Abstract
Expression and activity of several key drug metabolizing enzymes (DMEs) and transporters are altered in various pathophysiological conditions, leading to altered drug metabolism and disposition. This can have profound impact on the pharmacotherapy of widely used clinically relevant medications in terms of safety and efficacy by causing inter-individual variabilities in drug responses. This review article highlights altered drug disposition in inflammation and infectious diseases, and commonly encountered disorders such as cancer, obesity/diabetes, fatty liver diseases, cardiovascular diseases and rheumatoid arthritis. Many of the clinically relevant drugs have a narrow therapeutic index. Thus any changes in the disposition of these drugs may lead to reduced efficacy and increased toxicity. The implications of changes in DMEs and transporters on the pharmacokinetics/pharmacodynamics of clinically-relevant medications are also discussed. Inflammation-mediated release of pro-inflammatory cytokines and activation of toll-like receptors (TLRs) are known to play a major role in down-regulation of DMEs and transporters. Although the mechanism by which this occurs is unclear, several studies have shown that inflammation-associated cell-signaling pathway and its interaction with basal transcription factors and nuclear receptors in regulation of DMEs and transporters play a significant role in altered drug metabolism. Altered regulation of DMEs and transporters in a multitude of disease states will contribute towards future development of powerful in vitro and in vivo tools in predicting the drug response and opt for better drug design and development. The goal is to facilitate a better understanding of the mechanistic details underlying the regulation of DMEs and transporters in pathophysiological conditions.
Keywords: Inflammation, drug metabolizing enzymes, drug transporters, pharmacokinetics, Toll-like receptors, cytokines
1. INTRODUCTION
Drug metabolism can either lead to detoxification, bio-inactivation and/or elimination of drugs from the body. Metabolism can be broadly categorized into phases I and II. Phase I drug metabolizing enzymes (DMEs) primarily comprise of the Cytochrome (CYP) 450 family of enzymes. Within the 50 years after P450 discovery, tremendous research efforts on mammalian CYPs have shown that CYPs are not only involved in the biotransformation of drugs and xenobiotics but also to play a crucial role in the synthesis and metabolism of a variety of endogenous compounds such as steroids, fatty acids, prostaglandins, vitamins and bile acids. CYP3A4 is the most common isoform expressed in human liver and intestine accounting for ~30–60% of CYPs [1]. More than 50% of the currently marketed drugs are metabolized by CYP3A4 in humans [2]. Phase II metabolism consists of conjugation reactions such as glucuronidation, sulfation, glutathione conjugation or methylation forming polar metabolites leading to enhanced excretion [3, 4]. Drug transporters play a central role in the absorption, distribution, metabolism and elimination (ADME) processes of xenobiotics across the cellular barriers. They are broadly classified into uptake and efflux transporters which facilitate drug disposition in or out of the cells [5]. Major transporters include, but are not limited to: multidrug resistant gene/P-glycoprotein (MDR/P-gp), multidrug resistance associated protein (MRP1-3), breast cancer resistance protein (BCRP), organic anion transporting peptides (OATPs) and organic cationic transporters (OCTs) [5].
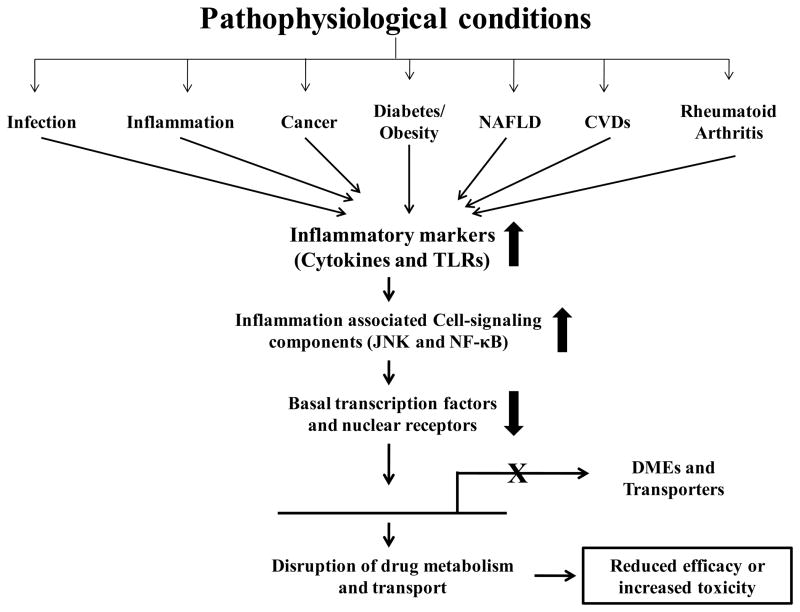
Several studies have shown that drug metabolism and transport is disrupted during pathophysiological conditions primarily due to reductions in gene expression of these enzymes and transporters (Fig. 1) [6, 7]. Altered drug metabolism can lead to adverse drug reactions which account for ~10% of hospitalized cases [8]. However, due to underreporting, the actual incidences may be much higher [9]. As early as 1960s, variations in drug metabolism were reported in patients or animals with diabetes [10], cancer [11], hepatitis [12] or influenza [13] along with a corresponding change in the PD of drugs [10, 14]. These early studies prompted researchers to further elucidate DME and transporter gene expression and PK/PD of clinically-relevant drugs in pathophysiological conditions such as obesity, rheumatoid arthritis, non-alcoholic fatty liver disease (NAFLD) and cardiovascular diseases (CVDs) such as hypertension, heart failure, or stroke. The possible mechanistic pathways regulating the DMEs and transporters during these pathophysiological conditions are discussed.
Fig. 1.
Schematic of regulation of drug disposition in pathophysiological conditions
Cell-signaling components including the transcription factors such as nuclear factor-κB (NF-κB) or CAAT enhancer-binding protein (C/EBP) [15, 16] and the xenobiotic nuclear receptors, pregnane X receptor (PXR) and constitutive androstane receptor (CAR), upon heterodimerization with the central nuclear receptor, retinoid X receptor (RXR)-α, regulate the gene expression of DMEs and transporters [17]. The orphan nuclear receptor, hepatocyte nuclear factor (HNF) 4α regulate the gene expression of PXR and CAR mediated xenobiotic induction of CYP3A4 [18]. The readers of this review will gain detailed understanding of hypothesis-driven mechanisms known to play a major role in altering the DMEs and transporters, and PK/PD (pharmacokinetics/pharmacodynamics) of clinically relevant medications in the above listed pathophysiological conditions.
2. INFECTION AND INFLAMMATION
2.1. Bacterial infections
2.1.1. Drug metabolizing enzymes
Most of the studies on regulation of DMEs have been documented with gram-negative bacteria. Clinically relevant cecal ligation and puncture (CLP) or inflammatory bowel disease (IBD) induced by Citrobacter rodentium (gram-negative pathogen) are the most frequently used models owing to their close resemblances in the progression and characteristics of human sepsis [19, 20]. Alterations in total hepatic microsomal CYP content and activities were reported in CLP rat or IBD mouse models [21, 22]. The rapid down-regulation of CYP2Cs and CYP3As after intraperitoneal (i.p) injection and CYP4As after oral injection of C. rodentium was quantitatively and qualitatively different, suggesting that the effects of oral infection are not due to bacterial translocation to the liver [22]. Infection of pigs with the gram-negative respiratory pathogen, Actinobacillus pleuropneumoniae, led to decreased microsomal metabolism of several CYP-dependent substrates 24 h after inoculation [23]. Similarly, gram-positive bacterial infections account for more than 50% of the total community acquired infections [24]. Listeriosis, caused by Listeria monocytogenes, is one of the most critical food-borne diseases in humans. L. monocytogenes induced CNS infection in rodents significantly down-regulated mRNA, protein and activity of hepatic CYPs [25].
The gram-negative and gram-positive bacterial components, lipopolysaccharide (LPS), and lipoteichoic acid (LTA), serve as sterile infection models by inducing inflammatory responses in animals [26, 27]. LPS injections down-regulate expression and activity of key hepatic, intestinal and renal DMEs in several animal species such as mice, rats or rabbits [28, 29]. The effect of LPS on CYP expression and activity is dependent on the route of administration at same dose of LPS [30]. We recently showed that LTA significantly down-regulated the gene expression of several phase I and phase II DMEs in mice [31].
2.1.2. Transporters
Changes in expression of drug transporters can have significant impact on the safety and efficacy of the drugs. LPS treatment of mice significantly down-regulated P-gp and Mrp2, major transporters involved in disposition of clinically relevant drugs such as colchicine, verapamil, daunorubicin, cyclosporin A and the abundant food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine (PhIP) [32, 33]. LPS-treated mice had significantly lower hepatic P-gp (30% of control) and increased P-gp expression in the kidney (140% controls) [34, 35].
2.1.3. PK/PD studies
As early as 1980’s, reduced clearances of clinically relevant drugs have been reported in humans infected with BCG vaccine, Streptococcus pneumoniae or Mycoplasma pulmonis [36–38]. LPS injections in animals and humans altered PK parameters such as maximum plasma concentration (Cmax, increase), area under the curve (AUC, increase), half-life (T1/2), volume of distribution (Vd) and clearance (CL, deccrease) of various drugs including cisplatin, antipyrine, theophylline, hexobarbital, gentamicin and vancomycin [39–42]. Humans infected with gram-positive bacteria such as Pseudomonas or Staphylococcus, led to an increase in Vd and dilution of antimicrobial agents in plasma and extracellular fluids, requiring careful monitoring of the dosage regimen [43]. PD changes were observed in turpentine oil-injected mice which showed high anti-tumor activity of gimatecan compared to the controls [44]. On the other hand, despite of very high plasma concentrations of the calcium channel blocker, verapmil, or potassium channel antagonists, sotalol or propranolol; no change in the PD was observed in inflamed animals [45, 46]. This discrepancy may be related to altered receptor-functioning or receptor-ligand binding in inflammation. Nevertheless, the above studies need further evaluations to delineate the disparities in altered drug metabolism caused by different bacterial infections or inflammation which has significant clinical implications for drug therapy in disease states.
2.2. Viral infections
2.2.1. Drug metabolizing enzymes
Viral infections result in the release of various inflammatory mediators from the immune cells [47]. Several studies have shown deleterious effects of viral infections such as mouse-adapted influenza virus [48], Newcastle disease virus [49], encephalomyocarditis virus [50], chronic active hepatitis and cirrhosis [51, 52] and HIV infection [53] on alteration of expression and activity of DMEs and oxidative pathways in animals and humans. Decreased levels of hepatic CYP1A2 were detected in children suffering from upper respiratory tract viral infections during an influenza outbreak [13]. With exceptions of CYP2D6 mRNA and CYP1A2 activity, other major CYPs such as CYP2C9, 2C19, and 3A4 in hepatitis C virus (HCV) infected PXB mice (mouse model with human hepatocytes) were comparable to the non-infected controls [54]. Recombinant adenovirus injections in Sprague-Dawley rats led to a significant down-regulation of renal CYP2E1 and hepatic CYP3A2 and CYP2C11 expression and activity, and induction of CYP4A protein expression [55, 56].
2.2.2. Drug transporters
A recent study showed that HIV-type 1 viral envelope glycoprotein gp120 decreased P-gp and Mrp expression levels in rat astrocytes [57]. However, due to the fact that HIV infected patients are on highly active antiretroviral therapy (HAART) consisting of numerous drugs, both, induction and suppression of drug transporters in HIV infection are reported [58]. Polyinosinic/polycytidylic acid [poly (I:C)] is widely as a model of in vivo viral-induced inflammation. Poly (I:C) can induce interferons (IFNs) and pro-inflammatory cytokines such as interleukin (IL)-6, IL-10, IL-12, and tumor necrosis factor (TNF)-α. A significant down-regulation of key maternal hepatic and placental drug transporters and their endogenous substrates was observed upon i.p injection of poly (I:C) in pregnant rats [59]. However, Abcb1b (ATP-binding cassette sub-family B member 1) and Abcc3 (ATP-binding cassette subfamily C) were significantly induced. A recent study in PXB mice infected with HCV reported significantly higher expression of MRP4 and OATP2B1 and lower expression of OCT1 compared to non-infected mice [54].
2.2.3. PK/PD Studies
During the 1982 influenza B outbreak in King County, Washington, 11 children whose asthma had previously been controlled with a stable theophylline dose, developed theophylline toxicity at this same dose [13]. These children had a significant decrease in CL and increase in T1/2 of theophylline. HIV infections could also lead to altered PK of levofloxacin and fluconazole [60, 61]. End-stage liver disease, which is largely the result of HCV infection, now accounts for up to 50% of deaths among persons with HIV-1 infection [62]. A clinical study in HIV-HCV-coinfected patients showed significantly lower nelfinavir oral clearances in HIV+ and HCV+ patients with and without cirrhosis compared to HIV+ and HCV-negative patients [63]. This presses the need for therapeutic drug monitoring in individualizing nelfinavir dosage in HIV-HCV-coinfected patients. In addition, an increase in AUC and Cmax of several anti-retrovirals are reported in HCV-infected patients with moderate liver impairment [64, 65]. Other studies also showed significantly higher AUC of docetaxel and reduced glomerular filtration rate, suggesting changes in renal CYP in rats injected with the recombinant adenovirus expressing β-galactosidase [56, 66].
2.3. Mechanisms for altered drug metabolism in infections and inflammation
Bacterial or viral infections lead to activation of Toll-like receptor (TLR) signaling pathway, which leads to the induction of pro-inflammatory cytokines, IL-1β, IL-6 and TNF-α in the immune cells. In the liver, TLRs are present on the cell surface of various immune cells (the resident macrophages or Kupffer cells) as well as the hepatocytes [67]. Out of the 13 TLRs identified in mammals, TLR4 is activated by the gram-negative component, LPS, and TLR2 is activated by the gram-positive component, LTA [68, 69]. We and others have shown down-regulation of Cyp3a11 and P-gp in LPS-sensitive TLR4 wild type (C3HeB/FeJ) mice could not be detected in TLR4-mutant (C3H/HeJ) mice [29]. Recent data from our group showed that down-regulation of gene expression of key hepatic phase I and phase II DMEs in TLR2+/+ mice by LTA was blocked in TLR2−/− mice [31]. We also observed that LTA down-regulated Mrp2, had no effect on Mrp3 and induced Mdr1b expression. Although, most of the studies have cited the role of Kupffer cell-derived TLRs in hepatic drug metabolism, we and others have also shown that LPS or LTA treatment of primary mouse hepatocytes can directly affect the DMEs via TLRs present on the hepatocytes, independent of cytokines [70, 71]. TLR-mediated signaling is initiated by the down-stream adaptor protein, Toll-interleukin 1 receptor domain containing adaptor protein (TIRAP) [72]. We showed that TIRAP was involved only in TLR2-mediated regulation of DME and transporter genes [71], and not by TLR4 [29].
Cytokines are involved in alteration of DMEs and transporters in vitro [73, 74]. LPS-treatment of primary rat cocultures of hepatocytes and Kupffer cells significantly suppressed phenobarbital-mediated induction of CYP2B1 [75]. This decrease was associated with a 5-fold induction in TNF-α released from the Kupffer cells in cocultures. In vitro studies with cytokine-treated rat or human hepatocytes led to decreased expression and activity of several drug transporters including efflux pumps such as P-gp, MRP2, 3 and 4, and BCRP, and uptake transporters such as OATP-B, OATP-C, and OATP-8 [76–78].
However, recent evidence suggests cytokines may not be playing a major role in regulation of DMEs. Earlier studies in TNF-α−/− and IL-6−/− knockout mice revealed that DMEs were still down-regulated [79, 80]. A recent study by Kinloch et al in TNFR1−/−, IL1R1−/− and Kupffer cell-depleted mice showed that only TNF-α, but not IL-1β or Kupffer cells, was involved in regulation of CYP3A11 and 3A25 in oral C. rodentium infected mice [81]. In addition, we showed that although down-regulation of DMEs was blocked in LTA-treated TIRAP−/− mice, hepatic cytokine gene expression remained unchanged [71].
Nitric oxide (NO), released from macrophages and hepatocytes during inflammation is also known to regulate DMEs [82]. However, contrasting results have been reported for the role of NO in regulation of DMEs in cytokine-treated primary rat hepatocytes [83, 84]. IL-1β and TNF-α-mediated down-regulation of CYP protein was NO dependent, but not in IL-6 mediated down-regulation [83]. NO was also shown to regulate the suppression of UGT activities in cytokine-treated hepatocytes [85].
Inflammation-mediated activation of NF-κB plays a significant role in down-regulation of DMEs [86, 87]. NF-κB can either indirectly regulate CYP gene expression through mutual repression between NF-κB and nuclear receptors, or can directly regulate CYP gene expression through binding to NF-κB response element in the promoter region of CYP genes [88]. Interaction of NF-κB with nuclear receptors during pathophysiological conditions can alter expression of DMEs [89]. Inflammation activates the mitogen activated protein kinase (MAPK), c-Jun-N-terminal kinase (JNK) which also regulates nuclear receptors and DMEs [90, 91]. Recent experiments in human gastric carcinoma and pancreatic carcinoma cell lines suggested a prominent role of JNK activation in down-regulation of P-gp protein expression [92]. However, further detailed studies using in vitro models such as cell lines or primary hepatocytes, and specific inhibitors of these cell signaling components will significantly contribute in understanding the mechanistic regulation of DMEs and transporters during inflammation.
It is known that down-regulation of DMEs and transporters during inflammation are associated with reduced expression of the regulatory nuclear receptors [29, 31, 93, 94]. We showed that down-regulation of nuclear receptors by LPS in TLR4+/+ or by LTA in TLR2+/+ mice was blocked in TLR4 mutant or TLR2−/− mice [29, 31, 71]. However, mRNA and protein expression of several CYPs did not differ in PXR−/− or PPAR−/− mice treated with LPS [95]. Similarly, it was shown that PXR was least important in regulating several efflux and uptake drug transporters using PXR wild type or PXR null mice treated with LPS [96]. However, the down-regulation of Bsep (bile salt export pump) and Mrp2 mRNA in IL6-treated wild type mice was attenuated in the PXR null mice. Thus, involvement of nuclear receptors in inflammation-mediated regulation of DMEs and transporters may depend on the nature of the inflammatory stimuli.
3. CANCER
3.1. Drug metabolizing enzymes
Owing to the fact that, most anticancer drugs have a very low or narrow therapeutic index, alteration of DMEs can lead to life-threatening adverse drug reactions or increased risk of treatment failure in patients undergoing chemotherapy. Decreased hepatic microsomal DME activity was detected in tumor bearing rats with Walker carcinosarcoma 256, where impaired metabolism of hexobarbital, strychnine and meprobamate was observed [11].
Due to difficulties in obtaining human liver tissue from cancer patients, an Engelbreth-Holm-Swarm (EHS) sarcoma mouse model bearing transgenic CYP3A4/lacZ gene was developed [97]. Reduced hepatic levels of the transgene-derived β-galactosidase, as quantified by o-nitrophenyl-β-D-galactopyranoside assay, and Cyp3a11 mRNA and protein was observed in these mice [97]. Tumors derived from the surface of the ovary account for the vast majority of ovarian tumors (approximately 80%). Altered gene expression ratio of CYP3A4/ABCB1 (P-gp) in cancer cells grown from epithelial ovarian tumors had significant contribution in altering docetaxel disposition [98]. On the other hand, there was no significant correlation in CYP2C8/ABCB1 ratio suggesting that paclitaxel disposition may require additional critical gene products. The expression of several phase II DMEs was also characterized in EHS tumor-bearing mice [97]. Out of 8 GSTs studied, six were reduced and two unchanged; SULT1A1 was increased while SULT2A1 and UGT2B5 were reduced, and no change was observed in UGT1A7. Tamoxifen remains the first-line targeted treatment for the estrogen receptor α-positive breast cancer patients and undergoes metabolism in the breast tissue which also consists of several DMEs [99]. In a study examining the role of methylation patterns of genes responsible for tamoxifen metabolism, higher methylation rate of N-acetyl transferase-1 (NAT1), a phase II DME gene, was observed in human breast cancer tissues compared to control breast tissues [100].
3.2. Drug transporters
Changes in the genetic variability in clinical specimens as well as over expression of ABC transporter family in tumors have been shown to play a critical role in multidrug resistance to several anticancer drugs [101–103]. A recent study showed significant reductions in the mRNA levels of Mdr2, Mrp2, Mrp3, Ntcp, Oatp 2, Bsep, Bcrp, whereas Mdr1a and Oatp1 remained unchanged [104].
3.3. PK/PD studies
Cancer-induced changes in the PK and PD profiles of several drugs have been documented since the late 1960s [14, 105]. In a clinical study, the absorption rate constant, apparent Vd and serum CL of penbutolol (antihypertensive drug) were significantly reduced in the cancer group [106]. PD effect (reduction in heart rate) of penbutolol did not vary statistically in respect to baseline values in cancer patients [106]. Reduction in the metabolism of omeprazole (CYP2C19 substrate) has also been observed in patients with advanced cancer [107]. Reduced CYP3A expression resulted in >2 fold increase in the sleep time in tumor bearing mice receiving the widely used sedative-hypnotic, midazolam (CYP3A specific substrate) [97].
3.4. Mechanisms of cancer-mediated altered drug metabolism
Since the 1800s, it was observed that chronic inflammation is frequently associated with the onset and progression of various cancers [108]. A strong association between cancer progression and induction of cytokines or acute phase reactive proteins in tumors is documented [109, 110]. For e.g. EHS tumor-bearing mice had significantly higher circulating plasma levels of IL-6 (25 pg/ml) compared to the control mice (below detection limit). IL-6 mediated activation of JNK was also evident in EHS tumor-bearing mice, which again prompts the important role of JNK in regulation of DMEs. Studies have shown that TLR expression is enhanced in tumor cells lines [111]. However, the role of TLRs in alteration of DMEs and transporters in cancer has never been investigated.
The role of NF-κB activation in acute inflammation has been suggested in carcinogenesis [112, 113]. Cancer-mediated alteration of DMEs and transporters may possibly be regulated by over-expression of NF-κB. A recent study highlighted the role of extra hepatic malignancies in down-regulation of PXR and CAR in tumor-bearing mice [114]. This study prompts to link the reduction in nuclear receptors with altered drug metabolism in cancer. However, additional studies with nuclear receptor knockout animal models with tumors will help identify their direct role in regulation of DMEs and transporters. Overall, all these studies imply that tumor-mediated inflammation may play an integral role in drug response and toxicity of various anticancer agents.
4. DIABETES AND OBESITY
4.1. Drug metabolizing enzymes
Another prevalent pathophysiological condition affecting millions of people in the world is the occurrence of diabetes and obesity. As per the latest statistical report, 366 million people in the world will have diabetes by 2030 [115]. Dixon et al demonstrated that alloxan-induced diabetes decreased hexobarbital, chlorpromazine, and codeine metabolism in male rats [10, 116]. Although, streptozotocin-induced diabetes in rats and hamsters significantly induced hepatic and renal CYP2E1 and 4A2 protein levels [117, 118], suggesting altered metabolism of ketones and fatty acids in diabetes, hepatic CYP2E1 protein levels remained unchanged in streptozotocin-induced diabetic mice livers [118, 119]. A recent study showed differential effects of alloxan-induced diabetes on protein expression and activity of CYP2E1 (increased) and CYP2B4 (decreased) in rabbits [120]. Altered gene expression of DMEs in genetically obese zucker fatty rats (reduction in CYP2B1/2 and Mrp3) and db/db mice (increase in CYP2B10) are also reported [121, 122]. Studies have reported interesting results on DME gene and protein expression for different diet-induced obese (DIO) animal models. E.g. Although Cyp3a11 gene and protein expression were significantly reduced in both long term (12 weeks) and short term treatment (1 week) of high fat diet (HFD), Cyp2c9 gene expression was significantly reduced only in the short term HFD treatment [123]. On the other hand, gold thioglucose induced obese mice had significant elevations in CYP2B10 and CYP4A10 gene expression [123]. We recently showed that mRNA levels of the phase II DMEs (Ugt1a1, Sult1a1, Sultn) were reduced ~30–60% in mice fed high-fat diet (HFD, 60% kcal fat for 14 weeks) compared to low fat diet (LFD, 10% kcal fat) mice [124]. RNA levels of Cyp2e1 and Cyp1a2 were unaltered in HFD mice. These findings indicate that regulation of CYPs is dependent on the model of diabetes and obesity, and is tissue, isoform and species-specific.
4.2. Drug transporters
Streptozotocin treatment in rats increased hepatic levels of Mdr2, leading to increased phospholipid secretion into bile [125]. Another study also showed that the hepatic expression of uptake transporters (Oatp1a1, 1a4, 1b2, 1a6, 2b1, and Ntcp) in diabetic mice decreased significantly compared to the wild type controls [126]. Our recent study showed no effect of high fat in DIO mice on gene expression of hepatic transporters (Mrp2 and 3, and Mdr1b) [124].
4.3. PK/PD studies
Obesity-associated alterations in phase II metabolism were reported in 1980’s. For e.g. clearances of oxazepam and lorazepam, widely used benzodiazepines and excreted as glucuronide conjugates, were significantly increased in obese patients [127]. Similarly, increased metabolism of chlorzoxazone (CYP2E1 substrate) to 6-hydroxychlorzoxazone was observed in obese individuals. This was attributed to increased CYP2E1 activity associated with obesity [128]. Animal studies performed using a diabetes mellitus rat model (induced by alloxan or streptozotocin treatment) have reported altered PK of drugs such as acetaminophen (APAP), chlorzoxazone, furosemide, and methotrexate [129–132]. A recent clinical study demonstrated accelerated clearance and a decrease in the AUC of levofloxacin in ambulatory obese individuals compared to the normal weight [133]. Although, no changes in PD of atracurium were reported in obese animals compared to lean control [134], triazolam-induced sedation in obese patients increased significantly compared to normal weight men [135]. We also observed similar disparities in the PD of midazolam, CYP3A substrate, (increased sleep time) and zoxazolamine, CYP2E1 substrate (no change) in DIO mice [124]. This can be attributed to decrease in CYP3A and no change in CYP2E1 expression. Thus, the differential effects of obesity on PD of drugs may depend on the DME, the drug or the target organ itself.
4.4. Mechanisms of altered drug metabolism in diabetes/obesity
The major pathophysiological manifestation in diabetes/obesity is characterized by low-level chronic and local inflammation, such as release or over expression of TNF-α and C-reactive protein in adipose tissue [136, 137]. However, the role of inflammation in regulation of DMEs and transporters in diabetes/obesity remains unclear. Hormonal regulation of DMEs in diabetes/obesity has also been addressed before [138]. Although an increase in mRNA or protein levels of CYP2E1 have been observed in obese patients [139], db/db mice showed no such effects [122]. This can possibly be due to hyperinsulinemia leading to a faster turnover (shorter CYP2E1 mRNA half-life) by insulin [140]. Various studies have shown that phosphatidylinositol-3-kinase (PI3K) signaling, using PI3K inhibitors, wortmannin and LY294002, ameliorated insulin-mediated decrease in CYP2E1 and phase II enzymes (α-GST) mRNA [141, 142].
Interestingly, lower expression of CAR and CYP2B in obese Zucker rats and ~2 fold induction in obese and genetically diabetic mice (db/db) on HFD [121, 123] were reported. This discrepancy in obese Zucker rats and db/db mice in regulating expression profiles of CYPs and nuclear receptors can be explained by the difference in the position of mutation of leptin receptor gene [143, 144]. We recently showed that expression of PXR and CAR; and protein levels of RXRα were significantly reduced in HFD mice [124]. Thus, a complex set of processes including but not limited to cytokines, nuclear receptors, insulin sensitization or downstream signaling molecules, may regulate DMEs and transporters in diabetes/obesity.
5. NON-ALCOHOLIC FATTY LIVER DISEASE
5.1. Drug metabolizing enzymes
Non-alcoholic fatty liver disease (NAFLD) is highly prevalent with an estimated world population between 14% and 24% being affected. NAFLD comprises of symptoms ranging from simple steatosis (fatty liver) to the more severe non-alcoholic steatohepatitis (NASH, fatty liver with infiltration of inflammatory cells) to progressive hepatic fibrosis and to cirrhosis [145]. Alteration of hepatic CYP2E1 was first noted in humans with NASH [146]. Later studies have shown significant contribution of NAFLD (comprising of both, simple stage fatty liver as well as NASH) on expression and activity of DMEs in animals [147–149]. Similarly, in vitro studies in primary human or animal hepatocyte cell cultures from steatotic or non-steatotic livers showed a profound impact of steatosis on the metabolic functionality of hepatocytes [150, 151]. Significant reductions in CYP1A2, 2C9, 2E1 and 3A4 activities in fat-overloaded hepatocytes were observed compared with control hepatocytes prepared from the same liver [147].
5.2. Drug transporters
Decreased mRNA and protein expression of uptake transporters such as NTCP, OATP1a1, 1a4, 1b2 and 2b1; and OAT 2 and 3 were observed in NAFLD [148].
5.3. PK/PD studies
Studies have shown interesting results with APAP PK in rats and humans with NAFLD. Children with NAFLD had significantly higher concentrations of APAP-glucuronide (APAP-G) in serum and urine compared with controls, with no significant differences in PK of APAP among the 2 groups [152]. Another study showed that biliary concentrations of APAP-sulfate (APAP-S), APAP-G, and APAP-glutathione were reduced in MCD (methionine- and choline-deficient) rats [153]. However, plasma levels of APAP-G were also elevated in MCD rats, similar to that observed in children [152]. A clinical study evaluated the effect of NAFLD on PK of silymarin [154]. The AUC0-24h for the sum of total silymarin flavonolignans was ~3–4 fold higher in patients with NAFLD (p<0.03), compared with healthy volunteers.
5.4. Mechanisms of altered drug metabolism in NAFLD
Several mechanisms have been proposed for the effect of NAFLD on altered drug metabolism. Deposition of fat in human hepatocytes can lead to a marked impairment in CYP mRNA and activity [155]. Fisher et al observed intense staining for IL-1β in steatotic livers, indicating that experimental steatosis and NASH results in increased hepatocellular inflammation [148]. Studies have shown ambiguous results on expression of nuclear receptors and transcription factors in NAFLD [149, 156, 157]. Except for PXR, which was significantly increased by 1.4 fold, the other nuclear receptors (AhR, CAR, PPARα and Nrf2) were not altered [147]. Therefore, various factors need to be taken into account for improved pharmacotherapy in patients with NAFLD.
6. CARDIOVASCULAR DISORDERS
6.1. Drug metabolizing enzymes
CYPs in humans are responsible for metabolizing a large number of cardiovascular medications, including β-blockers, calcium channel blockers and angiotensin receptor antagonists [158]. Alteration in DMEs could be of particular clinical relevance in patients with heart failure because these patients take more than 10 medications on average. Although, not detected in the normal human heart, failing hearts expressed CYP11B1 and 11B2 [159]. Surprisingly, an up-regulation in CYP2J2, 1B1, 2E1, 4A10 and 2F2 gene expression was reported in the failing heart [160]. Increased cardiac CYP11B2 mRNA was associated with increased myocardial fibrosis and the severity of left ventricular dysfunction in patients with heart failure [161]. It was shown that the production of testosterone metabolites, including dihydrotestosterone and androstenedione, was significantly increased in hypertrophic human hearts [162]. Transient ischemic attacks (TIA) are risk factors for strokes. A recent study showed that cerebral infarct size was reduced in TIA-preconditioned animals and CYP2C11 mRNA and protein were coincidentally increased in the brain after experimentally induced TIA [163]. Genetic polymorphisms of DMEs are commonly associated with heart failure and hypertension [164]. For e.g. a study in Japanese subjects reported that CYP2C9 wild type carriers had lower systolic blood pressure after losartan (metabolizes to the active metabolite EXP3174) therapy than poor metabolizers [165].
6.2. Drug transporters
A recent study demonstrated a selective disease-dependent regulation of the high-affinity carnitine transporter, OCTN2, in patients with dilated cardiomyopathy, whereas the other OCT(N)s were unaffected [166].
6.3. PK/PD studies
It was shown that lidocaine plasma clearance was significantly decreased in patients with cardiac failure and this was associated with decreased liver blood flow [167]. Another group also observed reduced plasma clearance of lignocaine in patients suffering from myocardial infarction without cardiac failure [168]. Thus, the mounting evidence for the effect of CVDs on DMEs and transporters needs to be extended for further PK/PD studies. Although antihistamines exert cardiovascular effects, the effect of chronic heart failure on PK of antihistamines yet remains to be investigated.
6.4. Mechanisms of altered drug metabolism in CVDs
Failing or hypertensive hearts are susceptible to infiltration by pro-inflammatory cytokines and reactive oxygen species induced by stress [169]. Studies have shown that increased circulating levels of TNF-α and IL-6 in patients with congestive heart failure were inversely proportional to CYP2C19 and CYP1A2 activity [170]. Similarly, down-regulation of OCTN2 expression in patients with dilated cardiomyopathy inversely correlated with cardiac CD3+ T-cell count [166]. In addition, cardiac cytokine release may affect OCTN2 expression during cardiomyopathy associated with inflammation.
7. RHEUMATOID ARTHRITIS (RA)
7.1. Drug metabolizing enzymes
Rheumatic diseases are estimated to affect up to 1.1% of the world’s population [171]. Various studies have shown that gene expressions of DMEs are altered in adjuvant arthritis (AA) rats [172, 173].
7.2. Drug transporters
Decreased activity of hepatic P-gp in the isolated perfused liver of AA rats was reported [174, 175]. Decrease in P-gp activity corresponded with the decreased levels of Mdr1a mRNA and P-gp protein in AA rats.
7.3. PK/PD studies
PK/PD changes such as elevated plasma levels of propranolol and prolongation of sleep time with pentobarbital were observed in AA rats compared to normal rats [176, 177]. Based on these early observations, recent studies have also shown altered PK of methotrexate, T-5557 (novel anti-inflammatory agent) and doxorubicin in AA animals [174, 178]. Although, a significant increase in the plasma concentrations of verapamil in rats and humans with underlying arthritis were reported, there were no changes in the PD of verapamil (prolongation of PR interval) [45, 179]. This discrepancy was then attributed to a decrease in the receptor-ligand affinity in inflammation [180, 181].
7.4. Mechanisms of altered drug metabolism in RA
AA animal models represent a systemic inflammatory disease with bone and cartilage changes similar to those observed in RA [182]. Down-regulation of hepatic P-gp in AA rats was attributed to elevated levels of cytokines such as TNF-α and IL-6 but not IL-1β [183]. Similarly, increased plasma concentrations of drugs in AA rats correlated with increased serum TNF-α level [179]. Several in vitro and in vivo studies have shown up-regulation of NF-κB in RA and osteoarthritis [184, 185]. It was recently demonstrated that PXR and CAR expression in small intestine was decreased in arthritis [186]. Significantly decreased bilirubin elimination in collagen-induced arthritis (CIA) rats compared to normal rats [187] was attributed to decreased expression of CAR in CIA rats. Overall these studies imply an involvement of inflammatory pathways in regulation of DMEs and transporters in arthritis.
8. CONCLUSION
A common theme of this chapter is that a multiplex of mechanisms are responsible for alterations of DMEs, transporters and PK/PD of drugs in different pathophysiological conditions. It is well-established that changes in gene expression of enzymes and transporters can lead to disruption in drug disposition in altered pathophysiological conditions including infection/inflammation, cancer, obesity, CVD, rheumatoid arthritis, etc. Studies show that induction of inflammatory mediators is an underlying factor common to all these pathophysiological conditions and may contribute to altered drug disposition in disease states. In addition, the generally accepted role of cytokines in alterations of DMEs and transporters needs further evaluation. We have established the involvement of Toll-like receptor signaling pathway in the regulation of DMEs and transporters, and our studies point to the role of cytokine-independent pathways in the liver. The role of transcription factors and nuclear receptors in the regulation of DMEs and transporters in disease states need further investigation, implying an urgent need to develop models for delineating the roles of individual inflammatory mediators or nuclear receptors in altered drug disposition in disease states. It is of utmost importance to study clinically relevant drugs with known adverse effects to determine if changes in metabolism lead to increased toxicity or reduced efficacy in some individuals with underlying illness in predicting and preventing undesirable sub-therapeutic effects.
References
- 1.Nebert DW, Russell DW. Clinical importance of the cytochromes P450. Lancet. 2002;360(9340):1155–1162. doi: 10.1016/S0140-6736(02)11203-7. [DOI] [PubMed] [Google Scholar]
- 2.Guengerich FP. Cytochrome P-450 3A4: regulation and role in drug metabolism. Annu Rev Pharmacol Toxicol. 1999:391–17. doi: 10.1146/annurev.pharmtox.39.1.1. [DOI] [PubMed] [Google Scholar]
- 3.Meyer UA. Overview of enzymes of drug metabolism. J Pharmacokinet Biopharm. 1996;24(5):449–459. doi: 10.1007/BF02353473. [DOI] [PubMed] [Google Scholar]
- 4.Jancova P, Anzenbacher P, Anzenbacherova E. Phase II drug metabolizing enzymes. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2010;154(2):103–116. doi: 10.5507/bp.2010.017. [DOI] [PubMed] [Google Scholar]
- 5.Mizuno N, Niwa T, Yotsumoto Y, Sugiyama Y. Impact of drug transporter studies on drug discovery and development. Pharmacol Rev. 2003;55(3):425–461. doi: 10.1124/pr.55.3.1. [DOI] [PubMed] [Google Scholar]
- 6.Kato R. Drug metabolism under pathological and abnormal physiological states in animals and man. Xenobiotica. 1977;7(1–2):25–92. doi: 10.3109/00498257709036242. [DOI] [PubMed] [Google Scholar]
- 7.Aitken AE, Richardson TA, Morgan ET. Regulation of drug-metabolizing enzymes and transporters in inflammation. Annu Rev Pharmacol Toxicol. 2006:46123–149. doi: 10.1146/annurev.pharmtox.46.120604.141059. [DOI] [PubMed] [Google Scholar]
- 8.Deng X, Luyendyk JP, Ganey PE, Roth RA. Inflammatory stress and idiosyncratic hepatotoxicity: hints from animal models. Pharmacol Rev. 2009;61(3):262–282. doi: 10.1124/pr.109.001727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pirmohamed M, James S, Meakin S, Green C, Scott AK, Walley TJ, Farrar K, Park BK, Breckenridge AM. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18 820 patients. BMJ. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dixon RL, Hart LG, Fouts JR. The metabolism of drugs by liver microsomes from alloxan-diabetic rats. J Pharmacol Exp Ther. 1961:1337–11. [PubMed] [Google Scholar]
- 11.Kato R, Frontino G. Vassanellip Decreased activities of liver microsomal drug-metabolizing enzymes in the rats bearing Walker carcinosarcoma. Experientia. 1963:1931–32. doi: 10.1007/BF02135343. [DOI] [PubMed] [Google Scholar]
- 12.Klotz U, McHorse TS, Wilkinson GR, Schenker S. The effect of cirrhosis on the disposition and elimination of meperidine in man. Clin Pharmacol Ther. 1974;16(4):667–675. doi: 10.1002/cpt1974164667. [DOI] [PubMed] [Google Scholar]
- 13.Kraemer MJ, Furukawa CT, Koup JR, Shapiro GG, Pierson WE, Bierman CW. Altered theophylline clearance during an influenza B outbreak. Pediatrics. 1982;69(4):476–480. [PubMed] [Google Scholar]
- 14.Kato R, Takanaka A, Oshima T. Drug metabolism in tumor-bearing rats. II. In vivo metabolisms and effects of drugs in tumor-bearing rats. Jpn J Pharmacol. 1968;18(2):245–254. doi: 10.1254/jjp.18.245. [DOI] [PubMed] [Google Scholar]
- 15.Zordoky BN, El-Kadi AO. Role of NF-kappaB in the regulation of cytochrome P450 enzymes. Curr Drug Metab. 2009;10(2):164–178. doi: 10.2174/138920009787522151. [DOI] [PubMed] [Google Scholar]
- 16.Gonzalez FJ, Lee YH. Constitutive expression of hepatic cytochrome P450 genes. FASEB J. 1996;10(10):1112–1117. doi: 10.1096/fasebj.10.10.8751713. [DOI] [PubMed] [Google Scholar]
- 17.Xie W. Nuclear Receptors in Drug Metabolism. 1. John Wiley & Sons, Inc; Hoboken, NJ: 2008. [Google Scholar]
- 18.Tirona RG, Lee W, Leake BF, Lan LB, Cline CB, Lamba V, Parviz F, Duncan SA, Inoue Y, Gonzalez FJ, Schuetz EG, Kim RB. The orphan nuclear receptor HNF4alpha determines PXR- and CAR-mediated xenobiotic induction of CYP3A4. Nat Med. 2003;9(2):220–224. doi: 10.1038/nm815. [DOI] [PubMed] [Google Scholar]
- 19.Wichterman KA, Baue AE, Chaudry IH. Sepsis and septic shock--a review of laboratory models and a proposal. J Surg Res. 1980;29(2):189–201. doi: 10.1016/0022-4804(80)90037-2. [DOI] [PubMed] [Google Scholar]
- 20.Higgins LM, Frankel G, Douce G, Dougan G, MacDonald TT. Citrobacter rodentium infection in mice elicits a mucosal Th1 cytokine response and lesions similar to those in murine inflammatory bowel disease. Infect Immun. 1999;67(6):3031–3039. doi: 10.1128/iai.67.6.3031-3039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Godellas CV, Williams JF, Fabri PJ. Mixed-function oxidase activity in sepsis. J Surg Res. 1995;59(6):783–786. doi: 10.1006/jsre.1995.1240. [DOI] [PubMed] [Google Scholar]
- 22.Chaluvadi MR, Kinloch RD, Nyagode BA, Richardson TA, Raynor MJ, Sherman M, Antonovic L, Strobel HW, Dillehay DL, Morgan ET. Regulation of hepatic cytochrome P450 expression in mice with intestinal or systemic infections of citrobacter rodentium. Drug Metab Dispos. 2009;37(2):366–374. doi: 10.1124/dmd.108.024240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Monshouwer M, Witkamp RF, Nijmeijer SM, Pijpers A, Verheijden JH, Van Miert AS. Selective effects of a bacterial infection (Actinobacillus pleuropneumoniae) on the hepatic clearances of caffeine, antipyrine, paracetamol, and indocyanine green in the pig. Xenobiotica. 1995;25(5):491–499. doi: 10.3109/00498259509061868. [DOI] [PubMed] [Google Scholar]
- 24.Martin GS, Mannino DM, Eaton S, Moss M. The epidemiology of sepsis in the United States from 1979 through 2000. N Engl J Med. 2003;348(16):1546–1554. doi: 10.1056/NEJMoa022139. [DOI] [PubMed] [Google Scholar]
- 25.Garcia Del Busto Cano E, Renton KW. Modulation of hepatic cytochrome P450 during Listeria monocytogenes infection of the brain. J Pharm Sci. 2003;92(9):1860–1868. doi: 10.1002/jps.10433. [DOI] [PubMed] [Google Scholar]
- 26.Ginsburg I. Role of lipoteichoic acid in infection and inflammation. Lancet Infect Dis. 2002;2(3):171–179. doi: 10.1016/s1473-3099(02)00226-8. [DOI] [PubMed] [Google Scholar]
- 27.Leemans JC, Vervoordeldonk MJ, Florquin S, van Kessel KP, van der Poll T. Differential role of interleukin-6 in lung inflammation induced by lipoteichoic acid and peptidoglycan from Staphylococcus aureus. Am J Respir Crit Care Med. 2002;165(10):1445–1450. doi: 10.1164/rccm.2106045. [DOI] [PubMed] [Google Scholar]
- 28.Sewer MB, Koop DR, Morgan ET. Endotoxemia in rats is associated with induction of the P4504A subfamily and suppression of several other forms of cytochrome P450. Drug Metab Dispos. 1996;24(4):401–407. [PubMed] [Google Scholar]
- 29.Ghose R, White D, Guo T, Vallejo J, Karpen SJ. Regulation of hepatic drug-metabolizing enzyme genes by Toll-like receptor 4 signaling is independent of Toll-interleukin 1 receptor domain-containing adaptor protein. Drug Metab Dispos. 2008;36(1):95–101. doi: 10.1124/dmd.107.018051. [DOI] [PubMed] [Google Scholar]
- 30.Shimamoto Y, Kitamura H, Hoshi H, Kazusaka A, Funae Y, Imaoka S, Saito M, Fujita S. Differential alterations in levels of hepatic microsomal cytochrome P450 isozymes following intracerebroventricular injection of bacterial lipopolysaccharide in rats. Arch Toxicol. 1998;72(8):492–498. doi: 10.1007/s002040050533. [DOI] [PubMed] [Google Scholar]
- 31.Ghose R, Guo T, Haque N. Regulation of gene expression of hepatic drug metabolizing enzymes and transporters by the Toll-like receptor 2 ligand, lipoteichoic acid. Arch Biochem Biophys. 2009;481(1):123–130. doi: 10.1016/j.abb.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dietrich CG, de Waart DR, Ottenhoff R, Schoots IG, Elferink RP. Increased bioavailability of the food-derived carcinogen 2-amino-1-methyl-6-phenylimidazo[4,5-b]pyridine in MRP2-deficient rats. Mol Pharmacol. 2001;59(5):974–980. doi: 10.1124/mol.59.5.974. [DOI] [PubMed] [Google Scholar]
- 33.Petrovic V, Teng S, Piquette-Miller M. Regulation of drug transporters during infection and inflammation. Mol Interv. 2007;7(2):99–111. doi: 10.1124/mi.7.2.10. [DOI] [PubMed] [Google Scholar]
- 34.Hartmann G, Kim H, Piquette-Miller M. Regulation of the hepatic multidrug resistance gene expression by endotoxin and inflammatory cytokines in mice. Int Immunopharmacol. 2001;1(2):189–199. doi: 10.1016/s0162-3109(00)00271-x. [DOI] [PubMed] [Google Scholar]
- 35.Hartmann G, Vassileva V, Piquette-Miller M. Impact of endotoxin-induced changes in P-glycoprotein expression on disposition of doxorubicin in mice. Drug Metab Dispos. 2005;33(6):820–828. doi: 10.1124/dmd.104.002568. [DOI] [PubMed] [Google Scholar]
- 36.Gray JD, Renton KW, Hung OR. Depression of theophylline elimination following BCG vaccination. Br J Clin Pharmacol. 1983;16(6):735–737. doi: 10.1111/j.1365-2125.1983.tb02253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sonne J, Dossing M, Loft S, Andreasen PB. Antipyrine clearance in pneumonia. Clin Pharmacol Ther. 1985;37(6):701–704. doi: 10.1038/clpt.1985.117. [DOI] [PubMed] [Google Scholar]
- 38.Modric S, Webb AI, Derendorf H. Pharmacokinetics and pharmacodynamics of tilmicosin in sheep and cattle. J Vet Pharmacol Ther. 1998;21(6):444–452. doi: 10.1046/j.1365-2885.1998.00177.x. [DOI] [PubMed] [Google Scholar]
- 39.Ishikawa M, Ohzeki R, Takayanagi Y, Sasaki K. Potentiation of cisplatin lethality by bacterial lipopolysaccharide pretreatment in mice. Res Commun Chem Pathol Pharmacol. 1990;70(3):375–378. [PubMed] [Google Scholar]
- 40.Hasegawa T, Nadai M, Wang L, Haghgoo S, Nabeshima T, Kato N. Influence of endotoxin and lipid A on the renal handling and accumulation of gentamicin in rats. Biol Pharm Bull. 1994;17(12):1651–1655. doi: 10.1248/bpb.17.1651. [DOI] [PubMed] [Google Scholar]
- 41.Shedlofsky SI, Israel BC, McClain CJ, Hill DB, Blouin RA. Endotoxin administration to humans inhibits hepatic cytochrome P450-mediated drug metabolism. J Clin Invest. 1994;94(6):2209–2214. doi: 10.1172/JCI117582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gous AG, Dance MD, Lipman J, Luyt DK, Mathivha R, Scribante J. Changes in vancomycin pharmacokinetics in critically ill infants. Anaesth Intensive Care. 1995;23(6):678–682. doi: 10.1177/0310057X9502300603. [DOI] [PubMed] [Google Scholar]
- 43.Pinder M, Bellomo R, Lipman J. Pharmacological principles of antibiotic prescription in the critically ill. Anaesth Intensive Care. 2002;30(2):134–144. doi: 10.1177/0310057X0203000203. [DOI] [PubMed] [Google Scholar]
- 44.Frapolli R, Zucchetti M, Sessa C, Marsoni S, Vigano L, Locatelli A, Rulli E, Compagnoni A, Bello E, Pisano C, Carminati P, D’Incalci M. Clinical pharmacokinetics of the new oral camptothecin gimatecan: the inter-patient variability is related to alpha1-acid glycoprotein plasma levels. Eur J Cancer. 2010;46(3):505–516. doi: 10.1016/j.ejca.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 45.Mayo PR, Skeith K, Russell AS, Jamali F. Decreased dromotropic response to verapamil despite pronounced increased drug concentration in rheumatoid arthritis. Br J Clin Pharmacol. 2000;50(6):605–613. doi: 10.1046/j.1365-2125.2000.00314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Guirguis MS, Jamali F. Disease-drug interaction: Reduced response to propranolol despite increased concentration in the rat with inflammation. J Pharm Sci. 2003;92(5):1077–1084. doi: 10.1002/jps.10381. [DOI] [PubMed] [Google Scholar]
- 47.Mannering GJ, Deloria LB. The pharmacology and toxicology of the interferons: an overview. Annu Rev Pharmacol Toxicol. 1986:26455–515. doi: 10.1146/annurev.pa.26.040186.002323. [DOI] [PubMed] [Google Scholar]
- 48.Corbett TH, Nettesheim P. Effect of PR-8 viral respiratory infection of benz[a]pyrene hydroxylase activity in BALB/c mice. J Natl Cancer Inst. 1973;50(3):779–782. doi: 10.1093/jnci/50.3.779. [DOI] [PubMed] [Google Scholar]
- 49.Singh G, Renton KW. Interferon-mediated depression of cytochrome P-450-dependent drug biotransformation. Mol Pharmacol. 1981;20(3):681–684. [PubMed] [Google Scholar]
- 50.Renton KW. Depression of hepatic cytochrome P-450-dependent mixed function oxidases during infection with encephalomyocarditis virus. Biochem Pharmacol. 1981;30(16):2333–2336. doi: 10.1016/0006-2952(81)90107-6. [DOI] [PubMed] [Google Scholar]
- 51.Schoene B, Fleischmann RA, Remmer H, von Oldershausen HF. Determination of drug metabolizing enzymes in needle biopsies of human liver. Eur J Clin Pharmacol. 1972;4(2):65–73. doi: 10.1007/BF00562499. [DOI] [PubMed] [Google Scholar]
- 52.Wilkinson GR. The effects of diet, aging and disease-states on presystemic elimination and oral drug bioavailability in humans. Adv Drug Deliv Rev. 1997;27(2–3):129–159. doi: 10.1016/s0169-409x(97)00040-9. [DOI] [PubMed] [Google Scholar]
- 53.Lee BL, Wong D, Benowitz NL, Sullam PM. Altered patterns of drug metabolism in patients with acquired immunodeficiency syndrome. Clin Pharmacol Ther. 1993;53(5):529–535. doi: 10.1038/clpt.1993.66. [DOI] [PubMed] [Google Scholar]
- 54.Kikuchi R, McCown M, Olson P, Tateno C, Morikawa Y, Katoh Y, Bourdet DL, Monshouwer M, Fretland AJ. Effect of hepatitis C virus infection on the mRNA expression of drug transporters and cytochrome p450 enzymes in chimeric mice with humanized liver. Drug Metab Dispos. 2010;38(11):1954–1961. doi: 10.1124/dmd.109.031732. [DOI] [PubMed] [Google Scholar]
- 55.Callahan SM, Ming X, Lu SK, Brunner LJ, Croyle MA. Considerations for use of recombinant adenoviral vectors: dose effect on hepatic cytochromes P450. J Pharmacol Exp Ther. 2005;312(2):492–501. doi: 10.1124/jpet.104.075374. [DOI] [PubMed] [Google Scholar]
- 56.Le HT, Boquet MP, Clark EA, Callahan SM, Croyle MA. Renal pathophysiology after systemic administration of recombinant adenovirus: changes in renal cytochromes P450 based on vector dose. Hum Gene Ther. 2006;17(11):1095–1111. doi: 10.1089/hum.2006.17.1095. [DOI] [PubMed] [Google Scholar]
- 57.Ronaldson PT, Bendayan R. HIV-1 viral envelope glycoprotein gp120 triggers an inflammatory response in cultured rat astrocytes and regulates the functional expression of P-glycoprotein. Mol Pharmacol. 2006;70(3):1087–1098. doi: 10.1124/mol.106.025973. [DOI] [PubMed] [Google Scholar]
- 58.Giraud C, Manceau S, Decleves X, Goffinet F, Morini JP, Chappuy H, Batteux F, Chouzenoux S, Yousif S, Scherrmann JM, Blanche S, Treluyer JM. Influence of development, HIV infection, and antiretroviral therapies on the gene expression profiles of ABC transporters in human lymphocytes. J Clin Pharmacol. 2010;50(2):226–230. doi: 10.1177/0091270009343696. [DOI] [PubMed] [Google Scholar]
- 59.Petrovic V, Piquette-Miller M. Impact of polyinosinic/polycytidylic acid on placental and hepatobiliary drug transporters in pregnant rats. Drug Metab Dispos. 2010;38(10):1760–1766. doi: 10.1124/dmd.110.034470. [DOI] [PubMed] [Google Scholar]
- 60.Goodwin SD, Gallis HA, Chow AT, Wong FA, Flor SC, Bartlett JA. Pharmacokinetics and safety of levofloxacin in patients with human immunodeficiency virus infection. Antimicrob Agents Chemother. 1994;38(4):799–804. doi: 10.1128/aac.38.4.799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tett S, Moore S, Ray J. Pharmacokinetics and bioavailability of fluconazole in two groups of males with human immunodeficiency virus (HIV) infection compared with those in a group of males without HIV infection. Antimicrob Agents Chemother. 1995;39(8):1835–1841. doi: 10.1128/aac.39.8.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bica I, McGovern B, Dhar R, Stone D, McGowan K, Scheib R, Snydman DR. Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clin Infect Dis. 2001;32(3):492–497. doi: 10.1086/318501. [DOI] [PubMed] [Google Scholar]
- 63.Regazzi M, Maserati R, Villani P, Cusato M, Zucchi P, Briganti E, Roda R, Sacchelli L, Gatti F, Delle Foglie P, Nardini G, Fabris P, Mori F, Castelli P, Testa L. Clinical pharmacokinetics of nelfinavir and its metabolite M8 in human immunodeficiency virus (HIV)-positive and HIV-hepatitis C virus-coinfected subjects. Antimicrob Agents Chemother. 2005;49(2):643–649. doi: 10.1128/AAC.49.2.643-649.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Veronese L, Rautaureau J, Sadler BM, Gillotin C, Petite JP, Pillegand B, Delvaux M, Masliah C, Fosse S, Lou Y, Stein DS. Single-dose pharmacokinetics of amprenavir, a human immunodeficiency virus type 1 protease inhibitor, in subjects with normal or impaired hepatic function. Antimicrob Agents Chemother. 2000;44(4):821–826. doi: 10.1128/aac.44.4.821-826.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Wyles DL, Gerber JG. Antiretroviral drug pharmacokinetics in hepatitis with hepatic dysfunction. Clin Infect Dis. 2005;40(1):174–181. doi: 10.1086/426021. [DOI] [PubMed] [Google Scholar]
- 66.Wonganan P, Zamboni WC, Strychor S, Dekker JD, Croyle MA. Drug-virus interaction: effect of administration of recombinant adenoviruses on the pharmacokinetics of docetaxel in a rat model. Cancer Gene Ther. 2009;16(5):405–414. doi: 10.1038/cgt.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Scott MJ, Liu S, Shapiro RA, Vodovotz Y, Billiar TR. Endotoxin uptake in mouse liver is blocked by endotoxin pretreatment through a suppressor of cytokine signaling-1-dependent mechanism. Hepatology. 2009;49(5):1695–1708. doi: 10.1002/hep.22839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Aliprantis AO, Yang RB, Mark MR, Suggett S, Devaux B, Radolf JD, Klimpel GR, Godowski P, Zychlinsky A. Cell activation and apoptosis by bacterial lipoproteins through toll-like receptor-2. Science. 1999;285(5428):736–739. doi: 10.1126/science.285.5428.736. [DOI] [PubMed] [Google Scholar]
- 69.Takeuchi O, Hoshino K, Kawai T, Sanjo H, Takada H, Ogawa T, Takeda K, Akira S. Differential roles of TLR2 and TLR4 in recognition of gram-negative and gram-positive bacterial cell wall components. Immunity. 1999;11(4):443–451. doi: 10.1016/s1074-7613(00)80119-3. [DOI] [PubMed] [Google Scholar]
- 70.Ferrari L, Peng N, Halpert JR, Morgan ET. Role of nitric oxide in down-regulation of CYP2B1 protein, but not RNA, in primary cultures of rat hepatocytes. Mol Pharmacol. 2001;60(1):209–216. doi: 10.1124/mol.60.1.209. [DOI] [PubMed] [Google Scholar]
- 71.Ghose R, Guo T, Vallejo JG, Gandhi A. Differential role of Toll-interleukin 1 receptor domain-containing adaptor protein in Toll-like receptor 2-mediated regulation of gene expression of hepatic cytokines and drug-metabolizing enzymes. Drug Metab Dispos. 2011;39(5):874–881. doi: 10.1124/dmd.110.037382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kagan JC, Medzhitov R. Phosphoinositide-mediated adaptor recruitment controls Toll-like receptor signaling. Cell. 2006;125(5):943–955. doi: 10.1016/j.cell.2006.03.047. [DOI] [PubMed] [Google Scholar]
- 73.Barker CW, Fagan JB, Pasco DS. Interleukin-1 beta suppresses the induction of P4501A1 and P4501A2 mRNAs in isolated hepatocytes. J Biol Chem. 1992;267(12):8050–8055. [PubMed] [Google Scholar]
- 74.Muntane-Relat J, Ourlin JC, Domergue J, Maurel P. Differential effects of cytokines on the inducible expression of CYP1A1, CYP1A2, and CYP3A4 in human hepatocytes in primary culture. Hepatology. 1995;22(4 Pt 1):1143–1153. [PubMed] [Google Scholar]
- 75.Milosevic N, Schawalder H, Maier P. Kupffer cell-mediated differential down-regulation of cytochrome P450 metabolism in rat hepatocytes. Eur J Pharmacol. 1999;368(1):75–87. doi: 10.1016/s0014-2999(98)00988-1. [DOI] [PubMed] [Google Scholar]
- 76.Sukhai M, Yong A, Kalitsky J, Piquette-Miller M. Inflammation and interleukin-6 mediate reductions in the hepatic expression and transcription of the mdr1a and mdr1b Genes. Mol Cell Biol Res Commun. 2000;4(4):248–256. doi: 10.1006/mcbr.2001.0288. [DOI] [PubMed] [Google Scholar]
- 77.Lee G, Piquette-Miller M. Cytokines alter the expression and activity of the multidrug resistance transporters in human hepatoma cell lines; analysis using RT-PCR and cDNA microarrays. J Pharm Sci. 2003;92(11):2152–2163. doi: 10.1002/jps.10493. [DOI] [PubMed] [Google Scholar]
- 78.Le Vee M, Gripon P, Stieger B, Fardel O. Down-regulation of organic anion transporter expression in human hepatocytes exposed to the proinflammatory cytokine interleukin 1beta. Drug Metab Dispos. 2008;36(2):217–222. doi: 10.1124/dmd.107.016907. [DOI] [PubMed] [Google Scholar]
- 79.Warren GW, Poloyac SM, Gary DS, Mattson MP, Blouin RA. Hepatic cytochrome P-450 expression in tumor necrosis factor-alpha receptor (p55/p75) knockout mice after endotoxin administration. J Pharmacol Exp Ther. 1999;288(3):945–950. [PubMed] [Google Scholar]
- 80.Warren GW, van Ess PJ, Watson AM, Mattson MP, Blouin RA. Cytochrome P450 and antioxidant activity in interleukin-6 knockout mice after induction of the acute-phase response. J Interferon Cytokine Res. 2001;21(10):821–826. doi: 10.1089/107999001753238060. [DOI] [PubMed] [Google Scholar]
- 81.Kinloch RD, Lee CM, van Rooijen N, Morgan ET. Selective role for tumor necrosis factor-alpha, but not interleukin-1 or Kupffer cells, in down-regulation of CYP3A11 and CYP3A25 in livers of mice infected with a noninvasive intestinal pathogen. Biochem Pharmacol. 2011;82(3):312–321. doi: 10.1016/j.bcp.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Morris SM, Jr, Billiar TR. New insights into the regulation of inducible nitric oxide synthesis. Am J Physiol. 1994;266(6 Pt 1):E829–839. doi: 10.1152/ajpendo.1994.266.6.E829. [DOI] [PubMed] [Google Scholar]
- 83.Carlson TJ, Billings RE. Role of nitric oxide in the cytokine-mediated regulation of cytochrome P-450. Mol Pharmacol. 1996;49(5):796–801. [PubMed] [Google Scholar]
- 84.Sewer MB, Morgan ET. Nitric oxide-independent suppression of P450 2C11 expression by interleukin-1beta and endotoxin in primary rat hepatocytes. Biochem Pharmacol. 1997;54(6):729–737. doi: 10.1016/s0006-2952(97)00226-8. [DOI] [PubMed] [Google Scholar]
- 85.Monshouwer M, Witkamp RF, Nujmeijer SM, Van Amsterdam JG, Van Miert AS. Suppression of cytochrome P450- and UDP glucuronosyl transferase-dependent enzyme activities by proinflammatory cytokines and possible role of nitric oxide in primary cultures of pig hepatocytes. Toxicol Appl Pharmacol. 1996;137(2):237–244. doi: 10.1006/taap.1996.0077. [DOI] [PubMed] [Google Scholar]
- 86.Abdulla D, Goralski KB, Del Busto Cano EG, Renton KW. The signal transduction pathways involved in hepatic cytochrome P450 regulation in the rat during a lipopolysaccharide-induced model of central nervous system inflammation. Drug Metab Dispos. 2005;33(10):1521–1531. doi: 10.1124/dmd.105.004564. [DOI] [PubMed] [Google Scholar]
- 87.Gilmore TD. Introduction to NF-kappaB: players, pathways, perspectives. Oncogene. 2006;25(51):6680–6684. doi: 10.1038/sj.onc.1209954. [DOI] [PubMed] [Google Scholar]
- 88.Pascussi JM, Dvorak Z, Gerbal-Chaloin S, Assenat E, Maurel P, Vilarem MJ. Pathophysiological factors affecting CAR gene expression. Drug Metab Rev. 2003;35(4):255–268. doi: 10.1081/dmr-120026394. [DOI] [PubMed] [Google Scholar]
- 89.Gu X, Ke S, Liu D, Sheng T, Thomas PE, Rabson AB, Gallo MA, Xie W, Tian Y. Role of NF-kappaB in regulation of PXR-mediated gene expression: a mechanism for the suppression of cytochrome P-450 3A4 by proinflammatory agents. J Biol Chem. 2006;281(26):17882–17889. doi: 10.1074/jbc.M601302200. [DOI] [PubMed] [Google Scholar]
- 90.Adam-Stitah S, Penna L, Chambon P, Rochette-Egly C. Hyperphosphorylation of the retinoid X receptor alpha by activated c-Jun NH2-terminal kinases. J Biol Chem. 1999;274(27):18932–18941. doi: 10.1074/jbc.274.27.18932. [DOI] [PubMed] [Google Scholar]
- 91.Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong AN. Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem. 1999;274(39):27545–27552. doi: 10.1074/jbc.274.39.27545. [DOI] [PubMed] [Google Scholar]
- 92.Zhou J, Liu M, Aneja R, Chandra R, Lage H, Joshi HC. Reversal of P-glycoprotein-mediated multidrug resistance in cancer cells by the c-Jun NH2-terminal kinase. Cancer Res. 2006;66(1):445–452. doi: 10.1158/0008-5472.CAN-05-1779. [DOI] [PubMed] [Google Scholar]
- 93.Synold TW, Dussault I, Forman BM. The orphan nuclear receptor SXR coordinately regulates drug metabolism and efflux. Nat Med. 2001;7(5):584–590. doi: 10.1038/87912. [DOI] [PubMed] [Google Scholar]
- 94.Ghose R, Zimmerman TL, Thevananther S, Karpen SJ. Endotoxin leads to rapid subcellular re-localization of hepatic RXRalpha: A novel mechanism for reduced hepatic gene expression in inflammation. Nucl Recept. 2004;2(1):4. doi: 10.1186/1478-1336-2-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Richardson TA, Morgan ET. Hepatic cytochrome P450 gene regulation during endotoxin-induced inflammation in nuclear receptor knockout mice. J Pharmacol Exp Ther. 2005;314(2):703–709. doi: 10.1124/jpet.105.085456. [DOI] [PubMed] [Google Scholar]
- 96.Teng S, Piquette-Miller M. The involvement of the pregnane X receptor in hepatic gene regulation during inflammation in mice. J Pharmacol Exp Ther. 2005;312(2):841–848. doi: 10.1124/jpet.104.076141. [DOI] [PubMed] [Google Scholar]
- 97.Charles KA, Rivory LP, Brown SL, Liddle C, Clarke SJ, Robertson GR. Transcriptional repression of hepatic cytochrome P450 3A4 gene in the presence of cancer. Clin Cancer Res. 2006;12(24):7492–7497. doi: 10.1158/1078-0432.CCR-06-0023. [DOI] [PubMed] [Google Scholar]
- 98.DeLoia JA, Zamboni WC, Jones JM, Strychor S, Kelley JL, Gallion HH. Expression and activity of taxane-metabolizing enzymes in ovarian tumors. Gynecol Oncol. 2008;108(2):355–360. doi: 10.1016/j.ygyno.2007.10.029. [DOI] [PubMed] [Google Scholar]
- 99.Williams JA, Phillips DH. Mammary expression of xenobiotic metabolizing enzymes and their potential role in breast cancer. Cancer Res. 2000;60(17):4667–4677. [PubMed] [Google Scholar]
- 100.Kim SJ, Kang HS, Jung SY, Min SY, Lee S, Kim SW, Kwon Y, Lee KS, Shin KH, Ro J. Methylation patterns of genes coding for drug-metabolizing enzymes in tamoxifen-resistant breast cancer tissues. J Mol Med (Berl) 2010;88(11):1123–1131. doi: 10.1007/s00109-010-0652-z. [DOI] [PubMed] [Google Scholar]
- 101.Robinson LJ, Roberts WK, Ling TT, Lamming D, Sternberg SS, Roepe PD. Human MDR 1 protein overexpression delays the apoptotic cascade in Chinese hamster ovary fibroblasts. Biochemistry. 1997;36(37):11169–11178. doi: 10.1021/bi9627830. [DOI] [PubMed] [Google Scholar]
- 102.Young LC, Campling BG, Voskoglou-Nomikos T, Cole SP, Deeley RG, Gerlach JH. Expression of multidrug resistance protein-related genes in lung cancer: correlation with drug response. Clin Cancer Res. 1999;5(3):673–680. [PubMed] [Google Scholar]
- 103.Hoffmeyer S, Burk O, von Richter O, Arnold HP, Brockmoller J, Johne A, Cascorbi I, Gerloff T, Roots I, Eichelbaum M, Brinkmann U. Functional polymorphisms of the human multidrug-resistance gene: multiple sequence variations and correlation of one allele with P-glycoprotein expression and activity in vivo. Proc Natl Acad Sci U S A. 2000;97(7):3473–3478. doi: 10.1073/pnas.050585397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sharma R, Kacevska M, London R, Clarke SJ, Liddle C, Robertson G. Downregulation of drug transport and metabolism in mice bearing extra-hepatic malignancies. Br J Cancer. 2008;98(1):91–97. doi: 10.1038/sj.bjc.6604101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rosso R, Dolfini E, Donelli MG. Prolonged effect of pentobarbital in tumor bearing rats. Eur J Cancer. 1968;4(1):133–135. doi: 10.1016/0014-2964(68)90078-9. [DOI] [PubMed] [Google Scholar]
- 106.Aguirre C, Troconiz IF, Valdivieso A, Jimenez RM, Gonzalez JP, Calvo R, Rodriguez-Sasiain JM. Pharmacokinetics and pharmacodynamics of penbutolol in healthy and cancer subjects: role of altered protein binding. Res Commun Mol Pathol Pharmacol. 1996;92(1):53–72. [PubMed] [Google Scholar]
- 107.Williams ML, Bhargava P, Cherrouk I, Marshall JL, Flockhart DA, Wainer IW. A discordance of the cytochrome P450 2C19 genotype and phenotype in patients with advanced cancer. Br J Clin Pharmacol. 2000;49(5):485–488. doi: 10.1046/j.1365-2125.2000.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357(9255):539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 109.Burke F, Balkwill FR. Cytokines in animal models of cancer. Biotherapy. 1996;8(3–4):229–241. doi: 10.1007/BF01877209. [DOI] [PubMed] [Google Scholar]
- 110.Burke F, Relf M, Negus R, Balkwill F. A cytokine profile of normal and malignant ovary. Cytokine. 1996;8(7):578–585. doi: 10.1006/cyto.1996.0077. [DOI] [PubMed] [Google Scholar]
- 111.Yu L, Chen S. Toll-like receptors expressed in tumor cells: targets for therapy. Cancer Immunol Immunother. 2008;57(9):1271–1278. doi: 10.1007/s00262-008-0459-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lind DS, Hochwald SN, Malaty J, Rekkas S, Hebig P, Mishra G, Moldawer LL, Copeland EM, 3rd, Mackay S. Nuclear factor-kappa B is upregulated in colorectal cancer. Surgery. 2001;130(2):363–369. doi: 10.1067/msy.2001.116672. [DOI] [PubMed] [Google Scholar]
- 113.Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2(4):301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- 114.Kacevska M, Downes MR, Sharma R, Evans RM, Clarke SJ, Liddle C, Robertson GR. Extrahepatic cancer suppresses nuclear receptor-regulated drug metabolism. Clin Cancer Res. 2011;17(10):3170–3180. doi: 10.1158/1078-0432.CCR-10-3289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–1053. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 116.Dixon RL, Hart LG, Rogers LA, Fouts JR. The Metabolism of Drugs by Liver Microsomes from Alloxan-Diabetic Rats: Long Term Diabetes. J Pharmacol Exp Ther. 1963:142312–317. [PubMed] [Google Scholar]
- 117.Shimojo N, Ishizaki T, Imaoka S, Funae Y, Fujii S, Okuda K. Changes in amounts of cytochrome P450 isozymes and levels of catalytic activities in hepatic and renal microsomes of rats with streptozocin-induced diabetes. Biochem Pharmacol. 1993;46(4):621–627. doi: 10.1016/0006-2952(93)90547-a. [DOI] [PubMed] [Google Scholar]
- 118.Chen TL, Chen SH, Tai TY, Chao CC, Park SS, Guengerich FP, Ueng TH. Induction and suppression of renal and hepatic cytochrome P450-dependent monooxygenases by acute and chronic streptozotocin diabetes in hamsters. Arch Toxicol. 1996;70(3–4):202–208. doi: 10.1007/s002040050261. [DOI] [PubMed] [Google Scholar]
- 119.Sakuma T, Honma R, Maguchi S, Tamaki H, Nemoto N. Different expression of hepatic and renal cytochrome P450s between the streptozotocin-induced diabetic mouse and rat. Xenobiotica. 2001;31(4):223–237. doi: 10.1080/00498250110046451. [DOI] [PubMed] [Google Scholar]
- 120.Arinc E, Arslan S, Adali O. Differential effects of diabetes on CYP2E1 and CYP2B4 proteins and associated drug metabolizing enzyme activities in rabbit liver. Arch Toxicol. 2005;79(8):427–433. doi: 10.1007/s00204-005-0654-8. [DOI] [PubMed] [Google Scholar]
- 121.Xiong H, Yoshinari K, Brouwer KL, Negishi M. Role of constitutive androstane receptor in the in vivo induction of Mrp3 and CYP2B1/2 by phenobarbital. Drug Metab Dispos. 2002;30(8):918–923. doi: 10.1124/dmd.30.8.918. [DOI] [PubMed] [Google Scholar]
- 122.Yoshinari K, Takagi S, Sugatani J, Miwa M. Changes in the expression of cytochromes P450 and nuclear receptors in the liver of genetically diabetic db/db mice. Biol Pharm Bull. 2006;29(8):1634–1638. doi: 10.1248/bpb.29.1634. [DOI] [PubMed] [Google Scholar]
- 123.Yoshinari K, Takagi S, Yoshimasa T, Sugatani J, Miwa M. Hepatic CYP3A expression is attenuated in obese mice fed a high-fat diet. Pharm Res. 2006;23(6):1188–1200. doi: 10.1007/s11095-006-0071-6. [DOI] [PubMed] [Google Scholar]
- 124.Ghose R, Omoluabi O, Gandhi A, Shah P, Strohacker K, Carpenter KC, McFarlin B, Guo T. Role of high-fat diet in regulation of gene expression of drug metabolizing enzymes and transporters. Life Sci. 2011;89(1–2):57–64. doi: 10.1016/j.lfs.2011.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.van Waarde WM, Verkade HJ, Wolters H, Havinga R, Baller J, Bloks V, Muller M, Sauer PJ, Kuipers F. Differential effects of streptozotocin-induced diabetes on expression of hepatic ABC-transporters in rats. Gastroenterology. 2002;122(7):1842–1852. doi: 10.1053/gast.2002.33582. [DOI] [PubMed] [Google Scholar]
- 126.Cheng Q, Aleksunes LM, Manautou JE, Cherrington NJ, Scheffer GL, Yamasaki H, Slitt AL. Drug-metabolizing enzyme and transporter expression in a mouse model of diabetes and obesity. Mol Pharm. 2008;5(1):77–91. doi: 10.1021/mp700114j. [DOI] [PubMed] [Google Scholar]
- 127.Abernethy DR, Greenblatt DJ, Divoll M, Shader RI. Enhanced glucuronide conjugation of drugs in obesity: studies of lorazepam, oxazepam, and acetaminophen. J Lab Clin Med. 1983;101(6):873–880. [PubMed] [Google Scholar]
- 128.O’Shea D, Davis SN, Kim RB, Wilkinson GR. Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone: a putative probe of CYP2E1 activity. Clin Pharmacol Ther. 1994;56(4):359–367. doi: 10.1038/clpt.1994.150. [DOI] [PubMed] [Google Scholar]
- 129.Watkins JB, 3rd, Sherman SE. Long-term diabetes alters the hepatobiliary clearance of acetaminophen, bilirubin and digoxin. J Pharmacol Exp Ther. 1992;260(3):1337–1343. [PubMed] [Google Scholar]
- 130.Park JH, Lee WI, Yoon WH, Park YD, Lee JS, Lee MG. Pharmacokinetic and pharmacodynamic changes of furosemide after intravenous and oral administration to rats with alloxan-induced diabetes mellitus. Biopharm Drug Dispos. 1998;19(6):357–364. doi: 10.1002/(sici)1099-081x(199809)19:6<357::aid-bdd114>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 131.Park JM, Moon CH, Lee MG. Pharmacokinetic changes of methotrexate after intravenous administration to streptozotocin-induced diabetes mellitus rats. Res Commun Mol Pathol Pharmacol. 1996;93(3):343–352. [PubMed] [Google Scholar]
- 132.Baek HW, Bae SK, Lee MG, Sohn YT. Pharmacokinetics of chlorzoxazone in rats with diabetes: Induction of CYP2E1 on 6-hydroxychlorzoxazone formation. J Pharm Sci. 2006;95(11):2452–2462. doi: 10.1002/jps.20698. [DOI] [PubMed] [Google Scholar]
- 133.Cook AM, Martin C, Adams VR, Morehead RS. Pharmacokinetics of intravenous levofloxacin administered at 750 milligrams in obese adults. Antimicrob Agents Chemother. 2011;55(7):3240–3243. doi: 10.1128/AAC.01680-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Varin F, Ducharme J, Theoret Y, Besner JG, Bevan DR, Donati F. Influence of extreme obesity on the body disposition and neuromuscular blocking effect of atracurium. Clin Pharmacol Ther. 1990;48(1):18–25. doi: 10.1038/clpt.1990.112. [DOI] [PubMed] [Google Scholar]
- 135.Derry CL, Kroboth PD, Pittenger AL, Kroboth FJ, Corey SE, Smith RB. Pharmacokinetics and pharmacodynamics of triazolam after two intermittent doses in obese and normal-weight men. J Clin Psychopharmacol. 1995;15(3):197–205. doi: 10.1097/00004714-199506000-00008. [DOI] [PubMed] [Google Scholar]
- 136.Hotamisligil GS, Shargill NS, Spiegelman BM. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- 137.Wellen KE, Hotamisligil GS. Inflammation, stress, and diabetes. J Clin Invest. 2005;115(5):1111–1119. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Thummel KE, Schenkman JB. Effects of testosterone and growth hormone treatment on hepatic microsomal P450 expression in the diabetic rat. Mol Pharmacol. 1990;37(1):119–129. [PubMed] [Google Scholar]
- 139.Lucas D, Farez C, Bardou LG, Vaisse J, Attali JR, Valensi P. Cytochrome P450 2E1 activity in diabetic and obese patients as assessed by chlorzoxazone hydroxylation. Fundam Clin Pharmacol. 1998;12(5):553–558. doi: 10.1111/j.1472-8206.1998.tb00985.x. [DOI] [PubMed] [Google Scholar]
- 140.De Waziers I, Garlatti M, Bouguet J, Beaune PH, Barouki R. Insulin down-regulates cytochrome P450 2B and 2E expression at the post-transcriptional level in the rat hepatoma cell line. Mol Pharmacol. 1995;47(3):474–479. [PubMed] [Google Scholar]
- 141.Woodcroft KJ, Hafner MS, Novak RF. Insulin signaling in the transcriptional and posttranscriptional regulation of CYP2E1 expression. Hepatology. 2002;35(2):263–273. doi: 10.1053/jhep.2002.30691. [DOI] [PubMed] [Google Scholar]
- 142.Kim SK, Novak RF. The role of intracellular signaling in insulin-mediated regulation of drug metabolizing enzyme gene and protein expression. Pharmacol Ther. 2007;113(1):88–120. doi: 10.1016/j.pharmthera.2006.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Chua SC, Jr, White DW, Wu-Peng XS, Liu SM, Okada N, Kershaw EE, Chung WK, Power-Kehoe L, Chua M, Tartaglia LA, Leibel RL. Phenotype of fatty due to Gln269Pro mutation in the leptin receptor (Lepr) Diabetes. 1996;45(8):1141–1143. doi: 10.2337/diab.45.8.1141. [DOI] [PubMed] [Google Scholar]
- 144.Lee GH, Proenca R, Montez JM, Carroll KM, Darvishzadeh JG, Lee JI, Friedman JM. Abnormal splicing of the leptin receptor in diabetic mice. Nature. 1996;379(6566):632–635. doi: 10.1038/379632a0. [DOI] [PubMed] [Google Scholar]
- 145.Reynaert H, Geerts A, Henrion J. Review article: the treatment of non-alcoholic steatohepatitis with thiazolidinediones. Aliment Pharmacol Ther. 2005;22(10):897–905. doi: 10.1111/j.1365-2036.2005.02682.x. [DOI] [PubMed] [Google Scholar]
- 146.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27(1):128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 147.Fisher CD, Jackson JP, Lickteig AJ, Augustine LM, Cherrington NJ. Drug metabolizing enzyme induction pathways in experimental non-alcoholic steatohepatitis. Arch Toxicol. 2008;82(12):959–964. doi: 10.1007/s00204-008-0312-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Fisher CD, Lickteig AJ, Augustine LM, Oude Elferink RP, Besselsen DG, Erickson RP, Cherrington NJ. Experimental non-alcoholic fatty liver disease results in decreased hepatic uptake transporter expression and function in rats. Eur J Pharmacol. 2009;613(1–3):119–127. doi: 10.1016/j.ejphar.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37(10):2087–2094. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Gomez-Lechon MJ, Donato MT, Castell JV, Jover R. Human hepatocytes in primary culture: the choice to investigate drug metabolism in man. Curr Drug Metab. 2004;5(5):443–462. doi: 10.2174/1389200043335414. [DOI] [PubMed] [Google Scholar]
- 151.Donato MT, Jimenez N, Serralta A, Mir J, Castell JV, Gomez-Lechon MJ. Effects of steatosis on drug-metabolizing capability of primary human hepatocytes. Toxicol In Vitro. 2007;21(2):271–276. doi: 10.1016/j.tiv.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 152.Barshop NJ, Capparelli EV, Sirlin CB, Schwimmer JB, Lavine JE. Acetaminophen pharmacokinetics in children with nonalcoholic fatty liver disease. J Pediatr Gastroenterol Nutr. 2011;52(2):198–202. doi: 10.1097/MPG.0b013e3181f9b3a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Lickteig AJ, Fisher CD, Augustine LM, Aleksunes LM, Besselsen DG, Slitt AL, Manautou JE, Cherrington NJ. Efflux transporter expression and acetaminophen metabolite excretion are altered in rodent models of nonalcoholic fatty liver disease. Drug Metab Dispos. 2007;35(10):1970–1978. doi: 10.1124/dmd.107.015107. [DOI] [PubMed] [Google Scholar]
- 154.Schrieber SJ, Wen Z, Vourvahis M, Smith PC, Fried MW, Kashuba AD, Hawke RL. The pharmacokinetics of silymarin is altered in patients with hepatitis C virus and nonalcoholic Fatty liver disease and correlates with plasma caspase-3/7 activity. Drug Metab Dispos. 2008;36(9):1909–1916. doi: 10.1124/dmd.107.019604. [DOI] [PubMed] [Google Scholar]
- 155.Donato MT, Lahoz A, Jimenez N, Perez G, Serralta A, Mir J, Castell JV, Gomez-Lechon MJ. Potential impact of steatosis on cytochrome P450 enzymes of human hepatocytes isolated from fatty liver grafts. Drug Metab Dispos. 2006;34(9):1556–1562. doi: 10.1124/dmd.106.009670. [DOI] [PubMed] [Google Scholar]
- 156.Lickteig AJ, Fisher CD, Augustine LM, Cherrington NJ. Genes of the antioxidant response undergo upregulation in a rodent model of nonalcoholic steatohepatitis. J Biochem Mol Toxicol. 2007;21(4):216–220. doi: 10.1002/jbt.20177. [DOI] [PubMed] [Google Scholar]
- 157.Hardwick RN, Fisher CD, Canet MJ, Lake AD, Cherrington NJ. Diversity in antioxidant response enzymes in progressive stages of human nonalcoholic fatty liver disease. Drug Metab Dispos. 2010;38(12):2293–2301. doi: 10.1124/dmd.110.035006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Abernethy DR, Flockhart DA. Molecular basis of cardiovascular drug metabolism: implications for predicting clinically important drug interactions. Circulation. 2000;101(14):1749–1753. doi: 10.1161/01.cir.101.14.1749. [DOI] [PubMed] [Google Scholar]
- 159.Young MJ, Clyne CD, Cole TJ, Funder JW. Cardiac steroidogenesis in the normal and failing heart. J Clin Endocrinol Metab. 2001;86(11):5121–5126. doi: 10.1210/jcem.86.11.7925. [DOI] [PubMed] [Google Scholar]
- 160.Tan FL, Moravec CS, Li J, Apperson-Hansen C, McCarthy PM, Young JB, Bond M. The gene expression fingerprint of human heart failure. Proc Natl Acad Sci U S A. 2002;99(17):11387–11392. doi: 10.1073/pnas.162370099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Satoh M, Nakamura M, Saitoh H, Satoh H, Akatsu T, Iwasaka J, Masuda T, Hiramori K. Aldosterone synthase (CYP11B2) expression and myocardial fibrosis in the failing human heart. Clin Sci (Lond) 2002;102(4):381–386. [PubMed] [Google Scholar]
- 162.Thum T, Borlak J. Testosterone, cytochrome P450, and cardiac hypertrophy. FASEB J. 2002;16(12):1537–1549. doi: 10.1096/fj.02-0138com. [DOI] [PubMed] [Google Scholar]
- 163.Johnston SC. Ischemic preconditioning from transient ischemic attacks? Data from the Northern California TIA Study. Stroke. 2004;35(11 Suppl 1):2680–2682. doi: 10.1161/01.STR.0000143322.20491.0f. [DOI] [PubMed] [Google Scholar]
- 164.Kivisto KT, Niemi M, Schaeffeler E, Pitkala K, Tilvis R, Fromm MF, Schwab M, Lang F, Eichelbaum M, Strandberg T. CYP3A5 genotype is associated with diagnosis of hypertension in elderly patients: data from the DEBATE Study. Am J Pharmacogenomics. 2005;5(3):191–195. doi: 10.2165/00129785-200505030-00005. [DOI] [PubMed] [Google Scholar]
- 165.Sekino K, Kubota T, Okada Y, Yamada Y, Yamamoto K, Horiuchi R, Kimura K, Iga T. Effect of the single CYP2C9*3 allele on pharmacokinetics and pharmacodynamics of losartan in healthy Japanese subjects. Eur J Clin Pharmacol. 2003;59(8–9):589–592. doi: 10.1007/s00228-003-0664-5. [DOI] [PubMed] [Google Scholar]
- 166.Grube M, Ameling S, Noutsias M, Kock K, Triebel I, Bonitz K, Meissner K, Jedlitschky G, Herda LR, Reinthaler M, Rohde M, Hoffmann W, Kuhl U, Schultheiss HP, Volker U, Felix SB, Klingel K, Kandolf R, Kroemer HK. Selective regulation of cardiac organic cation transporter novel type 2 (OCTN2) in dilated cardiomyopathy. Am J Pathol. 2011;178(6):2547–2559. doi: 10.1016/j.ajpath.2011.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Thomson PD, Rowland M, Melmon KL. The influence of heart failure, liver disease, and renal failure on the disposition of lidocaine in man. Am Heart J. 1971;82(3):417–421. doi: 10.1016/0002-8703(71)90308-5. [DOI] [PubMed] [Google Scholar]
- 168.Prescott LF, Adjepon-Yamoah KK, Talbot RG. Impaired Lignocaine metabolism in patients with myocardial infarction and cardiac failure. Br Med J. 1976;1(6015):939–941. doi: 10.1136/bmj.1.6015.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fliser D, Buchholz K, Haller H. Antiinflammatory effects of angiotensin II subtype 1 receptor blockade in hypertensive patients with microinflammation. Circulation. 2004;110(9):1103–1107. doi: 10.1161/01.CIR.0000140265.21608.8E. [DOI] [PubMed] [Google Scholar]
- 170.Frye RF, Schneider VM, Frye CS, Feldman AM. Plasma levels of TNF-alpha and IL-6 are inversely related to cytochrome P450-dependent drug metabolism in patients with congestive heart failure. J Card Fail. 2002;8(5):315–319. doi: 10.1054/jcaf.2002.127773. [DOI] [PubMed] [Google Scholar]
- 171.Harris M. Careers in nursing: caring for children with rheumatoid arthritis. Nursing (Lond) 1980;(20):880–881. [PubMed] [Google Scholar]
- 172.Projean D, Dautrey S, Vu HK, Groblewski T, Brazier JL, Ducharme J. Selective downregulation of hepatic cytochrome P450 expression and activity in a rat model of inflammatory pain. Pharm Res. 2005;22(1):62–70. doi: 10.1007/s11095-004-9010-6. [DOI] [PubMed] [Google Scholar]
- 173.Uno S, Kawase A, Tsuji A, Tanino T, Iwaki M. Decreased intestinal CYP3A and P-glycoprotein activities in rats with adjuvant arthritis. Drug Metab Pharmacokinet. 2007;22(4):313–321. doi: 10.2133/dmpk.22.313. [DOI] [PubMed] [Google Scholar]
- 174.Achira M, Totsuka R, Fujimura H, Kume T. Tissue-specific regulation of expression and activity of P-glycoprotein in adjuvant arthritis rats. Eur J Pharm Sci. 2002;16(1–2):29–36. doi: 10.1016/s0928-0987(02)00052-0. [DOI] [PubMed] [Google Scholar]
- 175.Achira M, Totsuka R, Kume T. Decreased activity of hepatic P-glycoprotein in the isolated perfused liver of the adjuvant arthritis rat. Xenobiotica. 2002;32(11):963–973. doi: 10.1080/0049825021000012664. [DOI] [PubMed] [Google Scholar]
- 176.Dipasquale G, Welaj P, Rassaert CL. Prolonged pentobarbital sleeping time in adjuvant-induced polyarthritic rats. Res Commun Chem Pathol Pharmacol. 1974;9(2):253–264. [PubMed] [Google Scholar]
- 177.Piquette-Miller M, Jamali F. Selective effect of adjuvant arthritis on the disposition of propranolol enantiomers in rats detected using a stereospecific HPLC assay. Pharm Res. 1993;10(2):294–299. doi: 10.1023/a:1018907431893. [DOI] [PubMed] [Google Scholar]
- 178.Achira M, Totsuka R, Fujimura H, Kume T. Decreased hepatobiliary transport of methotrexate in adjuvant arthritis rats. Xenobiotica. 2002;32(12):1151–1160. doi: 10.1080/0049825021000017939. [DOI] [PubMed] [Google Scholar]
- 179.Sattari S, Dryden WF, Eliot LA, Jamali F. Despite increased plasma concentration, inflammation reduces potency of calcium channel antagonists due to lower binding to the rat heart. Br J Pharmacol. 2003;139(5):945–954. doi: 10.1038/sj.bjp.0705202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Shore SA, Laporte J, Hall IP, Hardy E, Panettieri RA., Jr Effect of IL-1 beta on responses of cultured human airway smooth muscle cells to bronchodilator agonists. Am J Respir Cell Mol Biol. 1997;16(6):702–712. doi: 10.1165/ajrcmb.16.6.9191472. [DOI] [PubMed] [Google Scholar]
- 181.Laporte JD, Moore PE, Panettieri RA, Moeller W, Heyder J, Shore SA. Prostanoids mediate IL-1beta-induced beta-adrenergic hyporesponsiveness in human airway smooth muscle cells. Am J Physiol. 1998;275(3 Pt 1):L491–501. doi: 10.1152/ajplung.1998.275.3.L491. [DOI] [PubMed] [Google Scholar]
- 182.Williams RO, Feldmann M, Maini RN. Anti-tumor necrosis factor ameliorates joint disease in murine collagen-induced arthritis. Proc Natl Acad Sci U S A. 1992;89(20):9784–9788. doi: 10.1073/pnas.89.20.9784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Philippe L, Gegout-Pottie P, Guingamp C, Bordji K, Terlain B, Netter P, Gillet P. Relations between functional, inflammatory, and degenerative parameters during adjuvant arthritis in rats. Am J Physiol. 1997;273(4 Pt 2):R1550–1556. doi: 10.1152/ajpregu.1997.273.4.R1550. [DOI] [PubMed] [Google Scholar]
- 184.Handel ML, McMorrow LB, Gravallese EM. Nuclear factor-kappa B in rheumatoid synovium. Localization of p50 and p65. Arthritis Rheum. 1995;38(12):1762–1770. doi: 10.1002/art.1780381209. [DOI] [PubMed] [Google Scholar]
- 185.Mor A, Abramson SB, Pillinger MH. The fibroblast-like synovial cell in rheumatoid arthritis: a key player in inflammation and joint destruction. Clin Immunol. 2005;115(2):118–128. doi: 10.1016/j.clim.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 186.Kawase A, Yoshida I, Tsunokuni Y, Iwaki M. Decreased PXR and CAR inhibit transporter and CYP mRNA Levels in the liver and intestine of mice with collagen-induced arthritis. Xenobiotica. 2007;37(4):366–374. doi: 10.1080/00498250701230534. [DOI] [PubMed] [Google Scholar]
- 187.Kawase A, Tsunokuni Y, Iwaki M. Effects of alterations in CAR on bilirubin detoxification in mouse collagen-induced arthritis. Drug Metab Dispos. 2007;35(2):256–261. doi: 10.1124/dmd.106.011536. [DOI] [PubMed] [Google Scholar]