical matrices stabilized by enzymatically sensitive covalent crosslinks. Acta Biomater. 2006;2:241–51. doi: 10.1016/j.actbio.2005.12.008. [DOI] [PubMed] [Google Scholar]
- 43.Jeong KJ, Panitch A. Interplay between covalent and physical interactions within environment sensitive hydrogels. Biomacromolecules. 2009;10:1090–9. doi: 10.1021/bm801270k. [DOI] [PubMed] [Google Scholar]
- 44.Yamaguchi N, Kiick KL. Polysaccharide-poly(ethylene glycol) star copolymer as a scaffold for the production of bioactive hydrogels. Biomacromolecules. 2005;6:1921–30. doi: 10.1021/bm050003+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yamaguchi N, Chae BS, Zhang L, Kiick KL, Furst EM. Rheological characterization of polysaccharide-poly(ethylene glycol) star copolymer hydrogels. Biomacromolecules. 2005;6:1931–40. doi: 10.1021/bm0500042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang L, Furst EM, Kiick KL. Manipulation of hydrogel assembly and growth factor delivery via the use of peptide-polysaccharide interactions. J Control Release. 2006;114:130–42. doi: 10.1016/j.jconrel.2006.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tan HP, Zhou QX, Qi HF, Zhu D, Ma XX, Xiong DS. Heparin interacting protein mediated assembly of nano-fibrous hydrogel scaffolds for guided stem cell differentiation. Macromol Biosci. 2012;12:621–7. doi: 10.1002/mabi.201100502. [DOI] [PubMed] [Google Scholar]
- 48.Wieduwild R, Tsurkan M, Chwalek K, Murawala P, Nowak M, Freudenberg U, et al. Minimal peptide motif for non-covalent peptide-heparin hydrogels. J Am Chem Soc. 2013;135:2919–22. doi: 10.1021/ja312022u. [DOI] [PubMed] [Google Scholar]
- 49.Hong SY, Oh JE, Lee K-H. Effect of d-amino acid substitution on the stability, the secondary structure, and the activity of membrane-active peptide. Biochem Pharmacol. 1999;58:1775–80. doi: 10.1016/s0006-2952(99)00259-2. [DOI] [PubMed] [Google Scholar]
- 50.Yamaguchi N, Zhang L, Chae BS, Palla CS, Furst EM, Kiick KL. Growth factor mediated assembly of cell receptor-responsive hydrogels. J Am Chem Soc. 2007;129:3040. doi: 10.1021/ja0680358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim SH, Kiick KL. Cell-mediated delivery and targeted erosion of vascular endothelial growth factor-crosslinked hydrogels. Macromol Rapid Comm. 2010;31:1231–40. doi: 10.1002/marc.201000130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Vermonden T, Censi R, Hennink WE. Hydrogels for protein delivery. Chem Rev. 2012;112:2853–88. doi: 10.1021/cr200157d. [DOI] [PubMed] [Google Scholar]
- 53.Benoit DSW, Anseth KS. Heparin functionalized PEG gels that modulate protein adsorption for hMSC adhesion and differentiation. Acta Biomater. 2005;1:461–70. doi: 10.1016/j.actbio.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 54.Benoit DSW, Durney AR, Anseth KS. The effect of heparin-functionalized PEG hydrogels on three-dimensional human mesenchymal stem cell osteogenic differentiation. Biomaterials. 2007;28:66–77. doi: 10.1016/j.biomaterials.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 55.Nilasaroya A, Poole-Warren LA, Whitelock JM, Martens PJ. Structural and functional characterisation of poly(vinyl alcohol) and heparin hydrogels. Biomaterials. 2008;29:4658–64. doi: 10.1016/j.biomaterials.2008.08.011. [DOI] [PubMed] [Google Scholar]
- 56.Jeon O, Powell C, Solorio LD, Krebs MD, Alsberg E. Affinity-based growth factor delivery using biodegradable, photocrosslinked heparin-alginate hydrogels. J Control Release. 2011;154:258–66. doi: 10.1016/j.jconrel.2011.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oliviero O, Ventre M, Netti PA. Functional porous hydrogels to study angiogenesis under the effect of controlled release of vascular endothelial growth factor. Acta Biomater. 2012;8:3294–301. doi: 10.1016/j.actbio.2012.05.019. [DOI] [PubMed] [Google Scholar]
- 58.Nie T, Baldwin A, Yamaguchi N, Kiick KL. Production of heparin-functionalized hydrogels for the development of responsive and controlled growth factor delivery systems. J Control Release. 2007;122:287–96. doi: 10.1016/j.jconrel.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nie T, Akins RE, Kiick KL. Production of heparin-containing hydrogels for modulating cell responses. Acta Biomater. 2009;5:865–75. doi: 10.1016/j.actbio.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Robinson KG, Nie T, Baldwin AD, Yang EC, Kiick KL, Akins RE. Differential effects of substrate modulus on human vascular endothelial, smooth muscle, and fibroblastic cells. J Biomed Mater Res A. 2012;100A:1356–67. doi: 10.1002/jbm.a.34075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tae G, Kim YJ, Choi WI, Kim M, Stayton PS, Hoffman AS. Formation of a novel heparin-based hydrogel in the presence of heparin-binding biomolecules. Biomacromolecules. 2007;8:1979–86. doi: 10.1021/bm0701189. [DOI] [PubMed] [Google Scholar]
- 62.Il Choi W, Kim M, Tae G, Kim YH. Sustained release of human growth hormone from heparin-based hydrogel. Biomacromolecules. 2008;9:1698–704. doi: 10.1021/bm701391b. [DOI] [PubMed] [Google Scholar]
- 63.Kim M, Lee JY, Jones CN, Revzin A, Tae G. Heparin-based hydrogel as a matrix for encapsulation and cultivation of primary hepatocytes. Biomaterials. 2010;31:3596–603. doi: 10.1016/j.biomaterials.2010.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim M, Kim SE, Kang SS, Kim YH, Tae G. The use of de-differentiated chondrocytes delivered by a heparin-based hydrogel to regenerate cartilage in partial-thickness defects. Biomaterials. 2011;32:7883–96. doi: 10.1016/j.biomaterials.2011.07.015. [DOI] [PubMed] [Google Scholar]
- 65.Cai SS, Liu YC, Shu XZ, Prestwich GD. Injectable glycosaminoglycan hydrogels for controlled release of human basic fibroblast growth factor. Biomaterials. 2005;26:6054–67. doi: 10.1016/j.biomaterials.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 66.Pike DB, Cai SS, Pomraning KR, Firpo MA, Fisher RJ, Shu XZ, et al. Heparin-regulated release of growth factors in vitro and angiogenic response in vivo to implanted hyaluronan hydrogels containing VEGF and bFGF. Biomaterials. 2006;27:5242–51. doi: 10.1016/j.biomaterials.2006.05.018. [DOI] [PubMed] [Google Scholar]
- 67.Elia R, Fuegy PW, VanDelden A, Firpo MA, Prestwich GD, Peattie RA. Stimulation of in vivo angiogenesis by in situ crosslinked, dual growth factor-loaded, glycosaminoglycan hydrogels. Biomaterials. 2010;31:4630–8. doi: 10.1016/j.biomaterials.2010.02.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Freudenberg U, Hermann A, Welzel PB, Stirl K, Schwarz SC, Grimmer M, et al. A star-PEG-heparin hydrogel platform to aid cell replacement therapies for neurodegenerative diseases. Biomaterials. 2009;30:5049–60. doi: 10.1016/j.biomaterials.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 69.Zieris A, Prokoph S, Levental KR, Welzel PB, Grimmer M, Freudenberg U, et al. FGF-2 and VEGF functionalization of starPEG-heparin hydrogels to modulate biomolecular and physical cues of angiogenesis. Biomaterials. 2010;31:7985–94. doi: 10.1016/j.biomaterials.2010.07.021. [DOI] [PubMed] [Google Scholar]
- 70.Zieris A, Chwalek K, Prokoph S, Levental KR, Welzel PB, Freudenberg U, et al. Dual independent delivery of pro-angiogenic growth factors from starPEG-heparin hydrogels. J Control Release. 2011;156:28–36. doi: 10.1016/j.jconrel.2011.06.042. [DOI] [PubMed] [Google Scholar]
- 71.Richardson TP, Peters MC, Ennett AB, Mooney DJ. Polymeric system for dual growth factor delivery. Nat Biotechnol. 2001;19:1029–34. doi: 10.1038/nbt1101-1029. [DOI] [PubMed] [Google Scholar]
- 72.Baumann L, Prokoph S, Gabriel C, Freudenberg U, Werner C, Beck-Sickinger AG. A novel, biased-like SDF-1 derivative acts synergistically with starPEG-based heparin hydrogels and improves eEPC migration in vitro. J Control Release. 2012;162:68–75. doi: 10.1016/j.jconrel.2012.04.049. [DOI] [PubMed] [Google Scholar]
- 73.Prokoph S, Chavakis E, Levental KR, Zieris A, Freudenberg U, Dimmeler S, et al. Sustained delivery of SDF-1 alpha from heparin-based hydrogels to attract circulating pro-angiogenic cells. Biomaterials. 2012;33:4792–800. doi: 10.1016/j.biomaterials.2012.03.039. [DOI] [PubMed] [Google Scholar]
- 74.Welzel PB, Grimmer M, Renneberg C, Naujox L, Zschoche S, Freudenberg U, et al. Macroporous starPEG-heparin cryogels. Biomacromolecules. 2012;13:2349–58. doi: 10.1021/bm300605s. [DOI] [PubMed] [Google Scholar]
- 75.Jin R, Teixeira LSM, Dijkstra PJ, van Blitterswijk CA, Karperien M, Feijen J. Chondrogenesis in injectable enzymatically crosslinked heparin/dextran hydrogels. J Control Release. 2011;152:186–95. doi: 10.1016/j.jconrel.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 76.Joung YK, You SS, Park KM, Go DH, Park KD. In situ forming, metal-adhesive heparin hydrogel surfaces for blood-compatible coating. Colloid Surface B. 2012;99:102–7. doi: 10.1016/j.colsurfb.2011.10.047. [DOI] [PubMed] [Google Scholar]
- 77.Ernst S, Langer R, Cooney CL, Sasisekharan R. Enzymatic degradation of glycosaminoglycans. Crit Rev Biochem Mol. 1995;30:387–444. doi: 10.3109/10409239509083490. [DOI] [PubMed] [Google Scholar]
- 78.Nilasaroya A, Martens PJ, Whitelock JM. Enzy matic degradation of heparin-modified hydrogels and its effect on bioactivity. Biomaterials. 2012;33:5534–40. doi: 10.1016/j.biomaterials.2012.04.022. [DOI] [PubMed] [Google Scholar]
- 79.Lutolf MP, Lauer-Fields JL, Schmoekel HG, Metters AT, Weber FE, Fields GB, et al. Synthetic matrix metalloproteinase-sensitive hydrogels for the conduction of tissue regeneration: Engineering cell-invasion characteristics. P Natl Acad Sci USA. 2003;100:5413–8. doi: 10.1073/pnas.0737381100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kraehenbuehl TP, Ferreira LS, Zammaretti P, Hubbell JA, Langer R. Cell-responsive hydrogel for encapsulation of vascular cells. Biomaterials. 2009;30:4318–24. doi: 10.1016/j.biomaterials.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Patterson J, Hubbell JA. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials. 2010;31:7836–45. doi: 10.1016/j.biomaterials.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 82.Fairbanks BD, Schwartz MP, Halevi AE, Nuttelman CR, Bowman CN, Anseth KS. A versatile synthetic extracellular matrix mimic via thiol-norbornene photopolymerization. Adv Mater. 2009;21:5005. doi: 10.1002/adma.200901808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Anderson SB, Lin CC, Kuntzler DV, Anseth KS. The performance of human mesenchymal stem cells encapsulated in cell-degradable polymer-peptide hydrogels. Biomaterials. 2011;32:3564–74. doi: 10.1016/j.biomaterials.2011.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Burdick JA, Murphy WL. Moving from static to dynamic complexity in hydrogel design. Nat Commun. 2012;3:1269. doi: 10.1038/ncomms2271. [DOI] [PubMed] [Google Scholar]
- 85.Tsurkan MV, Levental KR, Freudenberg U, Werner C. Enzymatically degradable heparin-polyethylene glycol gels with controlled mechanical properties. Chem Commun. 2010;46:1141–3. doi: 10.1039/b921616b. [DOI] [PubMed] [Google Scholar]
- 86.Tsurkan MV, Chwalek K, Levental KR, Freudenberg U, Werner C. Modular starPEG-heparin gels with bifunctional peptide linkers. Macromol Rapid Comm. 2010;31:1529–33. doi: 10.1002/marc.201000155. [DOI] [PubMed] [Google Scholar]
- 87.Chwalek K, Levental KR, Tsurkan MV, Zieris A, Freudenberg U, Werner C. Two-tier hydrogel degradation to boost endothelial cell morphogenesis. Biomaterials. 2011;32:9649–57. doi: 10.1016/j.biomaterials.2011.08.078. [DOI] [PubMed] [Google Scholar]
- 88.Meng F, Hennink WE, Zhong Z. Reduction-sensitive polymers and bioconjugates for biomedical applications. Biomaterials. 2009;30:2180–98. doi: 10.1016/j.biomaterials.2009.01.026. [DOI] [PubMed] [Google Scholar]
- 89.Roy D, Cambre JN, Sumerlin BS. Future perspectives and recent advances in stimuli-responsive materials. Prog Polym Sci. 2010;35:278–301. [Google Scholar]
- 90.Cheng R, Feng F, Meng F, Deng C, Feijen J, Zhong Z. Glutathione-responsive nano-vehicles as a promising platform for targeted intracellular drug and gene delivery. J Control Release. 2011;152:2–12. doi: 10.1016/j.jconrel.2011.01.030. [DOI] [PubMed] [Google Scholar]
- 91.Choh SY, Cross D, Wang C. Facile synthesis and characterization of disulfide-cross-linked hyaluronic acid hydrogels for protein delivery and cell encapsulation. Biomacromolecules. 2011;12:1126–36. doi: 10.1021/bm101451k. [DOI] [PubMed] [Google Scholar]
- 92.Lallana E, Tirelli N. Oxidation-responsive polymers: which groups to use, how to make them, what to expect from them (biomedical applications). Macromol Chem Phys. 2013;214:143–58. [Google Scholar]
- 93.Baldwin AD, Kiick KL. Tunable degradation of maleimide-thiol adducts in reducing environments. Bioconjugate Chem. 2011;22:1946–53. doi: 10.1021/bc200148v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Baldwin AD, Kiick KL. Reversible maleimide-thiol adducts yield glutathione-sensitive poly(ethylene glycol)-heparin hydrogels. Polym Chem. 2013;4:133–43. doi: 10.1039/C2PY20576A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yeh CC, Hou MF, Wu SH, Tsai SM, Lin SK, Hou LA, et al. A study of glutathione status in the blood and tissues of patients with breast cancer. Cell Biochem Funct. 2006;24:555–9. doi: 10.1002/cbf.1275. [DOI] [PubMed] [Google Scholar]
- 96.Farokhzad OC, Langer R. Impact of nanotechnology on drug delivery. Acs Nano. 2009;3:16–20. doi: 10.1021/nn900002m. [DOI] [PubMed] [Google Scholar]
- 97.Lammers T, Hennink WE, Storm G. Tumour-targeted nanomedicines: principles and practice. Brit J Cancer. 2008;99:392–7. doi: 10.1038/sj.bjc.6604483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Gao WW, Chan JM, Farokhzad OC. pH-responsive nanoparticles for drug delivery. Mol Pharmaceut. 2010;7:1913–20. doi: 10.1021/mp100253e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gao JH, Gu HW, Xu B. Multifunctional magnetic nanoparticles: design, synthesis, and biomedical applications. Accounts Chem Res. 2009;42:1097–107. doi: 10.1021/ar9000026. [DOI] [PubMed] [Google Scholar]
- 100.Murphy CJ, Gole AM, Stone JW, Sisco PN, Alkilany AM, Goldsmith EC, et al. Gold nanoparticles in biology: beyond toxicity to cellular imaging. Accounts Chem Res. 2008;41:1721–30. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 101.Raemdonck K, Demeester J, De Smedt S. Advanced nanogel engineering for drug delivery. Soft Matter. 2009;5:707–15. [Google Scholar]
- 102.Smith MH, Lyon LA. Multifunctional nanogels for siRNA delivery. Accounts Chem Res. 2012;45:985–93. doi: 10.1021/ar200216f. [DOI] [PubMed] [Google Scholar]
- 103.Yuk SH, Oh KS, Cho SH, Lee BS, Kim SY, Kwak BK, et al. Glycol chitosan/heparin immobilized iron oxide nanoparticles with a tumor-targeting characteristic for magnetic resonance imaging. Biomacromolecules. 2011;12:2335–43. doi: 10.1021/bm200413a. [DOI] [PubMed] [Google Scholar]
- 104.Lee JH, Jung MJ, Hwang YH, Lee YJ, Lee S, Lee DY, et al. Heparin-coated superparamagnetic iron oxide for in vivo MR imaging of human MSCs. Biomaterials. 2012;33:4861–71. doi: 10.1016/j.biomaterials.2012.03.035. [DOI] [PubMed] [Google Scholar]
- 105.Lee K, Lee H, Bae KH, Park TG. Heparin immobilized gold nanoparticles for targeted detection and apoptotic death of metastatic cancer cells. Biomaterials. 2010;31:6530–6. doi: 10.1016/j.biomaterials.2010.04.046. [DOI] [PubMed] [Google Scholar]
- 106.Sapsford KE, Algar WR, Berti L, Gemmill KB, Casey BJ, Oh E, et al. Functionalizing nanoparticles with biological molecules: developing chemistries that facilitate nanotechnology. Chem Rev. 2013;113:1904–2074. doi: 10.1021/cr300143v. [DOI] [PubMed] [Google Scholar]
- 107.Chung YI, Kim SK, Lee YK, Park SJ, Cho KO, Yuk SH, et al. Efficient revascularization by VEGF administration via heparin-functionalized nanoparticle-fibrin complex. J Control Release. 2010;143:282–9. doi: 10.1016/j.jconrel.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 108.Boddohi S, Moore N, Johnson PA, Kipper MJ. Polysaccharide-based polyelectrolyte complex nanoparticles from chitosan, heparin, and hyaluronan. Biomacromolecules. 2009;10:1402–9. doi: 10.1021/bm801513e. [DOI] [PubMed] [Google Scholar]
- 109.Boddohi S, Almodovar J, Zhang H, Johnson PA, Kipper MJ. Layer-by-layer assembly of polysaccharide-based nanostructured surfaces containing polyelectrolyte complex nanoparticles. Colloid Surface B. 2010;77:60–8. doi: 10.1016/j.colsurfb.2010.01.006. [DOI] [PubMed] [Google Scholar]
- 110.Volpato FZ, Almodovar J, Erickson K, Popat KC, Migliaresi C, Kipper MJ. Preservation of FGF-2 bioactivity using heparin-based nanoparticles, and their delivery from electrospun chitosan fibers. Acta Biomater. 2012;8:1551–9. doi: 10.1016/j.actbio.2011.12.023. [DOI] [PubMed] [Google Scholar]
- 111.Tang DW, Yu SH, Ho YC, Mi FL, Kuo PL, Sung HW. Heparinized chitosan/poly(gamma-glutamic acid) nanoparticles for multi-functional delivery of fibroblast growth factor and heparin. Biomaterials. 2010;31:9320–32. doi: 10.1016/j.biomaterials.2010.08.058. [DOI] [PubMed] [Google Scholar]
- 112.Reyes-Ortega F, Rodriguez G, Aguilar MR, Lord M, Whitelock J, Stenzel MH, et al. Encapsulation of low molecular weight heparin (bemiparin) into polymeric nanoparticles obtained from cationic block copolymers: properties and cell activity. J Mater Chem B. 2013;1:850–60. doi: 10.1039/c2tb00194b. [DOI] [PubMed] [Google Scholar]
- 113.Zhao Y, Lord MS, Stenzel MH. A polyion complex micelle with heparin for growth factor delivery and uptake into cells. J Mater Chem B. 2013;1:1635–43. doi: 10.1039/c3tb00360d. [DOI] [PubMed] [Google Scholar]
- 114.Glimelius B, Busch C, Höök M. Binding of heparin on the surface of cultured human endothelial cells. Thromb Res. 1978;12:773–82. doi: 10.1016/0049-3848(78)90271-2. [DOI] [PubMed] [Google Scholar]
- 115.Shing Y, Folkman J, Sullivan R, Butterfield C, Murray J, Klagsbrun M. Heparin affinity -purification of a tumor-derived capillary endothelial cell-growth factor. Science. 1984;223:1296–9. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- 116.Chung YI, Kim JC, Kim YH, Tae G, Lee SY, Kim K, et al. The effect of surface functionalization of PLGA nanoparticles by heparin- or chitosan-conjugated Pluronic on tumor targeting. J Control Release. 2010;143:374–82. doi: 10.1016/j.jconrel.2010.01.017. [DOI] [PubMed] [Google Scholar]
- 117.Choi JH, Jang JY, Joung YK, Kwon MH, Park KD. Intracellular delivery and anti-cancer effect of self-assembled heparin-Pluronic nanogels with RNase A. J Control Release. 2010;147:420–7. doi: 10.1016/j.jconrel.2010.07.118. [DOI] [PubMed] [Google Scholar]
- 118.Joung YK, Jang JY, Choi JH, Han DK, Park KD. Heparin-conjugated pluronic nanogels as muiti-drug nanocarriers for combination chemotherapy. Mol Pharmaceut. 2013;10:685–93. doi: 10.1021/mp300480v. [DOI] [PubMed] [Google Scholar]
- 119.Bae KH, Mok H, Park TG. Synthesis, characterization, and intracellular delivery of reducible heparin nanogels for apoptotic cell death. Biomaterials. 2008;29:3376–83. doi: 10.1016/j.biomaterials.2008.04.035. [DOI] [PubMed] [Google Scholar]
- 120.Wang Y, Xin DC, Liu KJ, Zhu MQ, Xiang JN. Heparin-paclitaxel conjugates as drug delivery system: synthesis, self-assembly property, drug release, and antitumor activity. Bioconjugate Chem. 2009;20:2214–21. doi: 10.1021/bc8003809. [DOI] [PubMed] [Google Scholar]
- 121.Wang Y, Wang YQ, Xiang JN, Yao KT. Target-specific cellular uptake of taxol-loaded heparin-PEG-folate nanoparticles. Biomacromolecules. 2010;11:3531–8. doi: 10.1021/bm101013s. [DOI] [PubMed] [Google Scholar]
- 122.Li L, Huh KM, Lee YK, Kim SY. Biofunctional self-assembled nanoparticles of folate-PEG-heparin/PBLA copolymers for targeted delivery of doxorubicin. J Mater Chem. 2011;21:15288–97. [Google Scholar]
- 123.She WC, Li N, Luo K, Guo CH, Wang G, Geng YY, et al. Dendronized heparin-doxorubicin conjugate based nanoparticle as pH-responsive drug delivery system for cancer therapy. Biomaterials. 2013;34:2252–64. doi: 10.1016/j.biomaterials.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 124.Hasan J, Shnyder SD, Clamp AR, McGown AT, Bicknell R, Presta M, et al. Heparin octasaccharides inhibit angiogenesis in vivo. Clin Cancer Res. 2005;11:8172–9. doi: 10.1158/1078-0432.CCR-05-0452. [DOI] [PubMed] [Google Scholar]
- 125.Hostettler N, Naggi A, Torri G, Ishai-Michaeli R, Casu B, Vlodavsky I, et al. P-selectin- and heparanase-dependent antimetastatic activity of non-anticoagulant heparins. Faseb J. 2007;21:3562–72. doi: 10.1096/fj.07-8450com. [DOI] [PubMed] [Google Scholar]
- 126.Lee DY, Lee SW, Kim SK, Lee M, Chang HW, Moon HT, et al. Antiangiogenic activity of orally absorbable heparin derivative in different types of cancer cells. Pharm Res. 2009;26:2667–76. doi: 10.1007/s11095-009-9989-9. [DOI] [PubMed] [Google Scholar]
- 127.Kloxin AM, Kasko AM, Salinas CN, Anseth KS. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science. 2009;324:59–63. doi: 10.1126/science.1169494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Brantley JN, Wiggins KM, Bielawski CW. Unclicking the click: mechanically facilitated 1,3-dipolar cycloreversions. Science. 2011;333:1606–9. doi: 10.1126/science.1207934. [DOI] [PubMed] [Google Scholar]
- 129.Schultz KM, Baldwin AD, Kiick KL, Furst EM. Rapid rheological screening to identify conditions of biomaterial hydrogelation. Soft Matter. 2009;5:740–2. doi: 10.1039/b818178k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Schultz KM, Furst EM. Microrheology of biomaterial hydrogelators. Soft Matter. 2012;8:6198–205. [Google Scholar]