Abstract
Background
Palonosetron is a potent second generation 5- hydroxytryptamine-3 selective antagonist which can be administered by either intravenous (IV) or oral routes, but subcutaneous (SC) administration of palonosetron has never been studied, even though it could have useful clinical applications. In this study, we evaluate the bioavailability of SC palonosetron.
Patients and Methods
Patients treated with platinum-based chemotherapy were randomized to receive SC or IV palonosetron, followed by the alternative route in a crossover manner, during the first two cycles of chemotherapy. Blood samples were collected at baseline and 10, 15, 30, 45, 60, 90 minutes and 2, 3, 4, 6, 8, 12 and 24 h after palonosetron administration. Urine was collected during 12 hours following palonosetron. We compared pharmacokinetic parameters including AUC0–24h, t1/2, and Cmax observed with each route of administration by analysis of variance (ANOVA).
Results
From October 2009 to July 2010, 25 evaluable patients were included. AUC0–24h for IV and SC palonosetron were respectively 14.1 and 12.7 ng × h/ml (p = 0.160). Bioavalability of SC palonosetron was 118% (95% IC: 69–168). Cmax was lower with SC than with IV route and was reached 15 minutes following SC administration.
Conclusions
Palonosetron bioavailability was similar when administered by either SC or IV route. This new route of administration might be specially useful for outpatient management of emesis and for administration of oral chemotherapy.
Trial Registration
ClinicalTrials.gov NCT01046240
Introduction
Emesis remains one of the most relevant side effects of chemotherapy. It induces a decrease in health-related quality of life and it is often underestimated by physicians [1], [2]. 5-hydroxytryptamine-3 (5-HT3) inhibitors are universally recommended as part of standard anti-emetic premedication for moderate and highly emetogenic chemotherapy agents [3], [4]. Palonosetron (Aloxi; Italfarmaco Laboratories,) is a potent and highly selective 5-HT3 inhibitor with a prolonged half-life (40 hours), which has up to 30 times higher affinity for the receptor than first-generation 5-HT3 antagonists. In addition, it has weak antagonistic action against other 5-HT receptors [5]. The efficacy of palonosetron in the prevention of nausea and vomiting has been shown in several phase III studies [6]–[8].
Palonosetron, as the other 5-HT3 antagonists, can be administered by oral or intravenous (IV) route. However, these routes are inadequate for patients managed in the outpatient setting that cannot tolerate oral medication, due to vomiting or other reasons. Subcutaneous (SC) administration of palonosetron could be an attractive option for these patients and for those that receive oral chemotherapy and do not require an intravenous access. Theoretical advantages of SC route over IV delivery include its simpler administration, as well as its decreased complications and costs. In a previous study, we compared the administration of SC and IV granisetron and we found that both administration routes have similar bioavailability [9]. The objective of this study was to compare the bioavailability of SC and IV palonosetron, in order to establish the validity of SC administration for cancer patients. We performed a pharmacokinetic evaluation of SC and IV palonosetron, using a randomized crossover design. We hypothesized that bioavailability of SC palonosetron would not be inferior to that achieved by IV delivery.
Patients and Methods
Eligible patients had to be candidates to receive platinum-based chemotherapy. Additional inclusion criteria were: adequate bone marrow, renal and hepatic function, respectively defined by: absolute neutrophil count ≥1500/mm3 and platelets ≥100000/mm3; creatinine<1.5 mg/dl; and bilirubin, AST and ALT≤2 times x upper limit of normality. Patients must had ECOG performance status ≤2. Patients were not eligible in case of pregnancy or relevant concomitant diseases.
Chemotherapy was the same in both cycles for each patient. Patients were randomized to receive SC or IV palonosetron 250 µg during the first cycle and to crossover to the alternative route during the second one. For IV treatment, 250 µg of palonosetron were injected over 30 seconds. For SC treatment 250 µg of palonosetron were administered subcutaneously in the abdomen. Patients received 20 mg of intravenous dexamethasone and further anti-emetic treatment if necessary, although no additional doses of palonosetron were administered, to avoid pharmacokinetic interference. The protocol for this trial and supporting CONSORT checklist are available as supporting information; see Checklist S1 and Protocol S1.
The main endpoint was bioavailability (F). Even though the study was not designed to test clinical efficacy, patients evaluated their emetic symptoms by completing a diary. Toxicity was assessed using Common Toxicity Criteria for adverse events (CTCAE) version 3.0. (http://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcaev3.pdf).
All patients signed written informed consent before treatment. The protocol was approved by the Clinical Research Ethics Committee of Navarra and by the Spanish Agency for Medicines and Healthcare Products. The trial was registered in ClinicalTrials.gov (NCT01046240, URL: http://clinicaltrials.gov/ct2/show/NCT01046240?term=palonosetron+sadaba&rank=1).
Pharmacokinetic study
Blood samples (5 ml) were obtained at baseline (pre-dose), 10, 15, 30, 45, 60 minutes and 1.5, 2, 3, 4, 6, 8, 12 and 24 hours following administration of palonosetron. Blood was drawn in heparin tubes, centrifuged (4°C, 3500 r.p.m., 10 minutes) and frozen at −20°C until analysis. Urine was collected for 12 hours after treatment. Palonosetron levels were determined by a validated high performance liquid chromatography with mass/mass detection after liquid/liquid extraction of acidified plasma samples. The quantitation limit was 0.1 ng/ml. Calibration curves were prepared at a concentration range of 0. 1–100 ng/ml. Plasma concentrations were analyzed by a laboratory certified in Good Laboratory Practices.
Pharmacokinetic parameters were calculated by noncompartimental methods. All calculations were carried out using WinNonlin Professional Version 5.3 (Scientific Consulting, Inc., Mountain View, USA). AUC0–12h and AUC0–24h were calculated by the trapezoidal rule. Maximum concentration (Cmax) and time to maximum concentration (tmax) were obtained from experimental data. Half-life (t1/2) and terminal phase rate constant (ke) were determined by unweighted non-linear regression analysis of the terminal slope of the log-plasma concentration-time curve.
Statistical analysis
Twenty-five patients were required to have a power of 0.80 in order to conclude equivalence at the significance level 0.05 in total bioavailability of SC administration in relation to IV administration. We compared pharmacokinetic parameters by analysis of variance (ANOVA) including the factors sequence, period, formulation and study participant to the log-transformed parameters log(AUC) and log(Cmax). We estimated the relative bioavailability and the 90% confidence intervals (CIs) by the residual variance of the ANOVA [10]. Other pharmacokinetic parameters were analyzed by paired Student's t test or Wilcoxon test. Statistical analysis was performed using SPSS 15.0 and WinNonlin Pro 5.3. The emetic symptoms were compared by McNemar's test. The 95% Cis for proportions were calculated using Epiinfo 6.11.
Results
From October 2009 to July 2010, 25 evaluable patients were included. Four additional patients were not evaluable because of anaphylactic shock during administration of paclitaxel (1), volunteer decision to leave the study (1), death due to disease progression (1) and chemotherapy related neutropenia (1). Patient characteristics are described in table 1. Gender distribution was 18 male (72%) and 7 female (28%). Mean age was 58 years (SD = 12.4) and mean body mass index 27.2 kg/m2 (SD = 4.7).
Table 1. Patient characteristics.
| N | % | Mean | Range | |
| Patients | 25 | - | - | - |
| Age (years) | - | - | 58 | 31-74 |
| Sex | ||||
| Male | 18 | 72 | ||
| Female | 7 | 28 | - | - |
| Weight (kg) | - | - | 77 | 50.8-121 |
| Height (cm) | - | - | 167.6 | 153-182 |
| Body mass index (kg/m2) | - | - | 27.2 | 19.2-38.1 |
| ECOG | ||||
| 0 | 10 | 40 | - | - |
| 1 | 12 | 48 | - | - |
| 2 | 3 | 12 | - | - |
| Tumours | ||||
| NSCLC stage IV | 12 | 48 | - | - |
| SCLC | 2 | 8 | - | - |
| Bladder cancer | 5 | 20 | - | - |
| Pelvis kidney cancer | 1 | 4 | - | - |
| Tongue cancer | 1 | 4 | - | - |
| Nasopharynx cancer | 2 | 8 | - | - |
| Testicular cancer | 1 | 4 | - | - |
| Cancer of unknown origin | 1 | 4 | - | - |
| Platinum | ||||
| Cisplatin | 21 | 84 | - | - |
| Carboplatin | 4 | 16 | - | - |
| Dose of platinum (mg) | ||||
| Cisplatin | - | - | 131.5 | 48–165 |
| Carboplatin | - | - | 626.5 | 450–750 |
Pharmacokinetic assessment
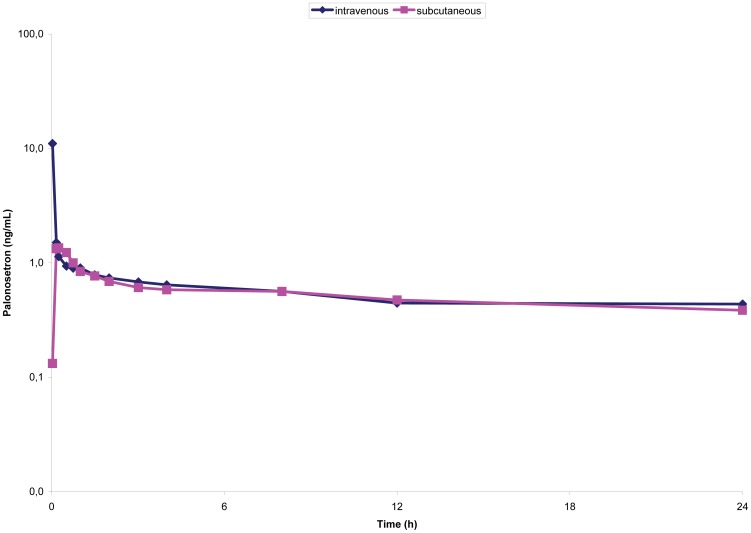
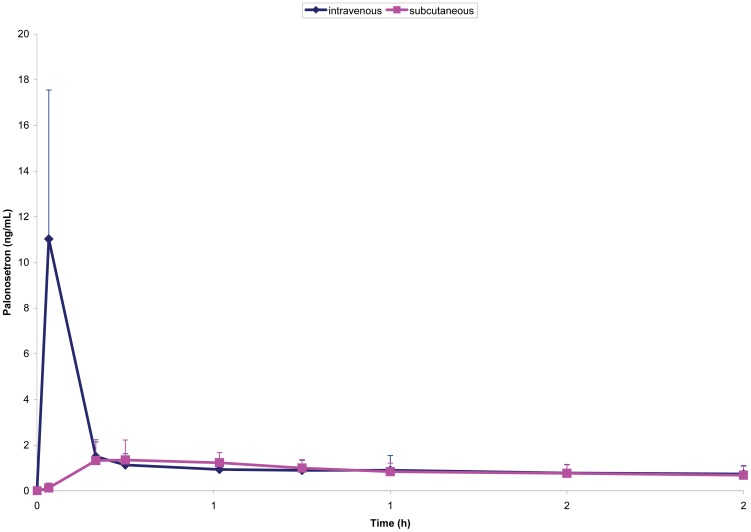
Pharmacokinetic parameters are presented in table 2. Maximum plasma concentrations were observed right at the end of the IV infusion and 15 minutes after SC administration. Cmax obtained after SC route was 15% (95% CI, 11–20%) of that one achieved by IV administration. Mean palonosetron plasma concentrations are presented on figures 1 and 2. AUC0–24h and urinary elimination (20% dose administrated) were similar between both routes, indicating similar bioavailability with a relative F of 1.18 (118%). Other pharmacokinetic parameters, such as t1/2 and ke were not statistically different.
Table 2. Pharmacokinetic characteristics of subcutaneous and intravenous palonosetron, compared by Student's t test for paired samples and Wilcoxon's test.
| IV | SC | p | |
| mean (± SD) | Mean (± SD) | ||
| AUC0-24h (ng × h/ml) | 14.10±6.73 | 12.68±6.70 | 0.160 |
| Cmax (ng/ml) | 11.88±7.38 | 1.91±1.09 | <0.001 |
| tmax (min) 1 | 1 (1–10) | 15 (10–32) | <0.001* |
| ke (h−1) | 0.095±0.117 | 0.075±0.061 | 0.527 * |
| t1/2 (h) | 12.71±10.21 | 14.68±9.79 | 0.527 * |
| C12h (ng/ml) | 0.487±0.292 | 0.459±0.289 | 0.671 |
| C24h (ng/ml) | 0.415±0.206 | 0.414±0.235 | 0.365 |
| Ae24h (%) | 19.48±9.99 | 22.24±8.50 | 0.660 |
IV: intravenous. SC: subcutaneous. AUC0–24h: area under the plasma drug concentration-time curve between 0 to 24 hours. n.s.s: non statistically significant. Cmax: maximum concentration. tmax: time to maximum concentration. ke: elimination constant t1/2: half life. C: concentration. Ae: amount of palonosetron eliminated by urine.
*: Wilcoxon's test.
: Median and range.
Figure 1. Palonosetron mean plasma levels (±SD) following a single 250 µg dose IV or SC (first 24 h, semilogarithmic graph).
Figure 2. Palonosetron mean plasma levels (±SD) following administration of a single 250 µg dose IV or SC (first two hours).
Efficacy and toxicity assessment
From 25 patients evaluable for antiemetic efficacy, 11 (44%) reported no differences in antiemetic control between both alternatives, 6 (24%) had less emesis with SC palonosetron and 8 (32%) presented better control with the IV route. These differences were not statistically significant.
Nine patients (36%) reported constipation, (5 grade 1 and 4 grade 2). Other reported adverse events potentially related with study drug were headache (2), diarrhoea (2), hiccups (2), dizziness (1), skin rash (1) and bruise in the injection site (1). All these events were grade 1 and 2 and none were significantly more frequent with either administration route.
Discussion
In this study, we have shown that palonosetron presents similar bioavailability when administered by either SC or IV route, confirming non-significant differences in AUC and urinary recovery between both routes. Therefore SC palonosetron seems a valid alternative to IV administration for control of emesis. This route could be of particular interest when conventional routes are difficult or impossible to use, for example, when heavy vomiting precludes oral intake or when IV administration is not possible in an outpatient setting. In addition, the SC route might be an interesting alternative for patients receiving oral chemotherapy that do not require IV medication.
Guidelines for management of emesis recommend the use of palonosetron with chemotherapy of moderate and high emetic potential (level 1, uniform consensus), and with chemotherapy regimens lasting over one day (level 2A, uniform consensus) [3], [4], [11]. We used a 250 µg dose of palonosetron since higher doses have not shown superior anti-emetic effect [12].
The observed t1/2 for the SC e IV routes were respectively 14.68 hours and 12.71 hours, within the range observed in previous studies [13], [14] Plasma palonosetron concentrations declined biexponentially after IV administration, with an initial rapid distribution phase followed by a slower elimination from the body. A Cmax value of 5.63 ng/ml (SD = 5.48) has been previously reported after IV administration of 3 µg/kg (168–270 µg) of palonosetron over 30 seconds [12]. Considering differences in dose and sampling time, this is consistent with the Cmax of 11.88 (SD = 7.38) ng/ml that we observed following IV administration. Absorption after SC administration of palonosetron was slow, and showed some influence of the absorption phase in the disposition of the drug. The maximum concentration was achieved 10–32 min after the dose, with an 85% reduction of Cmax achieved after IV injection. In a previous study, a 15 minute IV infusion of 250 µg of palonosetron reduced decreased by 40% Cmax as compared with a 30 second infusion [15]. It is unlikely that the differences in Cmax observed between both routes can affect clinical efficacy, because the higher plasma concentrations after IV injection just lasted a short period of time, inferior to 5 minutes. In addition, since antiemetics are usually administered 30 to 60 minutes before chemotherapy, this difference is unlikely to affect clinical efficacy under a prophylactic point of view. Nevertheless, it could favor the IV route for treatment of established emesis, although, as previously mentioned, higher doses of palonosetron have not demonstrated higher clinical efficacy than lower doses.
This trial was not designed to compare the efficacy of both alternatives, and therefore, no definitive conclusions on this issue can be established based on our results. Yet, 44% of the patients reported no differences in control of emesis between both routes of administration, while 24% and 32% reported better control with SC and IV palonosetron respectively. These results were not statistically significant, and therefore suggest that SC administration might have similar antiemetic efficacy than the IV route, but additional studies will be necessary to confirm such preliminary observation.
Local toxicity was mild, with only 1 patient presenting a local reaction, which consisted on a bruise. Systemic toxicity mainly consisted on grade 1–2 headache and constipation. These adverse effects have previously been reported with 5-HT3 antagonists, including palonosetron. While the rate of headache is similar to what has previously been described [16], the proportion of patients presenting constipation is somewhat higher [17]. Nevertheless, this is probably explained by the fact that 4 patients presented previous constipation.
Conclusion
SC administration of palonosetron has similar bioavailability than IV delivery. This is the first study that shows that SC palonosetron might be a valid alternative to IV administration. This new route of administration might be specially relevant for outpatient management of emesis in cancer patients and for oral chemotherapy regimens. Further studies are warranted to confirm the clinical value of SC palonosetron.
Supporting Information
CONSORT Checklist.
(DOC)
Trial Protocol.
(DOCX)
CONSORT Flow Diagram.
(DOC)
Acknowledgments
We are indebted to Mercedes Egaña, Iosune Goicoechea, Patricia Ibáñez, Elena Navarcorena, María Redín and Leyre Resano for their support in conducting the study.
This manuscript contains original material. Presented in part at the 35th and 37th Annual Meetings of the European Society of Medical Oncology, October 2010 (Milan, Italy) and October 2012 (Vienna, Austria) and at the 1st Simposium of the Spanish Society of Medical Oncology, October 2010 (Madrid, Spain).
Funding Statement
The authors have no support or funding to report.
References
- 1. Ballatori E, Roila F (2003) Impact of nausea and vomiting on quality of life in cancer patients during chemotherapy. Health Qual Life Outcomes 1: 46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Grunberg SM, Deuson RR, Mavros P, Geling O, Hansen M, et al. (2004) Incidence of chemotherapy-induced nausea and emesis after modern antiemetics. Cancer 100: 2261–2268. [DOI] [PubMed] [Google Scholar]
- 3. Basch E, Prestrud AA, Hesketh PJ, Kris MG, Feyer PC, et al. (2011) Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol 29: 4189–4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roila F, Herrstedt J, Aapro M, Gralla RJ, Einhorn LH, et al. (2010) Guideline update for MASCC and ESMO in the prevention of chemotherapy- and radiotherapy-induced nausea and vomiting: results of the Perugia consensus conference. Ann Oncol 21 Suppl 5v232–243. [DOI] [PubMed] [Google Scholar]
- 5. Wong EH, Clark R, Leung E, Loury D, Bonhaus DW, et al. (1995) The interaction of RS 25259–197, a potent and selective antagonist, with 5-HT3 receptors, in vitro. Br J Pharmacol 114: 851–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Aapro MS, Grunberg SM, Manikhas GM, Olivares G, Suarez T, et al. (2006) A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol 17: 1441–1449. [DOI] [PubMed] [Google Scholar]
- 7. Eisenberg P, Figueroa-Vadillo J, Zamora R, Charu V, Hajdenberg J, et al. (2003) Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer 98: 2473–2482. [DOI] [PubMed] [Google Scholar]
- 8. Gralla R, Lichinitser M, Van Der Vegt S, Sleeboom H, Mezger J, et al. (2003) Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol 14: 1570–1577. [DOI] [PubMed] [Google Scholar]
- 9. Gurpide A, Sadaba B, Martin-Algarra S, Azanza JR, Lopez-Picazo JM, et al. (2007) Randomized crossover pharmacokinetic evaluation of subcutaneous versus intravenous granisetron in cancer patients treated with platinum-based chemotherapy. Oncologist 12: 1151–1155. [DOI] [PubMed] [Google Scholar]
- 10. Chow SC, Liu JP (1992) On assessment of bioequivalence under a higher-order crossover design. J Biopharm Stat 2: 239–256. [DOI] [PubMed] [Google Scholar]
- 11. Garcia Gomez J, Perez Lopez ME, Garcia Mata J, Isla Casado D (2010) SEOM clinical guidelines for the treatment of antiemetic prophylaxis in cancer patients receiving chemotherapy. Clin Transl Oncol 12: 770–774. [DOI] [PubMed] [Google Scholar]
- 12. Eisenberg P, MacKintosh FR, Ritch P, Cornett PA, Macciocchi A (2004) Efficacy, safety and pharmacokinetics of palonosetron in patients receiving highly emetogenic cisplatin-based chemotherapy: a dose-ranging clinical study. Ann Oncol 15: 330–337. [DOI] [PubMed] [Google Scholar]
- 13. Saito M, Aogi K, Sekine I, Yoshizawa H, Yanagita Y, et al. (2009) Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol 10: 115–124. [DOI] [PubMed] [Google Scholar]
- 14. Stoltz R, Parisi S, Shah A, Macciocchi A (2004) Pharmacokinetics, metabolism and excretion of intravenous [l4C]-palonosetron in healthy human volunteers. Biopharm Drug Dispos 25: 329–337. [DOI] [PubMed] [Google Scholar]
- 15. Shah A, DeGroot T, Apseloff G (2006) Pharmacokinetic evaluation and safety profile of a 15-minute versus 30-second infusion of palonosetron in healthy subjects. J Clin Pharmacol 46: 1139–1145. [DOI] [PubMed] [Google Scholar]
- 16. Aapro MS (2007) Palonosetron as an anti-emetic and anti-nausea agent in oncology. Ther Clin Risk Manag 3: 1009–1020. [PMC free article] [PubMed] [Google Scholar]
- 17. Ruhlmann C, Herrstedt J (2010) Palonosetron hydrochloride for the prevention of chemotherapy-induced nausea and vomiting. Expert Rev Anticancer Ther 10: 137–148. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
CONSORT Checklist.
(DOC)
Trial Protocol.
(DOCX)
CONSORT Flow Diagram.
(DOC)