Abstract
Several groups undergo extended periods without sleep due to working conditions or mental illness. Such sleep deprivation (SD) can deleteriously affect attentional processes and disrupt work and family functioning. Understanding the biological underpinnings of SD effects may assist in developing sleep therapies and cognitive enhancers. Utilizing cross-species tests of attentional processing in humans and rodents would aid in mechanistic studies examining SD-induced inattention. We assessed the effects of 36 hours of: 1) Total SD (TSD) in healthy male and female humans (n=50); and 2) REM SD (RSD) in male C57BL/6 mice (n=26) on performance in the cross-species 5-Choice Continuous Performance Test (5C-CPT). The 5C-CPT includes target trials on which subjects were required to respond and non-target trials on which subjects were required to inhibit from responding. TSD-induced effects on human Psychomotor Vigilance Test (PVT) were also examined. Effects of SD were also examined on mice split into good and poor performance groups based on pre-deprivation scores. In the human 5C-CPT, TSD decreased hit rate and vigilance with trend-level effects on accuracy. In the PVT, TSD slowed response times and increased lapses. In the mouse 5C-CPT, RSD reduced accuracy and hit rate with trend-level effects on vigilance, primarily in good performers. In conclusion, SD induced impaired 5C-CPT performance in both humans and mice and validates the 5C-CPT as a cross-species translational task. The 5C-CPT can be used to examine mechanisms underlying SD-induced deficits in vigilance and assist in testing putative cognitive enhancers.
Keywords: attention, vigilance, CPT, Psychomotor Vigilance Test, bipolar disorder
1. INTRODUCTION
All species, including humans, require some state of sleep [1]. Despite the ubiquity of this phenomenon, much of the underlying mechanisms, long-term effects, and the actual function that sleep provides are still poorly understood. Nevertheless, it is well known that deprivation from sleep negatively affects general health and cognition in humans [2–4]. The extent to which sustained wakefulness impairs cognitive performance in particular seems to depend on the task at hand. For example, sleep deprivation (SD) has a more profound effect in tasks requiring the maintenance of attention than in tasks assessing working memory and executive functions [5].
The increasingly fast-paced nature of society requires people to work longer hours resulting in sleeping fewer hours per day with irregular patterns of sleep [6]. For example, several professions including piloting or the military require vigilance (attending to relevant stimuli over time), yet involve extended periods without sleep, which impairs vigilance [7, 8]. Moreover, certain psychiatric populations exhibit abnormal sleeping patterns, which may further impact their already deficient cognitive performance and possibly impair efficacy of some treatments (e.g. cognitive behavioral therapy). Patients with bipolar disorder for instance are well known for experiencing disrupted sleep patterns, SD, and concomitantly suffer from cognitive symptoms [9]. Furthermore, SD can precipitate manic and hypomanic episodes [10], yet benefit patients in depressive episodes [11, 12]. Investigating the mechanisms of SD-induced effects on behaviors including vigilance would aid in developing cognition-enhancing pharmaceuticals or behavioral countermeasures to cognitive deficits for certain professions and psychiatric disorders. While humans can be experimentally sleep deprived, animal models are more suitable for investigating underlying mechanisms of SD-induced deficits in vigilance. Additionally, SD may serve as an environmental challenge in animal models of psychiatric disorders [13, 14]. The limited cross-species tests of attention/vigilance in humans and animals hampers such investigations however.
Attentional performance during SD in humans has commonly been assessed using the Psychomotor Vigilance Test (PVT) [15]. This reaction time (RT) task requires responding to a visual cue (target stimulus) presented at pseudo-random intervals. Generally, RTs are slowed and more variable, while omissions are increased in humans subjected to SD [7]. SD-induced impaired performance has been observed in rats in a PVT analog [16] and the 5-choice serial reaction time task (5CSRTT), the latter of which requires responding in varied locations [17]. These tasks require only responses to target stimuli however, despite the important and distinct role that inhibiting from responding to irrelevant (non-target) stimuli has in attentional processes [18]. Specifically, with only target stimuli, separating attentional lapses from response fatigue is difficult. By including non-target stimuli, one can determine whether response rates are globally or specifically diminished due to inattention to relevant stimuli. Likewise, treatments that increase global responsiveness may not be useful when one’s environment is littered with irrelevant (non-target) stimuli. Hence, cross-species studies are required on the effects of SD on attentional performance that is specific to responding to relevant (target) stimuli.
The combination of both target and non-target stimuli is the hallmark of tests labeled as continuous performance tests (CPT; [18]). With the inclusion of non-target stimuli, CPTs measure vigilance and are the gold-standard tests of attention in psychiatric populations [19]. In the limited studies conducted on the effects of SD on CPT performance, several days of sleep restriction increased misses to target stimuli and reduced responses to non-target stimuli, thereby overall impairing vigilance and reducing responsiveness [20, 21]. Other studies using total SD (TSD) report modest but non-significantly increased misses to targets but no change in non-target responses after TSD in healthy subjects; however stronger attentional disruption is reported in methadone-maintained subjects [22, 23]. TSD primarily increased non-target responses compared to target responses in a go/nogo task however, despite this task not being a true CPT [24]. Determining the effects of SD on a cross-species vigilance task is required however, for examining putative underlying mechanisms.
The 5-choice (5C-)CPT, based on the 5CSRTT, was developed to assess vigilance in mice [25–27] and rats [28, 29], and is now available in humans [30], including in a fMRI setting [31]. Consistent with other CPTs, the 5C-CPT presents target stimuli to which the subject is required to respond as well as non-target stimuli, to which the subject is required to inhibit from responding. To date, no studies have assessed whether SD affects mouse or human performance in this cross-species CPT. Thus, the present studies investigated whether SD would affect 5C-CPT performance similarly in both mice and humans. We hypothesized that: a) 36 hours of TSD in humans; and b) 36 hours of rapid eye movement (REM) SD (RSD) in mice would similarly impair 5C-CPT performance. Since inter-individual differences were expected on mice 5C-CPT performance [27], and treatments can affect rodent performance differentially dependent upon baseline performance [32, 33], we split the animals in good and poor performers. Finally, to ensure the validity of our TSD protocol, we also assessed TSD-induced effects in the human PVT.
2. METHODS
2.1. Humans
Fifty human subjects (23 female) aged between 18 and 39 years were recruited through flyers, newspaper, and radio from the general San Diego community to participate in this study. Subjects were initially screened via telephone for eligibility. Informed consent was signed at an in-person screen, which included a complete medical history and a Structured Clinical Interview for DSM-IV. Inclusion criteria were at least 12 years of education, a consistent sleep-wake schedule (7–9 hours sleep each night), and for women to be tested in the early follicular phase of their menstrual cycle. Exclusion criteria were history of any sleep disorder, Axis I psychopathology or immediate family history of mood or psychotic disorders; head injury followed by unconsciousness, migraine headaches requiring treatment, seizures, neurological symptoms of the hand, wrist, or arm; current use of nicotine or in the past 2 years; current use of psychotropic medications, hormone-based birth control; high caffeine (>400 mg/day) or alcohol (>2 ounces/day) use; positive urine toxicology screen for illegal substances; hearing threshold above 45 dB(A) at 500–6000 Hz; non-responsiveness to startling stimuli or any other medical condition which might pose a health risk for the subject. Subjects were instructed to maintain a regular sleep-wake schedule at home for at least one week prior to the study, which was monitored with sleep diaries and actigraphy. Sleep monitoring on the first night of the study screened for unreported sleep disorders. This study was conducted at the VA San Diego with the approval of the IRBs of UCSD and VA and has therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
2.1.1. Total sleep deprivation
Subjects spent four nights and days in the laboratory: a) adaptation to the lab (night/day 0); b) normal sleep followed by a battery of testing including the PVT and then the 5C-CPT (night/day 1); c) sleep or TSD followed by a similar battery of testing (night/day 2); and d) sleep or TSD followed by a similar battery of testing as night/day 2 (night/day 3). Subjects were randomly assigned to one of three groups. Group 1 received normal sleep throughout the study; group 2 was sleep deprived for 36 hours prior to day 2; and group 3 was sleep deprived for 36 hours prior to day 3. Subjects assigned to group 1 were included in the ‘normal sleep’ group (n=18). Post-deprivation night data for subjects in groups 2 and 3 were collapsed into the TSD group (n=32). The data from group 1 used for analysis was taken from day 2 or 3 in order to match with subjects from groups 2 and 3 therefore minimizing practice effects as a putative confound. Sleep schedules were made as similar to those maintained at home as possible with sleep being monitored with a standard overnight polysomnogram, including EEG, EOG, and EMG. At each point, subjects were free to engage in activities such as reading, watching television, or socializing. No exercise more strenuous than walking was allowed, nor any form of stimulant. Light snacks and meals were provided. Lights were kept at a constant low level, with no sunlight introduced. Wakefulness was documented through 1) a staff-completed monitoring log every 15 min with subjects’ activities and mental status, and 2) actigraphy.
2.1.2. Psychomotor Vigilance Test
During the PVT, subjects were presented with a blank box in the middle of a screen. At pseudo-random intervals ranging from 2 to 10 s, a bright red light millisecond (ms) counter started to scroll, and subjects had to press the space bar to stop the counter as quickly as possible. After pressing the button, the counter displayed the achieved RT for 1 s, providing the subject with feedback on performance. The PVT task lasted 10 min and was programmed in Eprime (Psychology Software Tools (Sharpsburg, PA, USA)). Median RT, fastest and slowest 10% of RTs, and number of lapses (RTs > 500 ms) were measured.
2.1.3. Human 5C-CPT apparatus
The task appeared on a 56 cm CRT computer screen (60 cm from subject). Subjects used an arcade joystick to make responses. The joystick was spring-mounted so that it would return to the center after each response. A Dell PC with E-Prime2 software (Psychology Software Tools) was used for stimulus presentation and data acquisition.
2.1.4. Human 5C-CPT
A schematic of the paradigm is presented in Figure 1 and described elsewhere [30]. In brief, participants were briefly introduced to the task and were told that they would see 5 white lines (3 cm) in an arc on a black background. Subjects were instructed that if a white circle (≈2 cm) appeared behind a line (target stimuli), the joystick should be moved in that direction, but if circles appeared behind every line (non-target stimuli) they should inhibit from responding. Stimuli appeared for 100 ms with a response window of 1 s after the stimuli disappeared. A variable inter-trial interval (ITI; 0.5, 1, or 1.5 s) occurring 1 s after the stimulus of the previous trial was presented in a pseudo-random order between trials. Before the actual task, subjects were given a practice session, which consisted of 12 trials (10 target and 2 non-target stimuli randomly presented). The full task consisted of 270 trials (225 target and 45 non-target stimuli pseudo-randomly presented). Several measures were determined from this task (Table 1) and calculations based on hit rates (HR), false alarms (FA), FA rates (FAR), and correct rejections (CR) were made accordingly:
Figure 1. Schematic of the human and mouse 5C-CPT.
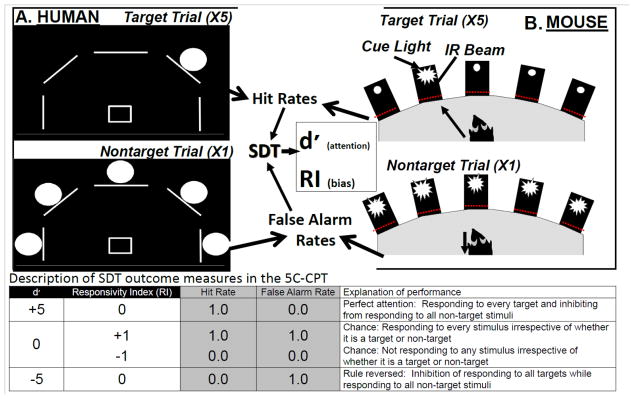
In both the human and mouse 5C-CPTs, there are 5 stimuli locations. For humans, stimuli are presented in 1 of 5 locations arrayed in an arc on a computer screen, and subjects respond using a 5-way joystick (A). For mice, stimuli are presented in 1 of 5 holes located in an arc at the rear of a 5-hole operant chamber and responses are recorded by infrared beams in each hole (B). The task design is the same in both cases, whereby: 1) a single stimulus represents a target trial to which subjects must respond; and 2) all 5 stimuli being presented simultaneously represents a non-target trial to which the subject must inhibit from responding. Target trials generate measures of hits and misses (target responses and omissions), which are used to calculate a subjects’ hit rate, while non-target trials generate measures of correct rejections and false alarms, which are used to calculate a subjects’ false alarm rate. Using signal detection theory (SDT), the non-parametric measure of vigilance (d′) and bias (responsivity index (RI)) are generated. The table provides examples of what permutations of hit and false alarm rates result in various d′ and RI levels and its interpretation.
Table 1.
Description of the behavioral measures used in the human and rodent 5C-CPTs.
| Measure | Description |
|---|---|
| Hit | Response to target stimulus in correct location |
| Miss | Non-response to target stimulus |
| Incorrect | Response to target stimulus but in wrong location |
| Correct Rejection (CR) | Correct non-response to non-target stimulus |
| False alarm (FA) | Incorrect response to non-target stimulus |
| Premature response | Response to no stimuli during the inter-trial interval |
| Mean reaction time (RT) | Mean latencies to correct responses |
| Variable RT | Standard deviation of the RT |
| Hit rate (HR) | Proportion of correct responses to target stimuli |
| False alarm rate (FAR) | Proportion of incorrect responses to non-target stimuli |
| Vigilance (d′) | Parametric measure examining the difference between Hit and False alarm rates to determine performance |
| Responsivity index (RI) | Non-parametric measure examining the combination of Hit and False alarm rates to determine responsivity to stimuli |
| Accuracy | Proportion of correct compared to incorrect responses |
| % Omissions | Percentage of misses/lapses |
Signal detection indices were calculated based upon these basic parameters to assess both sensitivity and responsivity indices:
d′ provides a parametric assessment of sensitivity to appropriate responding. The non-parametric response bias measure RIprovides a measure of the ‘tendency to respond’. Low numbers indicate a conservative response strategy, while high numbers indicate liberal responding [34, 35].
2.2. Animals
Male C57BL/6 mice (n=26) were 12–14 months old at the time of testing and weighed between 23–30 g. All animals were group housed (maximum 4/cage) and maintained in a temperature-controlled vivarium (21±1 °C) on a reversed day-night cycle (lights on at 7.00 PM, off at 7.00 AM) and tested during the dark phase of the day-night cycle between 8.00 AM and 11.00 AM. All mice had ad libitum access to water and were food-restricted at 85% of their free-feeding weight during periods of testing. All procedures were approved by the UCSD Institutional Animal Care and Use Committee. The UCSD animal facility meets all federal and state requirements for animal care and was approved by the American Association for Accreditation of Laboratory Animal Care.
2.2.1. REM sleep deprivation
Mice receiving normal sleep (n=13) and mice on RSD (n=13) were baseline matched on training performance as measured by their average d′ 3 days prior to testing. The conventional ‘inverted flower pot’ technique was used, originally designed by Jouvet et al. in 1964 [36] and still used in RSD studies in animals [13]. In brief, group-housed mice were sleep deprived by placing the same number of small inverted cups (4 cm diameter) as there were mice in the cage in a pool of water (37 C°; 2 cm height) for 36 hours prior to testing. Control animals had bigger inverted cups (7 cm diameter), which because of its size allowed for sleep, in a pool of water for the same period.
2.2.2. Mouse 5C-CPT
A schematic of the paradigm is presented in Figure 1 and described elsewhere [25, 27]. Consistent with the human task, mice were required to make a holepoke if 1 of the 5 holes lit up (target trials) in order to obtain a food reward, but inhibit from responding when all 5 holes lit up (non-target trials) in order to obtain a reward (see Supplemental Material and Methods). In brief, mice were progressively trained to conduct this task using simple choice progressing to use the entire 5-hole array and until performance was stable on d′ , % omissions, and RTs when tested for baseline performance over 3 days before SD (~70 5C-CPT training sessions). Measures were calculated as described for the human 5C-CPT (see Table 1 for measures).
2.3. Statistics
Human 5C-CPT performance was analyzed using the General Linear Model (GLM) with TSD and gender as between-subject factors and trial period as a within-subjects factor. Mouse 5C-CPT performance was analyzed using a repeated measure ANOVA with stimulus duration as a within-subject factor and RSD as a between-subject factor. Where appropriate, planned comparison Tukey post hoc analyses were conducted between groups and Cohen’s d effect sizes were calculated. Two animals from the RSD group were removed from statistical analyses because of a lack of responding (>95% omissions). In order to explore the effects of SD on individual differences in performance, a median split was conducted on vigilance performance (d′) measured during 3 days of baseline testing to group subjects into good and poor performers. Performance group was entered into the model as a between subject factor. The level of probability for statistical significance was set at 0.05. All statistics were performed using SPSS (19.0, Chicago, IL, USA).
3. RESULTS
3.1. Humans
3.1.1. Effects of TSD on PVT performance
The effects of TSD on PVT performance in humans are detailed in Table 2. In brief, TSD slowed overall RTs, including the fastest and slowest 10% of responses during the task. TSD also increased the number of attentional lapses (RTs > 500 ms).
Table 2.
Means, standard errors of the mean, and statistical comparison of human PVT performance after normal sleep vs. TSD.
| Variable | Normal sleep mean (s.e.m.) | TSD mean (s.e.m.) | d.f. | F | p-Value |
|---|---|---|---|---|---|
| Lapses | 0.4 (1.2) | 5.2 (0.9) | 1,48 | 10.5 | <0.005 |
| Median RT | 278.2 (8.2) | 312.2 (6.2) | 1,48 | 11.0 | <0.005 |
| Fastest 10% RT | 230.2 (5.6) | 245.6 (4.2) | 1,48 | 4.8 | <0.05 |
| Slowest 10% RT | 389.9 (154.8) | 816.0 (116.1) | 1,48 | 4.8 | <0.05 |
RT, reaction time (in milliseconds). TSD, total sleep deprivation.
3.1.2. Effects of TSD on human 5C-CPT performance
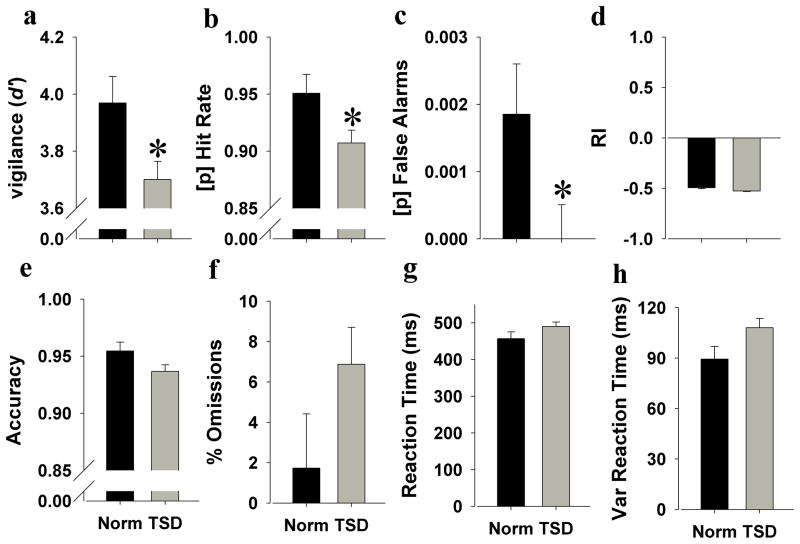
Because there were no interactions of TSD with trial period, gender, or baseline performance (F<1.8, ns), data were pooled and analyzed. As hypothesized, TSD impaired vigilance as measured by reduced d′ (F (1,42)=5.7, p<0.05; Cohen’s d = 0.6; Figure 2a). TSD also reduced hit (F (1,42)=4.8, p<0.05; Cohen’s d = 0.5; Figure 2b) and false alarm rates (F(1,42)=4.2, p<0.05; Cohen’s d = 0.15; Figure 2c). TSD tended to reduce responsivity as measured by reduced RI (F(1,42)=3.5, p<0.1; Cohen’s d = 0.2; Figure 2d), and tended to decrease accuracy (F(1,42)=3.4, p<0.1; Cohen’s d = 0.1; Figure 2e). There was no effect of TSD on omissions (F(1,42)=2.5, ns; Figure 2f). Interestingly, TSD did not affect RT(F (1,42)=2.2, ns; Figure 2g), but tended to increase variability of RT(F (1,42)=4.0, p<0.1; Figure 2h).
Figure 2. Effects of TSD on 5C-CPT performance in humans.
TSD impaired vigilance as measured by reduced d′ (a) with a large effect size (Cohen’s d = 0.6). This TSD-impaired vigilance was partially driven by reduced overall hit rate (b; Cohen’s d effect size = 0.5) and lower non-target responses (c; Cohen’s d effect size = 0.15). Humans during TSD were slightly less responsive (d) and also made slightly less target responses (e) compared to humans after normal sleep. No significant difference between normal sleep and TSD was observed on the number of omitted trials (f). Mean RTs did not differ (g), but humans during TSD exhibited slight increased variable RTs compared to humans after normal sleep (h). Data are presented as the mean ± SEM, *denotes p<0.05 when compared with humans after normal sleep.
3.2. Mice
3.2.1. Effects of RSD on mouse 5C-CPT performance
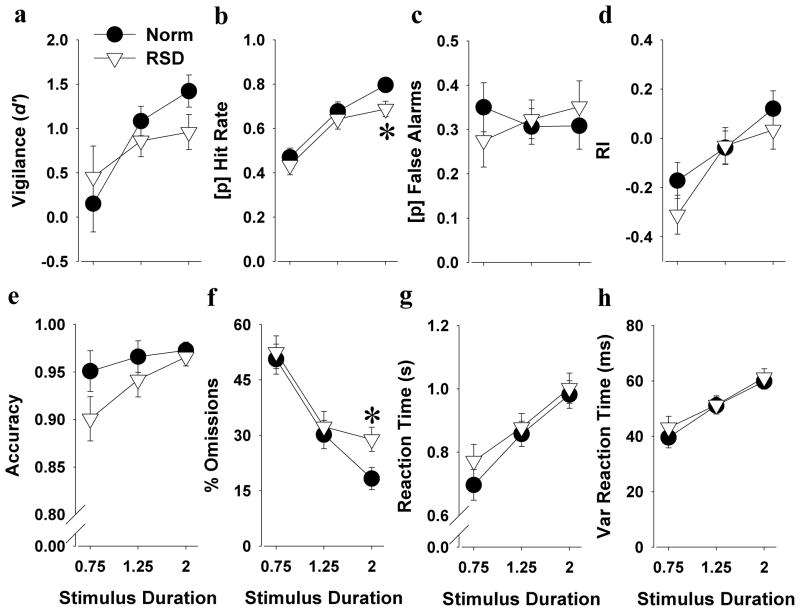
Interestingly, while longer stimulus durations improved hit rate in control mice (stimulus duration; F(2,22)=12.3, p<0.0001), this effect was not present in the RSD mice (stimulus duration; F<1, ns). Post hoc analyses revealed that RSD mice exhibited a reduced hit rate at the 2 s stimulus duration compared with control mice (p<0.05; Figure 3b). Similar benefits to lengthening the stimulus duration were observed in fewer omissions in control mice (stimulus duration; F(2,22)=12.0, p<0.0001) and again this effect was not present in the RSD mice (stimulus duration; F<1, ns). Post hoc analyses revealed that RSD mice exhibited increased omissions at the 2 s stimulus duration compared with control mice (p<0.05; Figure 3f). RSD tended to reduce accuracy (F(1,21)=3.5, p<0.1; Figure 3e), without interacting with stimulus duration. Overall, RSD did not affect d′, false alarms, RI, RTs, or vRTs (Figure 3). RSD also did not affect premature responses, but reduced the amount of trials completed in mice (F(1,21)=4.6, p<0.05), without interacting with stimulus duration (see Supplemental Table 1).
Figure 3. Effects of RSD on 5C-CPT performance in all C57BL/6 mice.
RSD had only subtle effects when looked at the overall group performance of mice in the 5C-CPT. Overall, mice seemed to perform better with longer stimulus duration (a-h). However, this effect was less pronounced in mice during RSD, where RSD decreased hit rate (b) and increased the amount of omissions (f) at the longest stimulus duration of 2 s. Data are presented as the mean ± SEM, *denotes p<0.05 when compared with mice after normal sleep.
3.3. Good and poor performing mice
Consistent with previous reports [27], inter-individual differences in performance were observed in mice, with several subjects performing at a low baseline level. Treatments can differentially affect rodent performances in operant tasks dependent upon baseline level of performance [32, 33]. Therefore, we investigated the effects of RSD in good vs. poor performing mice. Good and poor performers (n=12/12) were identified as described above (see 2.3. Statistics).
3.3.1. The effects of RSD on good performing mice in the 5C-CPT
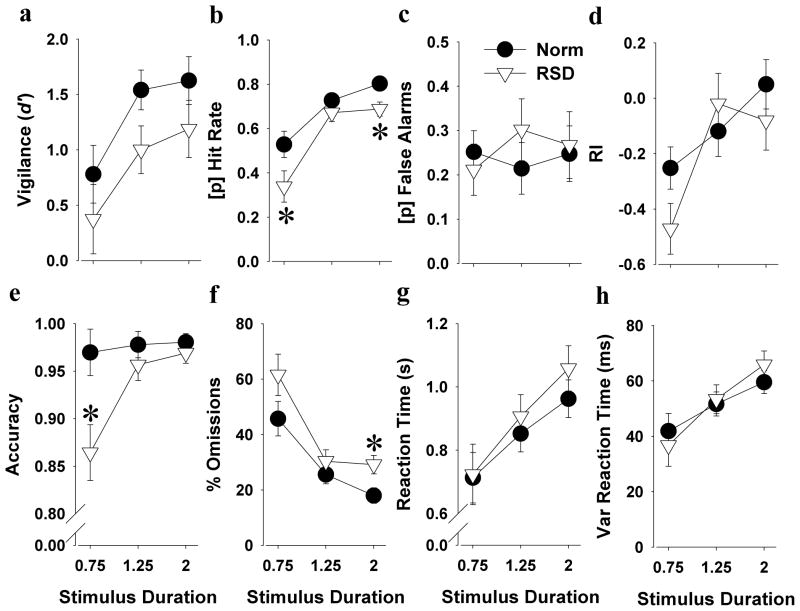
In good performing mice, RSD deleteriously affected accuracy (F(1,9)=5.7, p<0.05), with its effect tending to interact with stimulus duration (F(2,18)=4.6, p=0.051). RSD specifically reduced accuracy at the 0.75 s stimulus duration (p<0.05; Cohen’s d = 1.34; Figure 4e). For percentage omissions there was a trend effect of RSD (F(1,9)=3.5, p<0.1). Again, longer stimulus durations resulted in fewer omissions in control mice (stimulus duration; F(2,10)=12.3, p<0.005), but this effect was not present in the RSD mice (stimulus duration; F<1, ns), who exhibited more omissions at the 2 s stimulus duration compared with control mice (p<0.05; Cohen’s d = 1.79; Figure 4f). No main effect of RSD or interaction with stimulus duration was observed for d′. Longer stimulus durations tended to result in increased d′ in control mice however (stimulus duration; F(2,10)=3.3, p<0.1), with this effect not being present in RSD mice (stimulus duration; F<1, ns), who tended to exhibit reduced d′ at the 1.25 s stimulus duration compared with control mice (p<0.1; Cohen’s d = 1.18; Figure 4a). There was a trend effect of RSD impairing hit rate (F(1,9)=4.7, p<0.1). Although longer stimulus durations improved hit rate in control mice (stimulus duration; F(2,10)=17.3, p<0.005), this effect was not present in RSD mice (stimulus duration; F(2,6)=2.0, ns), whom exhibited a reduced hit rate at both the 0.75 (p<0.05; Cohen’s d = 1.28; Figure 4b) and the 2 s stimulus durations (p<0.05; Cohen’s d = 1.89) compared with control mice. For the RI, a trend stimulus duration by RSD interaction was observed (F(2,18)=3.6, p<0.1; Figure 4d), but no post hoc effect of RSD was observed. RSD did not affect RTs, vRTs, false alarms (Figure 4), trials completed, or percentage premature responses (See Supplemental Table 2).
Figure 4. Effects of RSD on 5C-CPT performance in good performing mice.
In the good performing subgroup of mice, RSD more severely impaired 5C-CPT performance. RSD negatively impacted vigilance as measured by slight reduced d′ at the 1.25 s stimulus duration (a). RSD decreased hit rate, specifically at the 0.75 s and 2 s stimulus durations (b), while leaving non-target responses unaffected (c). No effect of RSD was observed on responsiveness (d), but after RSD, mice made fewer target responses compared to mice after normal sleep, specifically at the 0.75 s stimulus duration (e). Although longer stimulus durations reduced the number of omitted trials in control mice, this effect was less pronounced in the mice after RSD, where RSD increased omissions at the highest stimulus duration (f). No effect of RSD was observed on both mean and variable RTs (g-h). Data are presented as the mean ± SEM, *denotes p<0.05 when compared with mice after normal sleep.
3.3.2. The effects of RSD on mice performing at low baseline levels in the 5C-CPT
The effects of RSD on poor performing mice in the 5C-CPT are detailed in Supplemental Table 3. In brief, RSD did not affect trials completed, percentage premature responses, RTs, vRTs, accuracy, false alarms, d′, or RI of these mice, overall, nor at any specific stimulus duration.
4. DISCUSSION
We report that 36 hours of TSD and RSD impaired 5C-CPT performance in humans and mice respectively. This SD-impaired 5C-CPT performance was driven by more misses of target stimuli, consistent with previous CPT studies in humans [20, 21]. TSD-induced attentional lapses in the PVT confirm the efficacy of the TSD procedure in humans. Importantly, the present data reveal that despite SD-induced reduced responsiveness of humans and mice overall, inattention specific to relevant stimuli was still observed, particularly in good performing subjects.
Over the last decade, the PVT has been used as the ‘gold standard’ to assess the effects of SD on alertness [4]. Broadly, PVT studies reliably find that SD slows RTs and increases lapses (omissions) of attention [7]. Similarly here, 36 hours of TSD slowed RTs and increased PVT lapses (RTs > 500 ms). This behavior has been associated with increased activation of the prefrontal region part of the ‘default mode network’, which is generally activated when subjects are at rest and not engaged in goal-directed behaviors [37]. Our PVT data confirm that our TSD protocol reliably affected attentional performance. Nevertheless, the TSD-induced reduction in PVT responsiveness could reflect generalized reduced responding rather than vigilance per se, since this distinction cannot be made using the PVT because it contains only target stimuli.
Specifically examining vigilance requires assessing both accurate responding to target stimuli as well as the inhibition of responding to non-target (irrelevant) stimuli. Using the 5C-CPT, we observed that TSD overall reduced target responding, as in the PVT, and also reduced non-target responding. Importantly, the greater decrease in target responding (as indicated by greater effect sizes) resulted in a lower d′ score of vigilance. Hence, these 5C-CPT findings indicate that TSD impairs attention beyond simply reducing responding as seen in the PVT, supporting the use of both stimulus types. Furthermore, mood questionnaires (PANAS) [38] completed by the subjects indicated that feelings of alertness correlated significantly with d′ in the 5C-CPT (r=0.42, p<0.005), but less with attentional lapses in the PVT (r=−0.31, p<0.05), whereas pleasantness correlated with PVT (p<0.05), but not 5C-CPT performance (p>0.1). These data support TSD-induced deficits in 5C-CPT as reflecting attentional dysfunction.
Consistent with the present results, mild cumulative sleep restriction impaired CPT performance of healthy controls and children with ADHD [21]. Joo et al. reported that impaired attention in subjects performing a complex CPT after 24 hours of TSD was also driven by reduced target responding, which was accompanied by increased non-target responses [20]. The discrepancy of SD effects on non-target responding between that report and the present study could have resulted from their small study population of only 6 young male adults and/or the complexity of the CPT used. In healthy and methadone-treated humans, a non-significant reduction in target responding was observed following 36 hours of TSD [22], which may have been limited by low sample sizes, practice effects, and/or poor performing subjects. In another study, SD did not significantly affect CPT performance in Korean medical residents and interns [23]. In the present studies using healthy subjects from the general population and matching post TSD-testing days to account for possible practice effects, we observed that 36 hours of TSD clearly impaired 5C-CPT performance.
Similar to our human study, 36 hours of RSD impaired performance in mice in the rodent 5C-CPT, an effect that was not observed in poor performing mice. Interestingly, the TSD-induced reduction in d′ and target responding in humans was primarily driven by affecting good performing humans, without significantly affecting poor performers (data not shown). RSD in good performing mice decreased their target (correct) responses, resulting in more omitted trials and a trend-level vigilance deficit as measured by reduced d′. Comparable results have been observed in the 5CSRTT where 10-hour TSD rats made fewer correct responses and omitted more trials compared to rats with normal sleep [17]. Additionally, 24 hours of TSD slowed responses and increased lapses in a rat PVT [16]. These tasks support our findings in the 5C-CPT. Because these tasks include only target trials however, and no measure of false alarm rates, a simple reduction in responding could not be discounted. When developing treatments by using these tasks, it would be unclear therefore if developed treatments simply increased responding to any presented (even irrelevant) stimuli. In contrast, the 5C-CPT measures both correct responses to target trials and failures to inhibit responding to non-target trials [25]. Hence, responsiveness can be dissociated from target responding and our data support that RSD affects attentive responding beyond simply reducing responding. To date, the rodent 5C-CPT has been successfully used to assess genetic and pharmacological manipulations on attentional measures in both rats [28, 29] and mice [25–27, 39]. Thus, the current study validates the 5C-CPT as a test suited for translational studies because SD manipulations induce similar 5C-CPT effects in both humans and mice. Consequently, the 5C-CPT will be useful to examine the mechanism(s) underlying SD-induced impairment of attentional performance.
A cross-species comparison between 5C-CPT performance of mice and humans revealed that 36 hours of SD decreased hit rate and vigilance and tended to decrease accuracy in humans, whereas it decreased accuracy and hit rate while tending to decrease vigilance in mice, primarily in those with a good baseline performance. The lack of SD-induced deleterious effect in poorly performing mice could be due to a floor effect wherein performance could not be made worse in these mice (see Supplemental Table 3). The stronger overall vigilance deficits observed in humans may have also resulted from the different SD technique used compared to mice. TSD in humans slowed RTs in the PVT and increased variable RTs in the 5C-CPT, but did not affect 5C-CPT RTs in mice. The ‘inverted flower pot’ technique used here in mice affects various forms of sleep including deep slow wave sleep [40], but primarily deprives animals from REM sleep [41, 42]. TSD also affects non-REM sleep and reduces the overall amount of sleep to a greater extent than RSD. Thus, the TSD we administered in humans may have exerted a stronger effect on attention than the RSD we administered in mice. The extended training used in mice, which may have resulted in greater use of procedural memory and hence different circuitry activation patterns, may have also resulted in some of the differences between species.
With cross-species similarity of SD effects on 5C-CPT performance, the mechanism(s) underlying these effects can be investigated. Some putative mechanisms have been tested, e.g. that microdialysis perfusion-induced elevation of basal forebrain adenosine, a key mediator of sleep homeostasis, impaired rat PVT performance [43]. Similarly, SD increased basal levels of adenosine in rats [44]. Furthermore, the adenosine antagonist caffeine is commonly consumed by humans to increase wakefulness. Serotonergic mechanisms could also be examined given that RSD for 24 hours increased serotonergic activity in the hypothalamus in rats [45]. Therefore, various mechanisms that may underlie SD-impaired 5C-CPT performance could be targeted in the future to improve attention following sleep loss.
The consistency of SD-induced impaired human and mouse 5C-CPT performance could also prove useful when investigating aspects of psychiatric disorders. As described above, SD can switch people with bipolar disorder into a mania episode. In fact, SD has been used to model mania in rodents [13, 46, 47]. Such studies are limited however because healthy humans do not become manic after SD [14]. Thus, people with bipolar disorder have an underlying sensitivity to SD-induction of mania [10]. Therefore, using the 5C-CPT and SD technique described here may enable the examination of susceptibility genotypes that result in impaired attention in bipolar disorder patients [48].
SD impaired 5C-CPT performance in both humans and mice, primarily by reducing target responding. The SD-induced deficits in mice were only significant in good performers and at longer stimulus durations. Mouse 5C-CPT performance consistently improved with longer stimulus durations. It is clear that SD disrupted the benefit of longer stimulus durations leading to pronounced effects at these durations. With larger sample sizes however, SD would likely impair performance at all stimulus durations. Besides smaller sample sizes after the median split, differences in training and TSD vs. RSD techniques discussed above could have also contributed to the limited effects observed in mice. In addition to not affecting all forms of sleep, the ‘flower pot’ technique can be stressful for animals [49], even more so in combination with food restriction [50]. Other techniques such as the gentle handling method [17] may therefore be useful in future studies. Future studies with larger sample sizes will be conducted in order to account for inter-individual differences and SD effects.
5. CONCLUSION
In conclusion, 36 hour SD deleteriously affected 5C-CPT performance of both humans and mice. Importantly, SD primarily reduced target responding in both species, with a smaller effect on reducing non-target responding, indicating that SD is primarily deleterious to vigilance and not overall responding. These data validate using the 5C-CPT as a cross-species test of vigilance. Therefore, the rodent and human 5C-CPTs can be used in the future across species to examine mechanisms underlying SD effects, susceptibility of psychiatric disorders to such effects, and test pro-vigilance medication for affected groups.
Supplementary Material
Acknowledgments
We thank Dr. Berend Olivier as well as Ms. Mahalah Buell and Ms. Elisa Tsan for their support. The human studies were supported by a Defense Medical Research and Development Program award #DM102425 and Clinical Center and Center of Excellence for Stress and Mental Health and the animal studies were supported by NIH grants R01-MH071916, R21-MH091571, as well as by the Veteran’s Administration VISN 22 Mental Illness Research, Education, and Clinical Center (MIRECC). The experiments comply with all US federal and California state requirements for animal care and were approved by the American Association for Accreditation of Laboratory Animal Care.
List of Abbreviations
- 5C-CPT
5-Choice Continuous Performance Test
- 5CSRTT
5-Choice Serial Reaction Time Task
- CR
correct rejection
- FA
false alarm
- FAR
FA rate
- HR
hit rate
- ITI
inter-trial interval
- PVT
Psychomotor Vigilance Test
- REM
rapid eye movement
- RI
responsivity index
- RSD
REM sleep deprivation
- RT
reaction time
- SD
sleep deprivation
- TSD
total sleep deprivation
- vRT
variable RT
Footnotes
The authors report no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Siegel JM. Do all animals sleep? Trends Neurosci. 2008;31:208–13. doi: 10.1016/j.tins.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cappuccio FP, D’Elia L, Strazzullo P, Miller MA. Sleep duration and all-cause mortality: a systematic review and meta-analysis of prospective studies. Sleep. 2010;33:585–92. doi: 10.1093/sleep/33.5.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hanlon EC, Van Cauter E. Quantification of sleep behavior and of its impact on the cross-talk between the brain and peripheral metabolism. Proc Natl Acad Sci U S A. 2011;108 (Suppl 3):15609–16. doi: 10.1073/pnas.1101338108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Killgore WD. Effects of sleep deprivation on cognition. Prog Brain Res. 2010;185:105–29. doi: 10.1016/B978-0-444-53702-7.00007-5. [DOI] [PubMed] [Google Scholar]
- 5.Lo JC, Groeger JA, Santhi N, Arbon EL, Lazar AS, Hasan S, et al. Effects of partial and acute total sleep deprivation on performance across cognitive domains, individuals and circadian phase. PLoS One. 2012;7:e45987. doi: 10.1371/journal.pone.0045987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akerstedt T, Nilsson PM. Sleep as restitution: an introduction. J Intern Med. 2003;254:6–12. doi: 10.1046/j.1365-2796.2003.01195.x. [DOI] [PubMed] [Google Scholar]
- 7.Lim J, Dinges DF. Sleep deprivation and vigilant attention. Ann N Y Acad Sci. 2008;1129:305–22. doi: 10.1196/annals.1417.002. [DOI] [PubMed] [Google Scholar]
- 8.Lim J, Dinges DF. A meta-analysis of the impact of short-term sleep deprivation on cognitive variables. Psychol Bull. 2010;136:375–89. doi: 10.1037/a0018883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mansell W, Pedley R. The ascent into mania: a review of psychological processes associated with the development of manic symptoms. Clin Psychol Rev. 2008;28:494–520. doi: 10.1016/j.cpr.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 10.Salvadore G, Quiroz JA, Machado-Vieira R, Henter ID, Manji HK, Zarate CA., Jr The neurobiology of the switch process in bipolar disorder: a review. J Clin Psychiatry. 2010;71:1488–501. doi: 10.4088/JCP.09r05259gre. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bunney BG, Bunney WE. Mechanisms of Rapid Antidepressant Effects of Sleep Deprivation Therapy: Clock Genes and Circadian Rhythms. Biol Psychiatry. 2012 doi: 10.1016/j.biopsych.2012.07.020. [DOI] [PubMed] [Google Scholar]
- 12.Kahn-Greene ET, Killgore DB, Kamimori GH, Balkin TJ, Killgore WD. The effects of sleep deprivation on symptoms of psychopathology in healthy adults. Sleep Med. 2007;8:215–21. doi: 10.1016/j.sleep.2006.08.007. [DOI] [PubMed] [Google Scholar]
- 13.Gessa GL, Pani L, Fadda P, Fratta W. Sleep deprivation in the rat: an animal model of mania. Eur Neuropsychopharmacol. 1995;5 (Suppl):89–93. doi: 10.1016/0924-977x(95)00023-i. [DOI] [PubMed] [Google Scholar]
- 14.Young JW, Henry BL, Geyer MA. Predictive animal models of mania: hits, misses and future directions. Br J Pharmacol. 2011;164:1263–84. doi: 10.1111/j.1476-5381.2011.01318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dinges DF, Powell JW. Microcomputer Analyses of Performance on a Portable, Simple Visual Rt Task during Sustained Operations. Behavior Research Methods Instruments & Computers. 1985;17:652–5. [Google Scholar]
- 16.Christie MA, McKenna JT, Connolly NP, McCarley RW, Strecker RE. 24 hours of sleep deprivation in the rat increases sleepiness and decreases vigilance: introduction of the rat-psychomotor vigilance task. J Sleep Res. 2008;17:376–84. doi: 10.1111/j.1365-2869.2008.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cordova CA, Said BO, McCarley RW, Baxter MG, Chiba AA, Strecker RE. Sleep deprivation in rats produces attentional impairments on a 5-choice serial reaction time task. Sleep. 2006;29:69–76. [PMC free article] [PubMed] [Google Scholar]
- 18.Riccio CA, Reynolds CR, Lowe P, Moore JJ. The continuous performance test: a window on the neural substrates for attention? Arch Clin Neuropsychol. 2002;17:235–72. [PubMed] [Google Scholar]
- 19.Borgaro S, Pogge DL, DeLuca VA, Bilginer L, Stokes J, Harvey PD. Convergence of different versions of the continuous performance test: clinical and scientific implications. J Clin Exp Neuropsychol. 2003;25:283–92. doi: 10.1076/jcen.25.2.283.13646. [DOI] [PubMed] [Google Scholar]
- 20.Joo EY, Yoon CW, Koo DL, Kim D, Hong SB. Adverse effects of 24 hours of sleep deprivation on cognition and stress hormones. J Clin Neurol. 2012;8:146–50. doi: 10.3988/jcn.2012.8.2.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gruber R, Wiebe S, Montecalvo L, Brunetti B, Amsel R, Carrier J. Impact of sleep restriction on neurobehavioral functioning of children with attention deficit hyperactivity disorder. Sleep. 2011;34:315–23. doi: 10.1093/sleep/34.3.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bracken BK, Trksak GH, Penetar DM, Tartarini WL, Maywalt MA, Dorsey CM, et al. Response inhibition and psychomotor speed during methadone maintenance: impact of treatment duration, dose, and sleep deprivation. Drug Alcohol Depend. 2012;125:132–9. doi: 10.1016/j.drugalcdep.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kim HJ, Kim JH, Park KD, Choi KG, Lee HW. A survey of sleep deprivation patterns and their effects on cognitive functions of residents and interns in Korea. Sleep Med. 2011;12:390–6. doi: 10.1016/j.sleep.2010.09.010. [DOI] [PubMed] [Google Scholar]
- 24.Drummond SP, Paulus MP, Tapert SF. Effects of two nights sleep deprivation and two nights recovery sleep on response inhibition. J Sleep Res. 2006;15:261–5. doi: 10.1111/j.1365-2869.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 25.Young JW, Light GA, Marston HM, Sharp R, Geyer MA. The 5-choice continuous performance test: evidence for a translational test of vigilance for mice. PLoS One. 2009;4:e4227. doi: 10.1371/journal.pone.0004227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harms LR, Turner KM, Eyles DW, Young JW, McGrath JJ, Burne TH. Attentional processing in C57BL/6J mice exposed to developmental vitamin D deficiency. PLoS One. 2012;7:e35896. doi: 10.1371/journal.pone.0035896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Young JW, Meves JM, Geyer MA. Nicotinic agonist-induced improvement of vigilance in mice in the 5-choice continuous performance test. Behav Brain Res. 2013;240:119–33. doi: 10.1016/j.bbr.2012.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Barnes SA, Young JW, Neill JC. D(1) receptor activation improves vigilance in rats as measured by the 5-choice continuous performance test. Psychopharmacology (Berl) 2012;220:129–41. doi: 10.1007/s00213-011-2460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barnes SA, Young JW, Neill JC. Rats tested after a washout period from sub-chronic PCP administration exhibited impaired performance in the 5-Choice Continuous Performance Test (5C-CPT) when the attentional load was increased. Neuropharmacology. 2012;62:1432–41. doi: 10.1016/j.neuropharm.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Young JW, Geyer MA, Rissling AJ, Sharp RF, Eyler LT, Asgaard G, et al. Reverse translation of the rodent 5C-CPT reveals that the impaired attention of people with schizophrenia is similar to scopolamine-induced deficits in mice. Translational Psychiatry. 2013 doi: 10.1038/tp.2013.82. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McKenna BS, Young JW, Dawes SE, Asgaard GL, Eyler LT. Bridging the bench to bedside gap: validation of a reverse-translated rodent continuous performance test using functional magnetic resonance imaging. Psychiatry Res. 2013 doi: 10.1016/j.pscychresns.2013.01.005. [DOI] [PubMed] [Google Scholar]
- 32.Granon S, Passetti F, Thomas KL, Dalley JW, Everitt BJ, Robbins TW. Enhanced and impaired attentional performance after infusion of D1 dopaminergic receptor agents into rat prefrontal cortex. J Neurosci. 2000;20:1208–15. doi: 10.1523/JNEUROSCI.20-03-01208.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Amitai N, Weber M, Swerdlow NR, Sharp RF, Breier MR, Halberstadt AL, et al. A novel visuospatial priming task for rats with relevance to Tourette syndrome and modulation of dopamine levels. Neurosci Biobehav Rev. 2012 doi: 10.1016/j.neubiorev.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Frey P, Colliver J. Sensitivity and responsivity measures for discrimination learning. Learn Motiv. 1973;4:327–42. [Google Scholar]
- 35.Sahgal A. Some limitations of indices derived from signal detection theory: evaluation of an alternative index for measuring bias in memory tasks. Psychopharmacology (Berl) 1987;91:517–20. doi: 10.1007/BF00216022. [DOI] [PubMed] [Google Scholar]
- 36.Jouvet D, Vimont P, Delorme F, Jouvet M. Study of Selective Deprivation of the Paradoxal Sleep Phase in the Cat. C R Seances Soc Biol Fil. 1964;158:756–9. [PubMed] [Google Scholar]
- 37.Drummond SP, Bischoff-Grethe A, Dinges DF, Ayalon L, Mednick SC, Meloy MJ. The neural basis of the psychomotor vigilance task. Sleep. 2005;28:1059–68. [PubMed] [Google Scholar]
- 38.Watson D, Clark LA, Tellegen A. Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol. 1988;54:1063–70. doi: 10.1037//0022-3514.54.6.1063. [DOI] [PubMed] [Google Scholar]
- 39.Young JW, Powell SB, Scott CN, Zhou X, Geyer MA. The effect of reduced dopamine D4 receptor expression in the 5-choice continuous performance task: Separating response inhibition from premature responding. Behav Brain Res. 2011;222:183–92. doi: 10.1016/j.bbr.2011.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grahnstedt S, Ursin R. Platform sleep deprivation affects deep slow wave sleep in addition to REM sleep. Behav Brain Res. 1985;18:233–9. doi: 10.1016/0166-4328(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 41.Kitka T, Katai Z, Pap D, Molnar E, Adori C, Bagdy G. Small platform sleep deprivation selectively increases the average duration of rapid eye movement sleep episodes during sleep rebound. Behav Brain Res. 2009;205:482–7. doi: 10.1016/j.bbr.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Kitahama K, Valatx JL. Instrumental and pharmacological paradoxical sleep deprivation in mice: strain differences. Neuropharmacology. 1980;19:529–35. doi: 10.1016/0028-3908(80)90022-2. [DOI] [PubMed] [Google Scholar]
- 43.Christie MA, Bolortuya Y, Chen LC, McKenna JT, McCarley RW, Strecker RE. Microdialysis elevation of adenosine in the basal forebrain produces vigilance impairments in the rat psychomotor vigilance task. Sleep. 2008;31:1393–8. [PMC free article] [PubMed] [Google Scholar]
- 44.Sims RE, Wu HH, Dale N. Sleep-wake sensitive mechanisms of adenosine release in the basal forebrain of rodents: an in vitro study. PLoS One. 2013;8:e53814. doi: 10.1371/journal.pone.0053814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Senthilvelan M, Ravindran R, Samson J, Devi RS. Serotonin turnover in discrete regions of young rat brain after 24 h REM sleep deprivation. Neurochem Res. 2006;31:81–4. doi: 10.1007/s11064-005-9139-7. [DOI] [PubMed] [Google Scholar]
- 46.Benedetti F, Fresi F, Maccioni P, Smeraldi E. Behavioural sensitization to repeated sleep deprivation in a mice model of mania. Behav Brain Res. 2008;187:221–7. doi: 10.1016/j.bbr.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 47.Szabo ST, Machado-Vieira R, Yuan P, Wang Y, Wei Y, Falke C, et al. Glutamate receptors as targets of protein kinase C in the pathophysiology and treatment of animal models of mania. Neuropharmacology. 2009;56:47–55. doi: 10.1016/j.neuropharm.2008.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Malkesman O, Austin DR, Chen G, Manji HK. Reverse translational strategies for developing animal models of bipolar disorder. Dis Model Mech. 2009;2:238–45. doi: 10.1242/dmm.001628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Suchecki D, Lobo LL, Hipolide DC, Tufik S. Increased ACTH and corticosterone secretion induced by different methods of paradoxical sleep deprivation. J Sleep Res. 1998;7:276–81. doi: 10.1046/j.1365-2869.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 50.Coenen AM, van Luijtelaar EL. Stress induced by three procedures of deprivation of paradoxical sleep. Physiol Behav. 1985;35:501–4. doi: 10.1016/0031-9384(85)90130-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.