Abstract
Objective
It is not possible with current hydrocortisone replacement to mimic the diurnal cortisol profile in patients with adrenal insufficiency. Previous attempts with modified release technology were unsuccessful. Our objective was to develop hydrocortisone formulations that recreate the diurnal cortisol profile using multi-particulate technology.
Design and Measurements
Screening by in-vitro dissolution profiles, pharmacokinetic testing in dexamethasone suppressed dogs and humans, and comparison to a reference population.
Setting
Field laboratories and clinical research facility.
Results
Formulations were generated using an enteric (delayed-release) design configuration with an extended (sustained-release) dissolution profile. In-vitro dissolution confirmed delayed and sustained hydrocortisone release. However, in dogs and humans, sustained release resulted in reduced bioavailability. A formulation, DIURF-006, was developed that maintained delayed release but omitted the sustained release functionality. Pharmacokinetic characterisation of DIURF-006 showed that, despite absence of a sustained release component, absorption was sufficiently sustained to deliver extended hydrocortisone absorption. In dexamethasone-suppressed volunteers (n=16) receiving a twice daily ‘toothbrush’ regimen (20mg at 23:00h and 10mg at 07:00h), DIURF-006 gave a similar cortisol profile to physiological cortisol levels: DIURF-006 vs physiological, Geomean AUC 5610 vs 4706 hr*nmol/l, Geomean Cmax 665 vs 594 nmol/l and Median Tmax 8.5h vs clock time 08:12 hours for peak cortisol. The relative bioavailability of DIURF-006 vs hydrocortisone was 89% and cortisol levels increased linearly with doses between 5 and 30mg.
Conclusion
A multi-particulate oral hydrocortisone formulation with only an enteric coat provides delayed and sustained absorption and when given in a ‘toothbrush’ regimen provides physiological cortisol exposure.
Keywords: multi-particulate, modified release hydrocortisone, cortisol diurnal rhythm
Introduction
Cortisol is an essential stress hormone and replacement with oral hydrocortisone is lifesaving in patients with congenital adrenal hyperplasia (CAH) and adrenal insufficiency. Cortisol has a diurnal rhythm regulated by the central circadian oscillator (body clock) located in the suprachiasmatic nucleus of the hypothalamus. Cortisol levels rise between 03:00h and 04:00h, peak within an hour of waking, fall over the day with a quiescent period starting around 19:00h and nadir occurring at 00:00h, midnight 1. This rhythm is an important metabolic signal for peripheral tissue clocks and loss of cortisol rhythmicity is associated with fatigue, depression and insulin resistance 2, 3.
It is a general endocrine principle to replace hormones to mimic physiological levels. However, the pharmacokinetics of oral immediate release hydrocortisone make it impossible to mimic the overnight rise in cortisol and patients with adrenal insufficiency wake with low cortisol levels at the time when their cortisol level should be peaking 4, 5. Patients with congenital adrenal hyperplasia have the additional problem that the increased early morning ACTH drive from the pituitary, in the absence of physiological cortisol feedback, results in a rise in adrenal androgens 6. Patients with adrenal insufficiency, both primary and secondary, still have an increased morbidity and mortality despite current replacement regimens 7-9, and a major complaint is fatigue 10. Patients with CAH, despite a large number of different glucocorticoid treatment regimens, also have poor health outcomes 11, 12. Preliminary evidence, using either intravenous or subcutaneous infusions of hydrocortisone, suggest that provision of circadian cortisol levels with an overnight rise can improve both biochemical control of CAH 13, 14 and quality of life (QoL) in adrenal insufficiency 15. In addition, as cortisol is an important secondary messenger between central and peripheral clocks, providing physiological diurnal cortisol replacement may be important to achieve metabolic homeostasis and improve health status. The challenge is to generate an oral modified release formulation of hydrocortisone that can mimic the overnight rise in cortisol.
Plenadren®, recently licensed in Europe, is a modified release formulation of hydrocortisone that provides the potential for once daily dosing 16. Plenadren® has an immediate release coating combined with an extended-release core, the concept being, the medication is taken first thing in the morning and provides once daily dosing. The time of Cmax for Plenadren® is similar to that for oral hydrocortisone, and Plenadren® provides an extended release of hydrocortisone such that there is no requirement for midday dosing. The bioavailability of Plenadren® is 20% less than oral hydrocortisone and so dose adjustment may be required. Plenadren® is only taken in the morning and therefore cannot replace the overnight rise in cortisol levels 17.
We have been investigating a modified release formulation of hydrocortisone, Chronocort®, with the aim of determining whether it is possible to replace overnight cortisol levels and more fully mimic the cortisol circadian rhythm. The challenge has been to design a formulation that would allow hydrocortisone to be released optimally within the small bowel where absorption would reliably take place and within an appropriate time window to replace overnight cortisol levels. Previously, the use of a proprietary modified release, electrostatic (tablet) coating technology from Phoqus Pharmaceuticals, enabled the development of prototype formulations of hydrocortisone. These formulations exhibited delayed and sustained release functionality afforded by permitting drug release from only one face of an insoluble tablet matrix. In brief, these formulations had an insoluble coating on the base and sides; essentially a bucket which contained sustained release hydrocortisone covered by an eroding layer that was exposed for dissolution in the gut 18. We tested these prototype formulations and demonstrated that one particular formulation, with a controlled delayed release of approximately 4 hours, had yielded a physiological overnight rise in cortisol levels 19. The same formulation, given as a single 30mg dose at night, to patients with CAH demonstrated improved control of androgens on waking although control was not maintained as cortisol levels fell during the day 20. Pharmacokinetic modelling suggested that this formulation given in a twice daily ‘toothbrush’ regimen: 15–20mg at 23:00h and 10mg at 07:00h, could reproduce physiological cortisol levels throughout the 24 hours 19. However, this formulation could not be progressed due to issues in scaling manufacture. Furthermore, the relative bioavailability of this formulation (with respect to the immediate release tablet) was <75%, which would have necessitated the use of a high-dose to achieve a replication of the circadian profile for cortisol via a ‘toothbrush’ regimen.
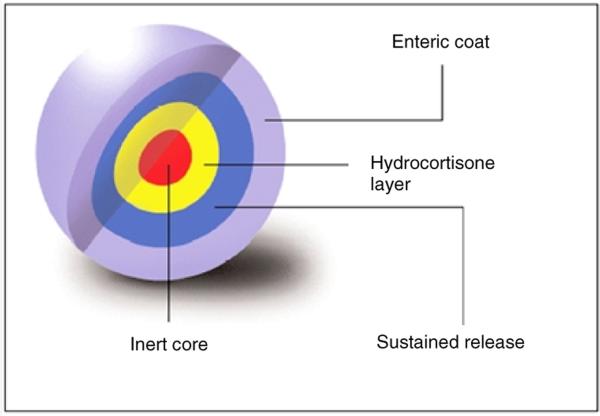
In view of the problems inherent with the Phoqus modified release formulations, the new Chronocort® formulations were developed using a scalable technology based on multi-particulates. The design configuration for this dosage form comprises an inert microcrystalline core coated with a drug layer and then further coated with polymeric layers that modify drug release (Figure 1). We have screened a number of multi-particulate formulations both in vitro and in vivo and chosen an optimal Chronocort® formulation, DIURF-006, which reproduces the overnight rise in cortisol. In this manuscript we present the development of DIURF-006.
Figure 1.
Cross-sectional diagram of the multi-particulate Chronocort® formulation
Subjects and Methods
Healthy reference population
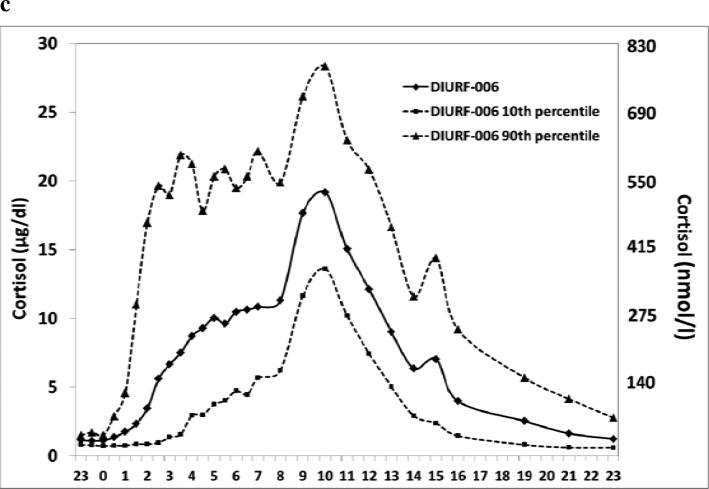
28 normal individuals who had undergone detailed, 24-hour, 20-minute, cortisol profiling provided data for definition of the physiological cortisol circadian rhythm as previously published 19. In this manuscript we used the cortisol levels measured by LC-MS/MS in this reference population.
Hydrocortisone Modified Release Capsules
The formulation of hydrocortisone modified release capsules was based on a multi-layered multi-particulate technology where the sustained release and enteric coats could be varied to provide differing release profiles (Figure 1). Essentially hydrocortisone was coated onto an inert microcrystalline core which was further covered with a sustained release layer and an enteric coat layer. The enteric coat layer was based on a pH trigger that would allow dissolution at a set point in the small bowel as the dosage form transited along the gastrointestinal system. The total dose of hydrocortisone could be modified by altering the fill-weight of the multi-particulates in a single capsule. The key differences between each of the prototype formulations of Chronocort® are shown in Table 1. As the initial formulation showed very poor bioavailability the sustained release coat was gradually decreased until in DIURF-006 there was no sustained release coat. Also the pH of the enteric coat was varied for different formulations.
Table 1.
Chronocort® formulations with varying delayed and sustained release coatings
| Formulation ID | Sustained release coat: thickness as % of drug core | Enteric coat: pH at which mean dissolution occurs |
|---|---|---|
| DIURF-000 | 25.0 | 6.8 |
| DIURF-001 | 20.0 | 6.5 |
| DIURF-002 | 12.5 | 6.5 |
| DIURF-003 | 12.5 | 6.0 |
| DIURF-004 | 10.0 | 6.8 |
| DIURF-005 | 8.0 | 6.8 |
| DIURF-006 | None | 6.8 |
Dissolution
An in vitro dissolution methodology using rotating basket apparatus (Apparatus I) was developed to best represent the expected dissolution conditions in vivo. Briefly, dissolution vessels were filled with 700 ml of USP simulated gastric fluid pH1.2 and pre-warmed to 37±0.5°C. In separate vessels, sufficient buffer to adjust the pH of the media (approximately 900 ml of 0.18M trisodium orthophosphate buffer for the first pH change and 300ml of the 0.23M trisodium orthophosphate buffer for the final pH change per dissolution) was pre-warmed. The Chronocort® capsule was added to each basket and once the media was at the required temperature, they were lowered into the media, with the speed set to 100 RPM. At 1 hour intervals 1-2 ml of sample was collected for analysis. After 2 hours the baskets were removed from the media and 150ml of the pre-warmed 0.18M buffer was added to each vessel. The pH of the media was altered as necessary with 2M HCl until a pH was obtained in the region 6.0 ±0.05. The baskets were lowered into the media and after a further hour the 3 hour time point was taken. The baskets were then removed from the media, and 50 ml of the pre-warmed 0.23M buffer was added (and adjusted as necessary), to give a final pH within the range of 7.2±0.5. The dissolution was then restarted and time points were taken every 2 hours until 23h and then one final time point was taken after 24 hours. The samples were vialled neat for analysis. The amount of hydrocortisone dissolved in the media was determined by HPLC analysis using reverse phase chromatography with UV detection at 254 nm.
Pharmacokinetic Analysis
In vivo studies were undertaken in dexamethasone suppressed dogs and healthy male volunteers. Four male beagle dogs were studied using a 3-period cross-over design with a 7-day washout period between each dosing period. For each dosing period, endogenous cortisol was suppressed with dexamethasone (dose:1 mg/10 kg BW, every 6 hours starting 6 hours before dosing with the test item and continuing until the last sampling). Administrations and blood extractions were carried out at Harlan Laboratories S.A. facilities (Spain). All the human studies in dexamethasone suppressed volunteers were open label, randomised studies, in healthy male volunteers aged 18 to 60 years. In the initial studies comparing formulations 6 volunteers were studied in each treatment group and in the studies of dose proportionality and the twice daily treatment regimen 16 volunteers were studied in each treatment group. The washout interval between all treatment periods was a minimum of a week. Each subject received 1 mg dexamethasone to suppress endogenous cortisol production at 18:00h and 22:00h on Day 1, and at 06:00h and 12:00h on Day 2. Human studies were undertaken at Simbec Research Ltd (UK).
Assays
In the initial study comparing DIURF-000 and immediate release hydrocortisone, cortisol was determined by fluorescence polarization immunoassay using an Abbott AxSYM automated system with an assay sensitivity of 1.1 μg/dl (30.4 nmol/l). All other cortisol measurements in the dog studies and comparison of different formulations including all studies with DIURF-006 were made by LC-MS/MS using an Applied Biosystems MDS Sciex API365 mass spectrometer with Perkin Elmer Series 200 LC system with an electrospray source in negative ionisation mode. Cortisol was measured in serum with the lower limit of quantitation (LLOQ) and upper limit of quantitation (ULOQ) of 0.50ng/ml and 250ng/ml respectively. Reproducibility (relative standard deviation) RSD% was equal to <15% at the LLOQ QC (cortisol 0.50ng/ml) and <10% at the low, medium and high QC level (cortisol 8ng/ml, 30ng/ml, 200ng/ml).
Statistics
Pharmacokinetic (PK) calculations derived from serum cortisol concentrations were performed using WinNonlin Professional V5.2.1 computer software. PK parameters are presented by treatment using n, geometric mean (except Tmax), arithmetic mean, arithmetic standard deviation (SD), arithmetic standard error (SE), 10th percentile and 90th percentile. Logarithmic transformed, Cmax and AUC0-t values were assessed for dose-proportionality by performing a regression analysis of the log-transformed parameter versus the log-transformed dose. For each parameter, a point estimate and 95% CI was calculated for the slope of the regression line.
Ethics
All animal procedures were checked and approved by the Animal Experimentation Ethics Committee at Harlan Laboratories S.A. All human studies were approved by the South East Wales Research Ethics Committee and all participants gave written informed consent. The study protocol was authorised by the Medicines and Healthcare products Regulatory Agency (MHRA).
Results
In vitro dissolution profiles
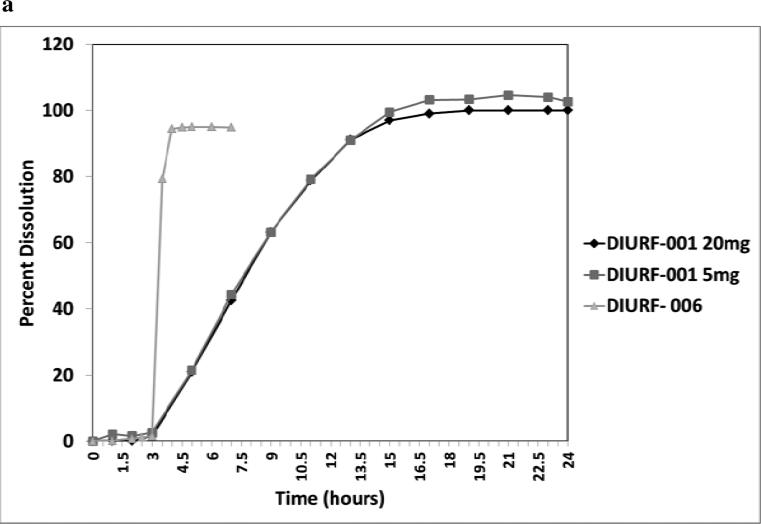
Previous pharmacokinetic analysis of modified release hydrocortisone suggested that to mimic the overnight rise in cortisol would require a delay in hydrocortisone release of between 2 to 4 hours and then sustained release over the next 6 to 7 hours. This would allow medication to be taken at around 23:00h with onset of hydrocortisone release at 01:00-03:00h and a cortisol peak at between 07:00 to 10:00h. Various formulations were made, as shown in Table 1, with a varying pH enteric coat and thickness of sustained release coat. At a pH of between 6.0 and 6.8 the enteric coat would dissolve from the middle of the small bowel to the terminal ileum. Figure 2 shows representative dissolution profiles for two formulations. DIURF-001 had an enteric coat pH of 6.5 and sustained release coat which was 20% weight for weight of the drug core and shows a dissolution profile with an approximately 3 hour delayed release followed by prolonged sustained release. In contrast, DIURF-006 had no sustained release coat but an enteric coat with a pH of 6.8 and again shows a delayed release of approximately 3 hours but then immediate release of hydrocortisone. These in vitro dissolution profiles would expect to translate in to different in vivo cortisol profiles.
Figure 2.
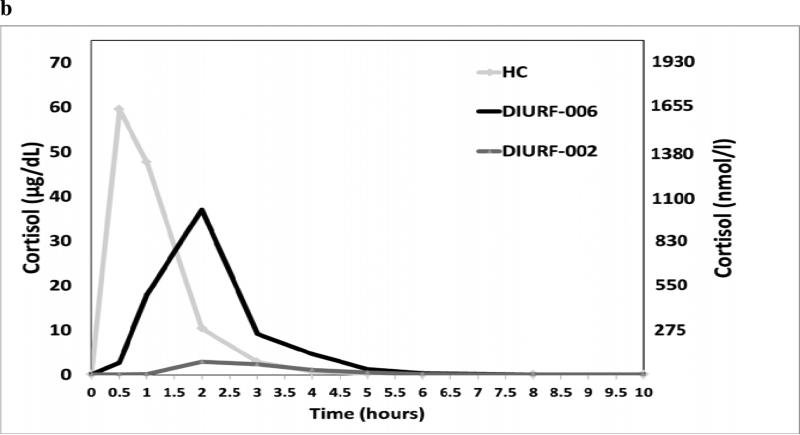
a: Representative dissolution profiles for DIURF-001 and DIURF-006. DIURF-001 has a sustained release coat and demonstrates sustained release in vitro whereas DIURF-006 has no sustained release coat and shows delayed but immediate release in vitro. 2b: PK profile for immediate release hydrocortisone (HC), DIURF-002 and DIURF-006 in dogs (Geomean ±SEM). DIURF-006 which has no sustained coating shows much better bioavailability than DIURF-002 which has sustained coating confirming that the sustained coating is the cause of reduced bioavailability.
Relative bioavailability of DIURF-000 to 003 in humans
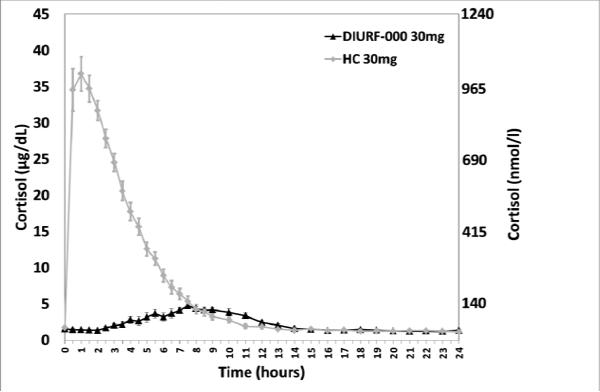
Formulation DIURF-000 was studied in 16 dexamethasone suppressed healthy male volunteers and the cortisol profile compared with that for immediate release hydrocortisone. As shown in figure 3 the relative bioavailability of 30mg DIURF-000 was <50% of that for 30mg hydrocortisone although the shape of the profile was that desired with the onset of cortisol rise occurring between 2-3 hours after capsule ingestion and the peak cortisol at 8-10 hours after ingestion. In view of the poor bioavailability three further formulations, DIURF-001, 002, & 003, were tested with reduced thickness of the sustained release coat (Table 1). Relative bioavailability was improved but remained <50% and the shape of the pharmacokinetic profile were similar to that for DIURF-000.
Figure 3.
Comparison of 30mg DIURF-000 and hydrocortisone in dexamethasone suppressed healthy volunteers. DIURF-000 shows delayed and sustained absorption profile but very poor bioavailability (Geomean ± SEM).
In vivo evaluation in dogs
In vivo dissolution studies were undertaken in a dog model to better understand exposure performance of the multi-particulate formulations in view of the low bioavailability in humans. Four male dexamethasone suppressed beagle dogs were dosed with 30mg hydrocortisone, DIURF-002 and DIURF-006 in a 3-period, cross-over design with a 7-day washout period between each dosing period. The intention was to investigate the impact of the delayed and sustained coats on the bioavailability and also to confirm in vivo hydrocortisone release from the core. The results in Figure 2b show that hydrocortisone was rapidly absorbed in the dogs, that both DIURF-002 and DIURF-006 showed delayed absorption but DIURF-002, which had a sustained coating, showed very reduced relative bioavailability, 8.5%, whereas DIURF-006, which was delayed but then released immediately, had 82% bioavailability.
Impact of sustained release coating on absorption in humans
The dog data suggested that the sustained release coating impaired bioavailability. Therefore three new formulations with varying thickness in sustained release coat were tested in dexamethasone suppressed healthy volunteers. DIURF-004 and -005 had a reduced thickness in the sustained release coat and DIURF-006 had no sustained release coating. The results are shown in Table 2 where it can be seen that reducing the sustained release coat did improve bioavailability, however, even the thin sustained release coat in DIURF-004 and -005, resulted in a relative bioavailability <50% compared to hydrocortisone. In contrast, DIURF-006, with only a delayed release enteric coat, gave >89% bioavailability relative to hydrocortisone. Surprisingly DIURF-006, which is essentially a delayed and immediate release formulation, gave a profile of delayed and sustained absorption with a Tmax 8.5 (5.5-11.0) hr very similar to all the other DIURF formulations which contained sustained release coats.
Table 2.
PK Characteristics of DIURF-000 to 006 compared to hydrocortisone and reference population
| Formulation & Normative data | Cmax nmol/l & μg/dL Geomean (10 & 90th Centile) | Tmax (hr) Median (10 & 90th Centile) | AUC0-24h hr*nmol/l & hr*μg/dL Geomean (10 & 90th Centile) |
|---|---|---|---|
| DIURF-000, 30mg (n=16)* | 176 (133-271) 6.4 (4.8 – 9.8) |
7.5 (5.3-10.0) | 1571 (1165-2049) 56.9 (42.2 – 74.3) |
| DIURF-001, 30mg (n=6) | 191 (153-257) 6.9 (5.5 – 9.3) |
7.5 (5.0-9.5) | 1607 (1279-2489) 58.2 (46.4 – 90.2) |
| DIURF-002, 30mg (n=6) | 221 (172-344) 8.0 (6.2 – 12.5) |
8.3 (5.0-10.0) | 1703 (1273-2713) 61.7 (46.1 – 98.3) |
| DIURF-003, 30mg (n=6) | 226 (178-289) 8.2 (6.5 – 10.5) |
6.8 (4.0-10.0) | 1975 (1396-3209) 71.6 (50.6 – 116.3) |
| DIURF-004, 30mg (n=6) | 273 (206-412) 9.9 (7.5 – 14.9) |
8.5 (6.5-10.0) | 2293 (1687-2987) 83.1 (61.1 – 108.2) |
| DIURF-005, 30mg (n=6) | 269 (137-391) 9.7 (5.0 – 14.2) |
10.0 (5.5-12.0) | 2236 (1362-3406) 81.0 (49.4 – 123.5) |
| DIURF-006, 30mg (n=6) | 693 (559-796) 25.1 (20.3 – 28.9) |
8.5 (5.5-11.0) | 4449 (3945-5315) 161.3 (143.0 – 192.6) |
| DIURF-006, 5mg (n=16) | 288 (213-485) 10.4 (7.7 – 17.6) |
6.0 (3.2-9.3) | 1586 (1030-2223) 57.5 (37.3 – 80.6) |
| DIURF-006, 10mg (n=16) | 440 (322-591) 15.9 (11.7 – 21.4) |
6.3 (4.2-11.9) | 2530 (1810-3600) 91.7 (65.6 – 130.5) |
| DIURF-006, 20mg (n=16) | 642 (513-855) 23.3 (18.6 – 31.0) | 6.0 (2.8-12.0) | 3919 (2816-5448) 142.0 (102.1 – 197.5) |
| DIURF-006, 20+10mg (n=16) | 665 (477-871) | 8.5 (3.2-12.5) | 5610 (4390-7974) |
| Dosing at 2300 & 0700h | 24.1 (17.3 – 31.6) | 203.3 (159.1 – 289.0) | |
| Hydrocortisone 30mg (n=16)* | 1142 (905-1476) 41.4 (32.8 – 53.5) |
1.0 (1.0-2.0) | 5007 (4191-6356) 185 (152.0 – 230.4) |
| Normative data (n=28) | 594 (409-973) 21.5 (14.8 – 35.3) |
0812 (0536 – 0936h)** | 4706 (3242-6588) 170.6(117.5 – 238.8) |
DIURF-000 and Hydrocortisone measured by IA not LCMS
Clock time
Dose-response and twice daily dosing with DIURF-006
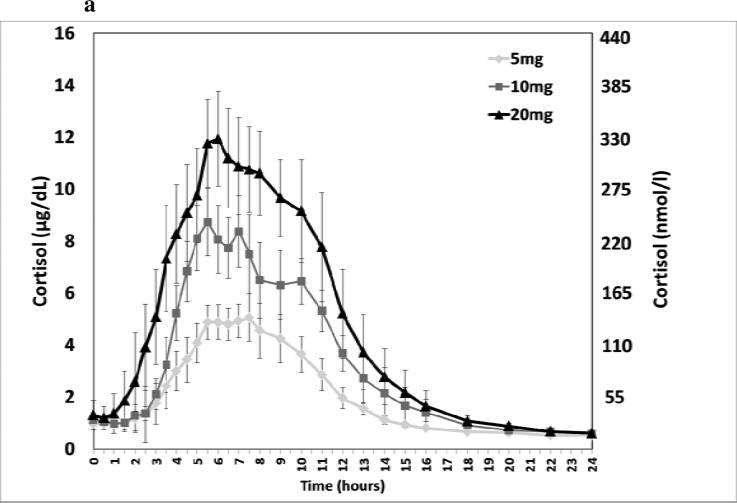
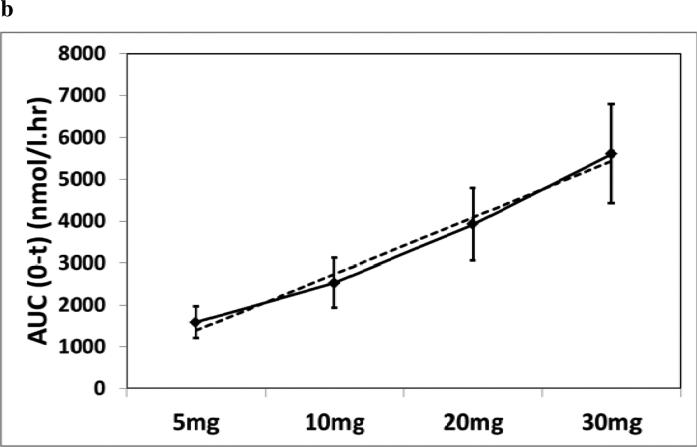
DIURF-006 had a PK profile that provided an overnight rise in cortisol with a Cmax similar to the physiological morning cortisol peak and Tmax compatible with the physiological time of the morning peak. DIURF-006 was therefore selected as the formulation to take forward into a pharmacokinetic study with single doses of 5, 10 & 20mg and also 30mg given in a split dose of 20mg at 23:00h and 10mg at 07:00h. Serum cortisol levels were shown to increase linearly with dose (Figure 4), although dose proportionality was not at unity as shown in the slope of the regression line: Cmax for 5, 10, 20 & 30 (20+10) mg was 10, 16, 23 & 24μg/dL (288, 440, 641, & 665 nmol/l) (Slope: 0.49, 95% CI 0.42-0.56) and AUC0-t was 57, 92, 142 & 203hr*μg/dL (1586, 2530, 3919 & 5610 hr*nmol/L) (Slope: 0.69, 95% CI 0.65-0.73). The AUC and Cmax for twice daily dosing with 20mg nocte and 10mg mane were similar to that reported for normal diurnal cortisol secretion (Table 2 and Figure 4).
Figure 4.
(a) Dose response of DIURF-006; 5, 10, & 20mg (Geomean ± SEM); (b) Dose proportionality for DIURF-006; 5, 10, & 20mg AUC (Geomean ± SD). Dotted line represents the regression line: Slope: 0.69, 95% CI 0.65-0.73; (c) Twice daily dosing at 23:00 & 07:00h with DIURF-006; 20 and 10mg in dexamethasone suppressed healthy male volunteers.
Discussion
We have characterised a modified release, multi-particulate, oral formulation of hydrocortisone that when given at night (23:00h) provides an overnight rise in cortisol. The same Chronocort® formulation, DIURF-006, when given as a twice daily ‘toothbrush’ regimen, 20mg at night (23:00h) and 10mg in the morning (07:00h) provided cortisol exposure similar to that seen in physiological cortisol levels in a healthy reference population and also to that seen in dexamethasone suppressed healthy volunteers after a single dose of 30mg hydrocortisone. The relative bioavailability of DIURF-006 to hydrocortisone was 89%, the Cmax on twice daily treatment was 665nmol/l (24μg/dL) compared to a physiological peak cortisol of 594nmol/l (22μg/dL) and the median Tmax equated to a clock time of 07:30h similar to the time of the normal physiological cortisol peak.
The challenge in replacing cortisol levels in adrenal insufficiency is creating a modified release formulation of hydrocortisone that mimics the normal circadian rhythm with an overnight cortisol rise and peak after waking. The difficulties to overcome being the short half-life of hydrocortisone combined with a limited window of opportunity for absorption in the small bowel. Our initial attempt used a proprietary modified-release technology to create delayed and sustained hydrocortisone release 19. This approach was successful in gaining an overnight rise in cortisol but bioavailability was reduced, the peak too early, and it was not possible to scale up that formulation to achieve consistent dissolution performance. However, based on these observations we proposed that delayed and sustained hydrocortisone release was essential to mimic the physiological overnight rise in cortisol. Unexpectedly, the work we now report with a multi-particulate formulation has shown that a delayed and immediate release multi-particulate formulation results in a delayed and sustained absorption profile. Our work in both dogs and humans showed that the inclusion of a sustained release layer led to reduced bioavailability. We suggest that as the formulation moves through the gut there is reduced water content, reduced mixing and reduced luminal exposure that results in a natural reduction in the rate of absorption of hydrocortisone without the need for a sustained release coat. This was demonstrated with formulation DIURF-006 which had only a delayed release coating and no sustained release layer.
The potential limitations of a modified release formulation of hydrocortisone are reduced bioavailability and increased variability in absorption21. Reduced bioavailability is found with the once daily Plenadren® preparation and also with previous tablet Chronocort® formulations; both formulations based on a monolith, modified-release, tablet presentation16, 19. The advantage of the multi-particulate formulation is that it provides more uniform drug release and a greater surface area for drug release and twice daily administration resulted in greater bioavailability. The variability of cortisol exposure for DIURF-006 was less than that of Plenadren® and immediate release hydrocortisone. For a (single) 30mg dose of DIURF-006, 30 mg Plenadren® and 30mg immediate release hydrocortisone the AUC±SD was: 4449±534, 3757±956 & 5007±939 hr*nmol/l 17.
One of the potential limitations of this study was the use of different cortisol assays. It is well recognised that the measurement of cortisol may vary between immunoassays 22, 23. LC-MS/MS is increasingly seen as a more sensitive measurement of cortisol as there is no interference from cortisol metabolites 24. LC-MS/MS was used in all our experiments when measuring cortisol except in the initial study of hydrocortisone and DIURF-000 where an immunoassay was used.
In defining normal cortisol levels we have considered that the important parameters include overall cortisol exposure (AUC), peak cortisol (Cmax) and time of peak cortisol (Tmax). Based on these parameters DIURF-006 administered 20mg at 23:00h and 10mg at 07:00h gave similar results to physiological cortisol levels: Geomean AUC 5610 vs 4706 hr*nmol/l (203 vs 171hr*μg/dL), Geomean Cmax 665 vs 594 nmol/l (24 vs 22μg/dL) and Median Tmax 8.5 vs 9.2 hours as measured from 23:00h. The AUC for 30mg DIURF-006 given as a split dose was greater than that seen after single dosing and the Cmax was lower. This would be expected as when cortisol levels exceed the capacity of cortisol binding protein, around 550nmol/l, so cortisol clearance increases 25. The time of Cmax after twice daily dosing was on average 8.5 hours after the first dose; a second peak occurred later reflecting the absorption of the morning dose combining with the levels from the evening dose. It would be expected that patients using this formulation would have titration of their dose according to body size which is a major predictor of cortisol levels 4. Dose formulations that have been tested are 5, 10 and 20mg which would allow dose titration. The timing of dosing could also be adjusted according to the patient's time of waking and sleeping.
In conclusion, a multi-particulate formulation of hydrocortisone with a delayed release enteric coating provides a delayed and sustained absorption of hydrocortisone. This formulation, given in a ‘toothbrush’ regimen, last thing at night and first thing in the morning, provides a pharmacokinetic profile similar to the normal physiological diurnal rhythm of cortisol.
Acknowledgements
This research was supported in part by the Intramural Research Program at the National Institutes of Health (NIH), Bethesda, US. W.A. receives grant support from the Medical Research Council UK (Program Grant G0900567). Richard Ross and Martin Whitaker are Directors of Diurnal Ltd and Hiep Huatan and Wiebke Arlt are consultants to Diurnal Ltd, Deborah Merke has a CRADA agreement through the NIH with Diurnal Ltd.
References
- 1.Krieger DT, Allen W, Rizzo F, Krieger HP. Characterization of the normal temporal pattern of plasma corticosteroid levels. Journal of Clinical Endocrinology & Metabolism. 1971;32:266–284. doi: 10.1210/jcem-32-2-266. [DOI] [PubMed] [Google Scholar]
- 2.Plat L, Leproult R, L'Hermite-Baleriaux M, Fery F, Mockel J, Polonsky KS, Van Cauter E. Metabolic effects of short-term elevations of plasma cortisol are more pronounced in the evening than in the morning. J Clin Endocrinol Metab. 1999;84:3082–3092. doi: 10.1210/jcem.84.9.5978. [DOI] [PubMed] [Google Scholar]
- 3.Spiegel K, Leproult R, Van Cauter E. Impact of sleep debt on metabolic and endocrine function. Lancet. 1999;354:1435–1439. doi: 10.1016/S0140-6736(99)01376-8. [DOI] [PubMed] [Google Scholar]
- 4.Mah PM, Jenkins RC, Rostami-Hodjegan A, Newell-Price J, Doane A, Ibbotson V, Tucker GT, Ross RJ. Weight-related dosing, timing and monitoring hydrocortisone replacement therapy in patients with adrenal insufficiency. Clinical Endocrinology. 2004;61:367–375. doi: 10.1111/j.1365-2265.2004.02106.x. [DOI] [PubMed] [Google Scholar]
- 5.Simon N, Castinetti F, Ouliac F, Lesavre N, Brue T, Oliver C. Pharmacokinetic evidence for suboptimal treatment of adrenal insufficiency with currently available hydrocortisone tablets. Clin Pharmacokinet. 2010;49:455–463. doi: 10.2165/11531290-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 6.Moeller H. Chronopharmacology of hydrocortisone and 9 alpha-fluorhydrocortisone in the treatment for congenital adrenal hyperplasia. Eur J Pediatr. 1985;144:370–373. doi: 10.1007/BF00441780. [DOI] [PubMed] [Google Scholar]
- 7.Bergthorsdottir R, Leonsson-Zachrisson M, Oden A, Johannsson G. Premature mortality in patients with Addison's disease: a population-based study. J Clin Endocrinol Metab. 2006;91:4849–4853. doi: 10.1210/jc.2006-0076. [DOI] [PubMed] [Google Scholar]
- 8.Tomlinson JW, Holden N, Hills RK, Wheatley K, Clayton RN, Bates AS, Sheppard MC, Stewart PM. Association between premature mortality and hypopituitarism. West Midlands Prospective Hypopituitary Study Group. Lancet. 2001;357:425–431. doi: 10.1016/s0140-6736(00)04006-x. [DOI] [PubMed] [Google Scholar]
- 9.Bensing S, Brandt L, Tabaroj F, Sjoberg O, Nilsson B, Ekbom A, Blomqvist P, Kampe O. Increased death risk and altered cancer incidence pattern in patients with isolated or combined autoimmune primary adrenocortical insufficiency. Clinical Endocrinology. 2008;69:697–704. doi: 10.1111/j.1365-2265.2008.03340.x. [DOI] [PubMed] [Google Scholar]
- 10.Lovas K, Loge JH, Husebye ES. Subjective health status in Norwegian patients with Addison's disease. Clinical Endocrinology. 2002;56:581–588. doi: 10.1046/j.1365-2265.2002.01466.x. [see comment] [DOI] [PubMed] [Google Scholar]
- 11.Arlt W, Willis DS, Wild SH, Krone N, Doherty EJ, Hahner S, Han TS, Carroll PV, Conway GS, Rees DA, Stimson RH, Walker BR, Connell JM, Ross RJ. Health status of adults with congenital adrenal hyperplasia: a cohort study of 203 patients. The Journal of clinical endocrinology and metabolism. 2010;95:5110–5121. doi: 10.1210/jc.2010-0917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Finkielstain GP, Kim MS, Sinaii N, Nishitani M, Van Ryzin C, Hill SC, Reynolds JC, Hanna RM, Merke DP. Clinical characteristics of a cohort of 244 patients with congenital adrenal hyperplasia. The Journal of clinical endocrinology and metabolism. 2012;97:4429–4438. doi: 10.1210/jc.2012-2102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Merza Z, Rostami-Hodjegan A, Memmott A, Doane A, Ibbotson V, Newell-Price J, Tucker GT, Ross RJ. Circadian hydrocortisone infusions in patients with adrenal insufficiency and congenital adrenal hyperplasia. Clinical Endocrinology. 2006;65:45–50. doi: 10.1111/j.1365-2265.2006.02544.x. [DOI] [PubMed] [Google Scholar]
- 14.Bryan SM, Honour JW, Hindmarsh PC. Management of altered hydrocortisone pharmacokinetics in a boy with congenital adrenal hyperplasia using a continuous subcutaneous hydrocortisone infusion. The Journal of clinical endocrinology and metabolism. 2009;94:3477–3480. doi: 10.1210/jc.2009-0630. [DOI] [PubMed] [Google Scholar]
- 15.Lovas K, Husebye ES. Continuous subcutaneous hydrocortisone infusion in Addison's disease. Eur J Endocrinol. 2007;157:109–112. doi: 10.1530/EJE-07-0052. [DOI] [PubMed] [Google Scholar]
- 16.Johannsson G, Nilsson AG, Bergthorsdottir R, Burman P, Dahlqvist P, Ekman B, Engstrom BE, Olsson T, Ragnarsson O, Ryberg M, Wahlberg J, Biller BM, Monson JP, Stewart PM, Lennernas H, Skrtic S. Improved cortisol exposure-time profile and outcome in patients with adrenal insufficiency: a prospective randomized trial of a novel hydrocortisone dual-release formulation. The Journal of clinical endocrinology and metabolism. 2012;97:473–481. doi: 10.1210/jc.2011-1926. [DOI] [PubMed] [Google Scholar]
- 17.Duocort Plenadren. European public assessment reports (EPAR) 2011 EMEA/H/C/2185. [Google Scholar]
- 18.Newell-Price J, Whiteman M, Rostami-Hodjegan A, Darzy K, Shalet S, Tucker GT, Ross RJ. Modified-release hydrocortisone for circadian therapy: a proof-of-principle study in dexamethasone-suppressed normal volunteers. Clinical Endocrinology. 2008;68:130–135. doi: 10.1111/j.1365-2265.2007.03011.x. [DOI] [PubMed] [Google Scholar]
- 19.Debono M, Ghobadi C, Rostami-Hodjegan A, Huatan H, Campbell MJ, Newell-Price J, Darzy K, Merke DP, Arlt W, Ross RJ. Modified-release hydrocortisone to provide circadian cortisol profiles. The Journal of clinical endocrinology and metabolism. 2009;94:1548–1554. doi: 10.1210/jc.2008-2380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verma S, Vanryzin C, Sinaii N, Kim MS, Nieman LK, Ravindran S, Calis KA, Arlt W, Ross RJ, Merke DP. A pharmacokinetic and pharmacodynamic study of delayed- and extended-release hydrocortisone (Chronocort) vs. conventional hydrocortisone (Cortef) in the treatment of congenital adrenal hyperplasia. Clin Endocrinol (Oxf) 2010;72:441–447. doi: 10.1111/j.1365-2265.2009.03636.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lennernas H, Skrtic S, Johannsson G. Replacement therapy of oral hydrocortisone in adrenal insufficiency: the influence of gastrointestinal factors. Expert opinion on drug metabolism & toxicology. 2008;4:749–758. doi: 10.1517/17425255.4.6.749. [DOI] [PubMed] [Google Scholar]
- 22.Clark PM, Neylon I, Raggatt PR, Sheppard MC, Stewart PM. Defining the normal cortisol response to the short Synacthen test: implications for the investigation of hypothalamic-pituitary disorders. Clinical Endocrinology. 1998;49:287–292. doi: 10.1046/j.1365-2265.1998.00555.x. [DOI] [PubMed] [Google Scholar]
- 23.Roberts RF, Roberts WL. Performance characteristics of five automated serum cortisol immunoassays. Clinical Biochemistry. 2004;37:489–493. doi: 10.1016/j.clinbiochem.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 24.Kushnir MM, Neilson R, Roberts WL, Rockwood AL. Cortisol and cortisone analysis in serum and plasma by atmospheric pressure photoionization tandem mass spectrometry. Clinical Biochemistry. 2004;37:357–362. doi: 10.1016/j.clinbiochem.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Tunn S, Mollmann H, Barth J, Derendorf H, Krieg M. Simultaneous measurement of cortisol in serum and saliva after different forms of cortisol administration. Clinical Chemistry. 1992;38:1491–1494. [PubMed] [Google Scholar]