Abstract
Background
FEV1 is universally used as a measure of severity in COPD. Current thresholds are based on expert opinion and not on evidence.
Objectives
We aimed to identify the best FEV1 (% predicted) and dyspnea (mMRC) thresholds to predict 5-yr survival in COPD patients.
Design and Methods
We conducted a patient-based pooled analysis of eleven COPD Spanish cohorts (COCOMICS). Survival analysis, ROC curves, and C-statistics were used to identify and compare the best FEV1 (%) and mMRC scale thresholds that predict 5-yr survival.
Results
A total of 3,633 patients (93% men), totaling 15,878 person-yrs. were included, with a mean age 66.4±9.7, and predicted FEV1 of 53.8% (±19.4%). Overall 975 (28.1%) patients died at 5 years. The best thresholds that spirometrically split the COPD population were: mild ≥70%, moderate 56–69%, severe 36–55%, and very severe ≤35%. Survival at 5 years was 0.89 for patients with FEV1≥70 vs. 0.46 in patients with FEV1 ≤35% (H.R: 6; 95% C.I.: 4.69–7.74). The new classification predicts mortality significantly better than dyspnea (mMRC) or FEV1 GOLD and BODE cutoffs (all p<0.001). Prognostic reliability is maintained at 1, 3, 5, and 10 years. In younger patients, survival was similar for FEV1 (%) values between 70% and 100%, whereas in the elderly the relationship between FEV1 (%) and mortality was inversely linear.
Conclusions
The best thresholds for 5-yr survival were obtained stratifying FEV1 (%) by ≥70%, 56–69%, 36–55%, and ≤35%. These cutoffs significantly better predict mortality than mMRC or FEV1 (%) GOLD and BODE cutoffs.
Introduction
According to the Global Burden of Disease Study, in 2010 chronic obstructive pulmonary disease (COPD) was the third leading cause of death worldwide and the ninth combining the years of life lost or lived with disability (DAILYs).[1], [2] COPD is characterized by an airflow limitation and therefore spirometry remains the essential test to diagnose the disease. Classically, COPD severity has been graded by postbronchodilator FEV1 expressed as percent of predicted values (FEV1 %).[3] More recently, several multidimensional indices have shown a better survival prediction than the isolated FEV1 (%). These include the original BODE index, which incorporates dyspnea measured with the modified scale of Medical Research Council (mMRC), Body Mass Index (BMI), FEV1, and exercise capacity assessed with the 6-minute walking distance (6MWD), as well as further modifications of this index, such as the BODEx (replacement of exercise capacity with severe exacerbations).[4]–[6] Other multidimensional prognostic indices are ADO (age, dyspnea, and FEV1), SAFE (quality of life measured by Saint George's Respiratory Questionnaire, FEV1, and 6MWD), and DOSE (dyspnea, smoking status, FEV1, and prior exacerbation history), among others.[7]–[9]
These indices have been constructed by adding different variables – such as dyspnea, exercise capacity, exacerbations, and age – to different categories of FEV1 values.[10] However, different thresholds of FEV1 and dyspnea are used in different staging systems and with different guidelines.[11]–[12] To date, the most widely used cutoff values are those proposed by the Global Obstructive Lung Disease (GOLD) and the ATS/ERS guidelines (mild≥80%, moderate 50–79%, severe 30–49% and very severe <30%).[13] However, the BODE index uses the old ATS standards (≥65%, 50–64% 36–49% and ≤35%), while the DOSE index uses a different cutoff (>50%, 30–49% and <30%).[4], [9] To the best of our knowledge the majority of these classifications are selected arbitrarily, based on cut-offs selected by expert opinion, and it is not known which of them best discriminates among different levels of mortality risk. Additionally, there are few studies comparing the usefulness of FEV1 in different age groups and the influence of dyspnea on survival assessment.[14]–[16]
The aim of the present study was to identify the best thresholds for FEV1 (%) and dyspnea measured with the mMRC to predict 5-yr survival in COPD patients, divided by subsets of age, using a pooled-analysis of individual patient data from eleven Spanish COPD cohorts (The COllaborative COhorts to assess Multicomponent Indices of COPD in Spain-COCOMICS study).[10]
Methods
Ethics Statement
All participants gave their informed written consent to participate, and their respective ethics committees approved each study (Hospital Galdekao-Usarsolo, Navarra Clinic University Hospital, Requena Hospital, Universitary Hospital Mútua de Terrassa, Universitary Hospital of Valme and Universitary Hospital Miquel Servet).
Study design
The COCOMICS study is a pooled-analysis of individual patient-data from eleven Spanish COPD patient cohorts. The methodology has been described in detail elsewhere.[10], [17] Briefly a common data set with age, gender, spirometry, comorbidity, previous severe exacerbations, and follow-up among other variables was provided by the principal investigator of each of the participating cohorts.[6], [18]–[26] Previous severe exacerbations were defined as those requiring emergency room visit with or without subsequent hospitalization during the previous year. All-cause mortality at 5 years was defined as the primary outcome. Postbronchodilator forced spirometry was performed according to the guidelines of the American Thoracic Society/European Respiratory Society consensus.[27] Dyspnea was assessed using mMRC dyspnea scale.[5] Comorbidities were quantified by means of the Charlson index, excluding COPD, without adjustment for age.[28] All cohorts were previously published although with different follow-up periods; the references of original articles are available in the Online Appendix. All data were quality controlled centrally, and a homogeneous template to translate all coding was applied.
Statistical analysis
Qualitative variables were expressed as absolute and relative frequencies, while quantitative variables were summarized as mean and standard deviation in the case of symmetry, and median otherwise. Comparison among continuous variables was made using the robust means comparison Student-Welch test, under symmetry, and the non-parametric Mann-Whitney U test otherwise. The Fisher exact test was used in order to check independence among categorical variables. We focused all analyses on time to death for all causes. Standard Cox semi-parametric proportional hazard models, all of them stratified by cohort, were used to study time-to-death data.[29] This methodology does not impose parametric restrictions on how the continuous covariate and the studied outcome are associated. In addition, the goodness-of-fit quality of the considered models was measured using the area under the incidence/dynamic ROC curve [30], AUC. The R package risksetROC, freely available in the R CRAN (www.r-project.org), was used for developing the computations. All the comparisons between the curves were performed using the L1-measure (given two functions, f and g, the L1-measure is defined by L1(f,g) = ∫ |f(t)−g(t)|dt). The general Bootstrap Algorithm (gBA) [31] was used in order to approximate the respective P-values. The gBA method allows developing complex hypotheses preserving the original data structure and without assuming any additional hypothesis (just the one considered null). Finally, optimal % FEV1 and dyspnea thresholds were computed for maximizing the AUC at five years follow-up. In a first stage, the threshold which leads to the Youden index was computed and then, on each one of the two resulting sub-populations and with the same criteria, a new threshold was computed. In all analyses, P-values below 0.05 were considered for statistical significance. The free software R.2.15 was used for developing the analysis.
Results
A total of 3,633 subjects with COPD (93% men) were included in the analysis, totaling the experience of 15,878 person-years. The mean age was 66.4 (SD ±9.7). At study entry, smoking exposure was substantial (53.4±26.5 pack-years), with 71.0% former smokers, and 27.9% current smokers. Most participants had moderate to severe airflow limitation with a predicted FEV1 (%) of 53.8%±19.4%, and a Charlson index of 2.1±1.5. Patients over 65 had significantly more comorbidities measured with the Charlson index than young people (≤64 years) [1.83 (1.44) vs. 2.17 (1.60); p<0.0001].
The main characteristics of the population cohorts are presented in Table 1. On average women were younger (59.8±11.0 years vs. 66.9±9.5 years) and more frequently current smokers (43.3% vs. 26.8%) than men (both p<0.05). After 5 years, 975 (28.1%) subjects had died. A significantly greater mortality was observed in older patients and women. Lower levels of FEV1 (%) and BMI, greater dyspnea, poorer quality of life measured with the Saint George's Respiratory Questionnaire (SGRQ), shorter distance walked in 6 x′ walking-test, and severe exacerbations of COPD during the previous year were also associated with a statistically significant five-year increased mortality (Table 1).
Table 1. Demographic and clinical characteristics.
| Number (SD) o % | TotalN = 3,633 | AliveN = 1,133 | DeadN = 975 | P | HR | CI 95% |
| Age | 66.4±9.7 | 64.0±9.3 | 70.8±8.7 | <0.001 | 1.07 | 1.06–1.08 |
| Gender (men) | 3,389 (93.3) | 1,040 (91.8) | 953 (97.7) | <0.001 | 2.99 | 2.10–4.24 |
| SmokingYesNonFormer | 26.9%1.2%71.9% | 32.2%0.4%67.4% | 20.8%2.1%77.1% | <0.0010.001<0.001 | 0.702.161 (Ref) & | (0.61–0.80)(1.43–3.27)– |
| SmokingPacks/year | 53.4±26.5 | 55.4±27.3 | 56.6±26.8 | 0.101 | 1.01 | (1.00–1.08) |
| Charlson Index | 2.1±1.56 | 2.00±1.38 | 2.47±1.87 | <0.001 | 1.18 | (1.14–1.23) |
| FVC (%) predicted | 80.2±22.6 | 88.6±22.4 | 69.0±20.7 | <0.001 | 0.97 | (0.97–0.98) |
| FEV1 (%) predicted | 53.8±19.4 | 58.4±20.6 | 44.6±16.5 | <0.001 | 0.97 | (0.96–0.97) |
| FEV1/FVC ratio | 51.9±11.8 | 51.3±11.9 | 48.6±12.0 | <0.001 | 0.97 | (0.97–0.98) |
| Dyspnea (mMRC)01234 | 17.6%33.7%27.8%23.7%7.2% | 18.8%30.3%31.4%11.9%7.6% | 10.0%24.4%29.8%21.5%14.3% | <0.0010.0070.495<0.001<0.001 | 1 (Ref) &1.241.792.683.25 | –(0.99–1.55)(1.44–2.23)(2.13–3.39)(2.52–4.20) |
| Dyspnea (mMRC) # | 1.59±1.14 | 1.03±1.20 | 2.03±1.20 | <0.001 | 1.47 | (1.40–1.54) |
| SGRQ* | 42.7±20.4 | 43.1±20.2 | 47.6±19.2 | 0.003 | 1.01 | (1.01–1.02) |
| BMI (Kg/m2) | 27.9±4.98 | 27.7±4.82 | 27.1±4.99 | 0.018 | 0.97 | (0.96–0.98) |
| 6-minute walking test (meters) | 397. 2±130.5 | 409.2±134.1 | 330.6±124.4 | <0.001 | 0.996 | (0.995–0.996) |
| Previous severe exacerbations ¥ | 0.89±1.82 | 0.91±2.22 | 1.28±1.86 | <0.001 | 1.07 | (1.05–1.09) |
Five-year mortality.
Reference group;
mMRC: dyspnea measured with the modified Medical Research Council;
Health related Quality of life, measured with Saint George's Respiratory Questionnaire;
Number of hospitalizations for COPD exacerbation in the previous year.
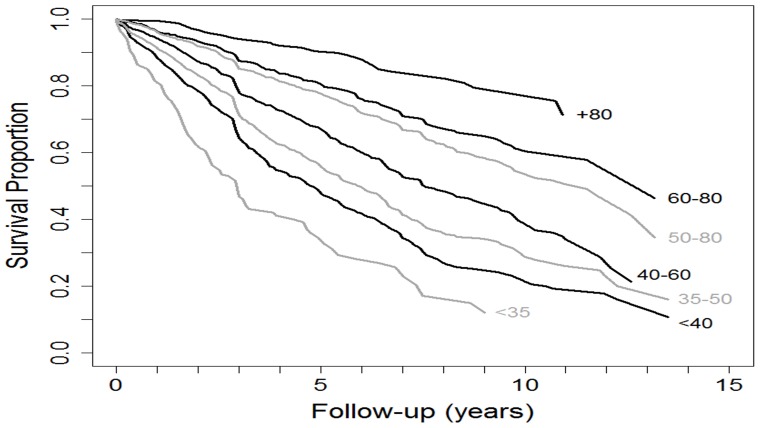
Mortality during short, medium and long-term follow-up – from one to 10 years - was consistently associated with FEV1 (%) (Figure 1, Table 2). The best FEV1 thresholds (%) in the entire cohort to predict 5-year mortality were mild≥70%, moderate 56–69%, severe 35–55%, and very severe ≤35%. Figure 2 shows graphically the risk of mortality at the different cutoffs of FEV1 (%). Of note, patients with an FEV1 (%) lower than 35% had a hazard ratio (HR) for mortality that was 6 times higher than the reference group with FEV1≥75% [95% Confidence Interval (CI): 4.69–7.74].
Figure 1. Kaplan-Meier survival curves for different thresholds of FEV1 up to 15 years.
Hazard ratios and 95% confidence intervals for different thresholds of FEV1 :1) >80% reference group, 2) 60–79% (1.5; 1.06–2.11), 3) 50–79% (1.74; 1.25–2.43), 3) 41–59% (2.38; 1.7–3.32),4) 35–49% (2.92; 2.1–4.07),5)<40% (3.54; 2.53–4.95), 6)<35% (5.18; 3.53–7.61)
Table 2. Area under the curve (AUC) to predict 1, 3, 5, and 10-yr survival at different staging spirometry thresholds, dyspnea levels (mMRC) and time.
| YEARS | 1 | 3 | 5 | 10 |
| COCOMICS | 0.643 | 0.650 | 0.657 | 0.654 |
| GOLD | 0.635 | 0.639 | 0.647 | 0.639 |
| Old ATS (BODE) | 0.643 | 0.650 | 0.653 | 0.649 |
| mMRC (Dyspnea) | 0.623 | 0.620 | 0.625 | 0.614 |
| P | 0.013 | 0.006 | 0.004 | 0.006 |
GOLD: Global Obstructive Lung Disease classification. ATS: American Thoracic Society classification. BODE: Body Mass Index, Obstruction (measured with old ATS classification), Dyspnea and Exercise. mMRC: Dyspnea measured with the modified Medical Research Council scale.
Figure 2. Spline inverse of the 5-yr hazard ratio of death to identify spirometry thresholds of severity (70%–55%–35%).
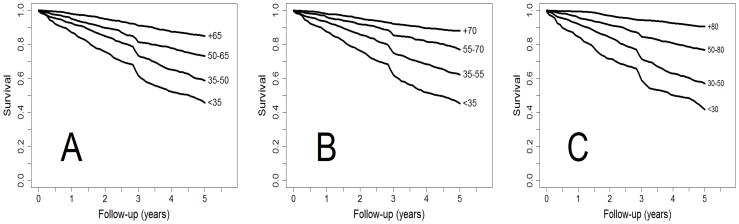
The probability of survival at 5 years was 0.89 (95% C.I.:0.86–0.92) in patients with higher levels of FEV1 (%) (>70%) in contrast to 0.46 (95% C.I.:0.42–0.51) for patients with FEV1 (%) <35%. Kaplan-Meier curves for different thresholds of FEV1 (%) and 5-year mortality in the present study compared with GOLD and old ATS-BODE cutoffs are displayed in Figure 3.
Figure 3. Kaplan-Meier survival curves of time to death by different staging spirometry thresholds: A) new COCOMICS, B) GOLD, and C) old-ATS/BODE.
For all comparisons, the predictive ability of the new cutoff points was higher than that of the previous cutoffs used in the GOLD document and slightly better than those used in the BODE index (old ATS). Hazard Ratios and their 95%CI between the different cutoffs of the COCOMIX study, GOLD, and BODE are presented in Table 3. Prognostic reliability of new thresholds is maintained at 1, 3, 5, and 10 years (Table 2).
Table 3. 5-yr hazard ratios of death at different staging spirometry thresholds.
| Mild | Moderate | Severe | Very severe | P | |
| GOLD | >80%1 (ref.) | 51–80%2.51 (1.83–3.44) | 30–49%5.06 (3.71–6.90) | <30%7.83 (5.65–10.87) | <0.001 |
| Old ATS (BODE) | ≥65%1 (ref.) | 50–64%1.95 (1.61–2.35) | 36–49%3.02 (2.53–3.62) | ≤35%4.62 (3.87–5.53) | <0.001 |
| COCOMIX | ≥70%1 (ref.) | 56–69%1.91 (1.51–2.41) | 35–55%3.40 (2.76–4.20) | ≤35%5.57 (4.48–6.91) | <0.001 |
GOLD: Global Obstructive Lung Disease classification. ATS: American Thoracic Society classification. BODE: Body Mass Index, Obstruction (measured with old ATS classification), Dyspnea and Exercise. Hazard Ratios and 95% Confidence Intervals between different cutoffs of the COCOMIX study, GOLD, and BODE classifications.
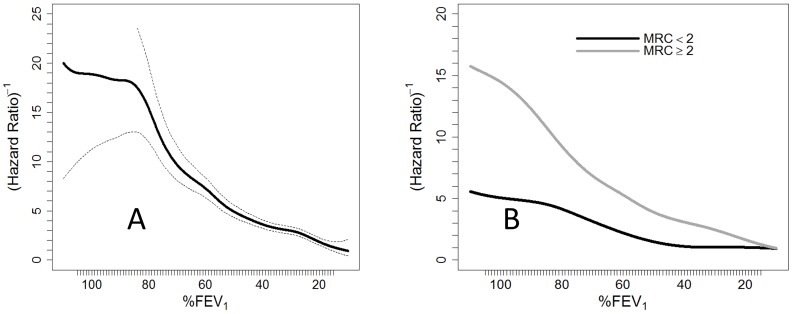
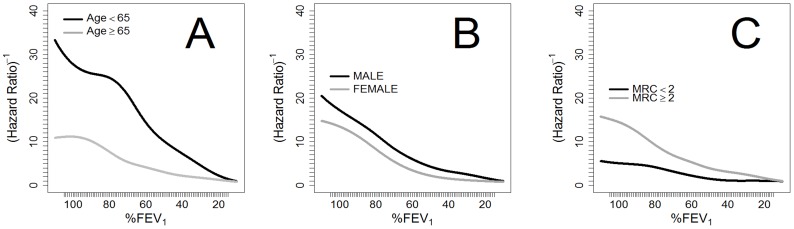
The prognostic value of FEV1 (%) differed according to age. In patients 65 or older, we observed an inverse progressive relationship between mortality and lung function, while in individuals younger than 64, mortality was similar in the interval of FEV1% between 75% and 100%.
Among patients with lower levels of dyspnea (mMRC≤1), overall survival at 5 years was 75.6% (95% CI: 73.2–78.1), for those who also had lower dyspnea an FEV1 (%) >90% survival at 5 years was 92.1% (95% CI: 86.5–98.1). In contrast, patients with higher levels of dyspnea (mMRC≥2) had a 5-year survival of 56.0% (95% CI: 53.3–58.9). No differences existed in the predictive ability of FEV1 (%) by gender. The relationship between FEV1 (%), age, gender and dyspnea are graphically displayed in Figure 4.
Figure 4. Spline inverse of the 5-yr hazard ratio for FEV1 and death, adjusted for: A) age, B) gender and C) dyspnea.
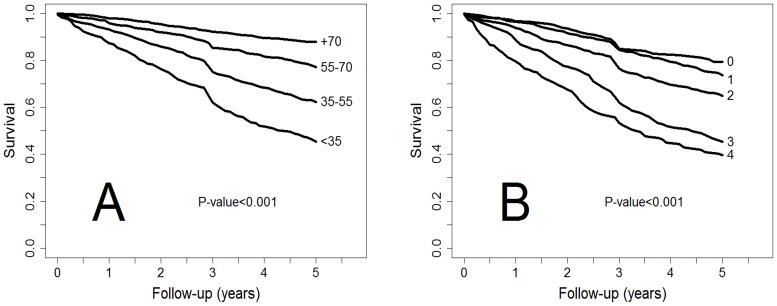
Of note, comparisons between Kaplan-Meier curves for the different levels of FEV1 (%) and dyspnea (Figure 5) showed that FEV1 (%) was a significantly better predictor of survival than degree of dyspnea (p<0.001). Similarly, the new cutoffs of FEV1 (%) were significantly better predictors of survival at 1, 3, 5, and 10 years than the levels of dyspnea measured with the mMRC (Table 3) (Figure 5).
Figure 5. Kaplan-Meier survival curves up to five years for different thresholds of A) FEV1 according to new COCOMICS and B) mMRC.
The quality of the models is measured from the AUC in the incidence/dynamic ROC curves.FEV1 is a better predictor of 5-year survival (p<0.005).
Discussion
The current study was conducted in a large sample of patients over the entire spectrum of COPD severity with long-term follow-up, and it shows that the proposed new spirometric thresholds to predict 5-year mortality (mild≥70%, moderate 56–69%, severe 35–55%, and very severe ≤35%) were significantly better predictors of survival than those used in the GOLD, and slightly better than those used in the BODE index (old ATS). This improvement in predictive capacity was also verified in both the short- and long-term follow-up (1 to 10 years). The study design – a pooled-analysis of individual patient-data from several cohorts – the large sample size and the different degrees of severity of the patients in the different cohorts guarantee a high external validity of the results.
Traditionally, lung function, measured with the FEV1 after a bronchodilator test, has been the most widely recognized variable associated with mortality in COPD. Furthermore, FEV1 is a good predictor of mortality even in the general population, and it is also considered the most important variable to evaluate the severity of COPD.[32] In addition, the decline in FEV1 over time has been used to evaluate the progression of the disease, although wide individual variability exist.[33] Until the last decade, different scientific societies and clinical guidelines had proposed different thresholds of postbronchodilator FEV1 expressed as percentage of predicted values to classify the severity of the disease. However these cutoffs were selected for pragmatic and educational reasons based on expert opinion, which explains the existing discrepancies in the proposed values.[3], [11] To the best of our knowledge, the present study is the first in which the different recommended thresholds were obtained from a cohort study looking for improved sensitivity and specificity points validated for mortality.
One important observation of our study is that although patients with lower levels of FEV1 (<35%) had a mortality that was 6 times higher than the patients with better FEV1 (≥75%), 5-year survival of patients with worse FEV1 (%) is almost 50%. In other words, higher values of FEV1 are associated with lower mortality, but a low FEV1, even below 35% predicted, does not exclude prolonged survival. These data are consistent with previous studies and highlight the conclusion that isolated FEV1 should not be used as an exclusive predictor of prognosis.[23], [34]
Accordingly, the new multicomponent indices have shown better predictive capacity for survival than FEV1 alone. The two common variables included in all multidimensional prognostic indices in COPD are respiratory function – measured with postbronchodilator FEV1 (%)– and dyspnea.[4], [6], [7], [9] However the cutoffs used are different among them. BODE index uses the old ATS values (≥65%, 50–64% 36–49%, and ≤35%), while the DOSE index uses a different cutoff (≥50%, 30–49%, and <30%), and the updated ADO index a 5 point scale (≥81%, ≥65–80, ≥50–64, ≥36–49 and ≤35%).[35] In contrast, the new GOLD document preserves its previous cutoffs for FEV1 (mild≥80%, moderate 50–79%, severe 30–49%, and very severe <30%), together with a combined COPD risk assessment evaluated with previous spirometric classification divided into 2 groups (FEV1%≥50% and ≤49%) along with the individual patient history of exacerbations in the preceding year (0–1 and ≥2 or 1 severe exacerbation). The evaluation of symptoms is measured with the COPD Assessment Test (CAT) (CAT<10 and CAT≥10) or dyspnea assessed by the mMRC scale (0–1 and ≥2), although the classification of COPD produced by the mMRC and CAT scores may differ.[3], [29] Additionally, several groups have demonstrated that the new GOLD multidimensional system classification does not improve prognostic reliability compared with the previous classification based only on spirometric severity for the prediction of mortality and hospitalizations. Indeed, mortality at 3 years was higher in GOLD group B (more symptoms, less risk) than in group C (more risk, fewer symptoms).[17], [36]–[38] A possible explanation is that patients in group B have more comorbidities, and therefore more symptoms despite better spirometric values.[39]–[40]
In the present study, the best cutoff for mMRC dyspnea scale was the same as that proposed in GOLD multidimensional system (mMRC≤1 or ≥2), and this confirmed that dyspnea is an excellent prognostic predictor of survival. In patients with lower levels of dyspnea, overall survival at 5 years was 75.6% while patients with higher levels of dyspnea survival decreased to 56.0%. However our study contradicts the findings reported by Nishimura et al. who reported that dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD.[15] The most plausible reason for this discrepancy is that Nishimura's study was based on only 183 patients with severe impairment of FEV1 (mean 41%), while COCOMICS includes many more patients with a wider range of FEV1 values.
In our study, the predictive ability of FEV1 for 5-year mortality was similar between genders, but the relationship between FEV1 values and survival was different between patients younger than 65 years and those that were older. In younger patients, mortality was similar for values of FEV1 between 75% and 100%, drawing an initial curved plateau, with a progressively decreasing survival below these values. In elderly patients (≥65 years), we observed an inverse progressive relationship between mortality and lung function. Elderly patients also had more comorbidities and higher mortality during follow-up. The importance of comorbidities in COPD patients and their prognostic implications have been increasingly recognized in the last decade.[41]–[43] Heart disease, lung cancer, hypertension, musculoskeletal disorders, and diabetes, among many other diseases, are common in COPD patients, and several epidemiological studies have shown that lung function impairment is associated with an increased risk of comorbid diseases.[44] Previous studies highlighted how comorbidities were more closely related with mortality in older patients, while pulmonary function seemed to be more important in younger patients.[14] Several of these comorbidities affect spirometric values, diabetes and metabolic syndrome, and can lead to a somewhat restrictive pattern, with significantly lower FEV1 and FVC values than in non-diabetics, even after adjustment for age, sex, BMI, smoking status, diabetes duration, and HbA1c levels. Similarly heart failure, coronary artery disease, osteoporosis, hypertension, atrial fibrillation, and muscular or hormonal disorders are related with a reduced forced expiratory volume in spirometry.[45]–[48] However, all our patients met criteria for a pulmonary obstruction pattern, and decreased FEV1 is a recognized predictor of mortality not only in COPD but also in other diseases, and even in the general population.[49]
Our study has several strengths and limitations. Among the former are the large number of subjects included and the long follow-up period, including nearly 16,000 person-yrs. Both are essential to study mortality in a broad spectrum of COPD severity. Second, the follow-up information is very accurate, with few participants lost in follow-up. Third, all cohorts were recruited in Spain, and all investigators followed the same COPD clinical guidelines for pharmacological and non-pharmacological treatment.
However, several limitations need to be acknowledged. Firstly, the variables studied were measured at inclusion from each patient and our analyses assumed that the patients' condition did not change from baseline. It was not possible to make an analysis of time-dependent variables to assess changes in medication, smoking habits and other factors. Although some COPD variables show stability and repeatability, these analyses had no regular monitoring and re-staging.
Secondly, all our participants were Caucasian with a clear predominance of males, reflecting the epidemiology of COPD in Spain; therefore, our results should be extrapolated with caution in other populations.[50]
In conclusion our study performed in a large pooled-analysis of individual patient-data showed that the new spirometric thresholds (mild ≥70%, moderate 56–69%, severe 35–55%, and very severe ≤35%) predicted 5-year mortality significantly better than those used in the GOLD strategy and BODE index, and this improvement in predictive capacity was also verified in both short- and long-term follow-up (1 to 10 years).
Acknowledgments
We sincerely thank the PLOS ONE Reviewers for their constructive criticism and many suggestions that indeed improved our original submission. We declare that we are open for any re-analyses requested from PLOS ONE reviewers and readers under the terms established in the COCOMICS data sharing agreement document. We thank Tom Yohannan (profesional medical copy-editor) for his editorial assistance.
Funding Statement
The COCOMICS study was financed in part with a grant of the Spanish Society of Pneumology and Thoracic surgery coded with the number 057/12.SEPAR 2013. PMC was supported by research grant MTM2011-23204 from the Spanish Ministerio de Ciencia e Innovación (FEDER support included). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Murray CJ, Vos T, Lozano R, Naqhavi M, Flaxman AD, et al. (2012) Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 380: 2197–223. [DOI] [PubMed] [Google Scholar]
- 2. Lozano R, Naghavi M, Foreman K, Kim S, Shibuya K, et al. (2012) Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. 380: 2095–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Vestbo J, Hurd SS, Agustí AG, Jones PW, Vogelmeier C, et al. (2013) Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 187: 347–65. [DOI] [PubMed] [Google Scholar]
- 4. Celli BR, Cote CG, Marin JM, Casanova C, Montes de Oca M, et al. (2004) The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 350: 1005–1012. [DOI] [PubMed] [Google Scholar]
- 5. Bestall JC, Paul EA, Garrod R, Garnham R, Jones PW, et al. (1999) Usefulness of the Medical Research Council (MRC) dyspnoea scale as a measure of disability in patients with chronic obstructive pulmonary disease. Thorax 54: 581–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Soler-Cataluna JJ, Martinez-Garcia MA, Sanchez LS, Tordera MO, Sanchez PR (2009) Severe exacerbations and BODE index: two independent risk factors for death in male COPD patients. Respir Med 103: 692–699. [DOI] [PubMed] [Google Scholar]
- 7. Puhan MA, Garcia-Aymerich J, Frey M, ter Riet G, Anto JM, et al. (2009) Expansion of the prognostic assessment of patients with chronic obstructive pulmonary disease: the updated BODE index and the ADO index. Lancet 374: 704–711. [DOI] [PubMed] [Google Scholar]
- 8. Azarisman MS, Fauzi MA, Faizal MP, Azami Z, Roslina AM, et al. (2007) The SAFE (SGRQ score, air-flow limitation and exercise tolerance) index: a new composite score for the stratification of severity in chronic obstructive pulmonary disease. Postgrad Med J 83: 492–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Jones RC, Donaldson GC, Chavannes NH, Kida K, Dickson-Spillmann M, et al. (2009) Derivation and validation of a composite index of severity in chronic obstructive pulmonary disease – the dose index. Am J Respir Crit Care Med 180: 1189–1195. [DOI] [PubMed] [Google Scholar]
- 10. Marin JM, Alfageme I, Almagro P, Casanova C, Esteban C, et al. (2013) Multicomponent indices to predict survival in COPD: The COllaborative COhorts to assess multicomponent indices of COPD in Spain-COCOMICS study. Eur Respir J 42: 323–32. [DOI] [PubMed] [Google Scholar]
- 11. BTS guidelines for the management of chronic obstructive pulmonary disease (1997) The COPD Guidelines Group of the Standards of Care Committee of the BTS. Thorax 52 Suppl 5 S1–28. [PMC free article] [PubMed] [Google Scholar]
- 12. Miravitlles M, Soler-Cataluña JJ, Calle M, Molina J, Almagro P, et al. (2012) Pharmacological treatment of stable COPD. Arch Bronconeumol 48: 247–257. [DOI] [PubMed] [Google Scholar]
- 13. Celli BR, MacNee W (2004) Standards for the diagnosis and treatment of patients with COPD: a summary of the ATS/ERS position paper. Eur Respir J 23: 932–946. [DOI] [PubMed] [Google Scholar]
- 14. Burgel PR, Paillasseur JL, Peene B, Dusser D, Roche N, et al. (2012) Two distinct chronic obstructive pulmonary disease (COPD) phenotypes are associated with high risk of mortality. PLoS One 7: e51048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Nishimura K, Izumi T, Tsukino M, Oga T (2002) Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 121: 1434–40. [DOI] [PubMed] [Google Scholar]
- 16. Oga T, Tsukino M, Hajiro T, Ikeda A, Nishimura K (2012) Analysis of longitudinal changes in dyspnea of patients with chronic obstructive pulmonary disease: an observational study. Respir Res 13: 85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Soriano JB, Alfageme I, Almagro P, Casanova C, Esteban C, et al. (2013) Distribution and prognostic validity of the new global initiative for chronic obstructive lung disease grading classification. Chest 143: 694–702. [DOI] [PubMed] [Google Scholar]
- 18. Esteban C, Quintana JM, Aburto M, Moraza J, Egurrola M, et al. (2008) Predictors of mortality in patients with stable COPD. J Gen Intern Med 23: 1829–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. de Torres JP, Bastarrika G, Zagaceta J, Sáiz-Mendiguren R, Alcaide AB, et al. (2011) Emphysema presence, severity, and distribution has little impact on the clinical presentation of a cohort of patients with mild to moderate COPD. Chest 139: 36–42. [DOI] [PubMed] [Google Scholar]
- 20. Soler-Cataluña JJ, Martínez-García MA, Román Sánchez P, Salcedo E, Navarro M, et al. (2005) Severe acute exacerbations and mortality in patients with chronic obstructive pulmonary disease. Thorax 60: 925–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Alfageme I, Reyes N, Merino M, Reina A, Gallego J, et al. (2010) The effect of airflow limitation on the cause of death in patients with COPD. Chron Respir Dis 7: 135–45. [DOI] [PubMed] [Google Scholar]
- 22. Casanova C, Cote C, de Torres JP, Aguirre-Jaime A, Marin JM, et al. (2005) Inspiratory-to-total lung capacity ratio predicts mortality in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 171: 591–7. [DOI] [PubMed] [Google Scholar]
- 23. Almagro P, Calbo E, Ochoa de Echagüen A, Barreiro B, Quintana S, et al. (2002) Mortality after hospitalization for COPD. Chest 121: 1441–8. [DOI] [PubMed] [Google Scholar]
- 24. Sanjaume M, Almagro P, Rodríguez-Carballeira M, Barreiro B, Heredia JL, et al. (2009) Post-hospital mortality in patients re-admitted due to COPD. Utility of BODE index.Rev Clin Esp 209: 364–70. [DOI] [PubMed] [Google Scholar]
- 25. Almagro P, Salvadó M, Garcia-Vidal C, Rodríguez-Carballeira M, Cuchi E, et al. (2012) Pseudomonas aeruginosa and mortality after hospital admission for chronic obstructive pulmonary disease. Respiration 84: 36–43.14. [DOI] [PubMed] [Google Scholar]
- 26. Marin JM, Soriano JB, Carrizo SJ, Boldova A, Celli BR (2010) Outcomes in patients with chronic obstructive pulmonary disease and obstructive sleep apnea: the overlap syndrome. Am J Respir Crit Care Med 182: 325–31. [DOI] [PubMed] [Google Scholar]
- 27. Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, et al. (2005) Standardisation of spirometry. Eur Respir J 26: 319–338. [DOI] [PubMed] [Google Scholar]
- 28. Charlson ME, Szatrowski TP, Peterson J, Gold J (1994) Validation of a combined comorbidity index. J Clin Epidemiol 47: 1245–1251. [DOI] [PubMed] [Google Scholar]
- 29.Cox DR, Oakes D (1984) Analysis of Survival Data. London, England: Chapman & Hall.
- 30. Heagerty PJ, Zheng Y (2005) Survival model predictive accuracy and ROC curves. Biometrics 61: 92–105. [DOI] [PubMed] [Google Scholar]
- 31. Martínez-Camblor P, Corral N (2012) A general bootstrap algorithm for hypothesis testing. J Statist Plann Inference 142: 589–600. [Google Scholar]
- 32. Hole DJ, Watt GC, Davey-Smith G, Hart CL, Gillis CR, et al. (1996) Impaired lung function and mortality risk in men and women: findings from the Renfrew and Paisley prospective population study. BMJ 313: 711–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vestbo J, Edwards LD, Scanlon PD, Yates JC, Agusti A, et al. (2011) Changes in forced expiratory volume in 1 second overtime in COPD. N Engl J Med 365: 1184–92. [DOI] [PubMed] [Google Scholar]
- 34. Postma DS, Sluiter HJ (1989) Prognosis of chronic obstructive pulmonary disease: the Dutch experience. Am Rev Respir Dis 140: S100–5. [DOI] [PubMed] [Google Scholar]
- 35.Puhan MA, Hansel NN, Sobradillo P, Enright P, Lange P, et al. (2012) Large-scale international validation of the ADO index in subjects with COPD: an individual subject data analysis of 10 cohorts. BMJ Open 2: .e002152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Kim S, Oh J, Kim YI, Ban HJ, Kwon YS, et al. (2013) Differences in classification of COPD group using COPD assessment test (CAT) or modified Medical Research Council (mMRC) dyspnea scores: a cross-sectional analyses. BMC Pulm Med 13: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Johannessen A, Nilsen RM, Storebø M, Gulsvik A, Eagan T, et al. (2013) Comparison of 2011 and 2007 GOLD Guidelines for Predicting Mortality and Hospitalization. Am J Respir Crit Care Med 188: 602–8. [DOI] [PubMed] [Google Scholar]
- 38. Lange P, Marott JL, Vestbo J, Olsen KR, Ingebrigtsen TS, et al. (2012) Prediction of the Clinical Course of Chronic Obstructive Pulmonary Disease, Using the New GOLD Classification. Am J Respir Crit Care Med 186: 975–981. [DOI] [PubMed] [Google Scholar]
- 39. Agusti A, Edwards LD, Celli B, Macnee W, Calverley PM, et al. (2013) Characteristics, stability and outcomes of the 2011 GOLD COPD groups in the ECLIPSE cohort. Eur Respir J 42: 636–46. [DOI] [PubMed] [Google Scholar]
- 40.Díez-Manglano J, Barquero-Romero J, Almagro P, Cabrera FJ, Lopez-Garcia F, et al. (2013) COPD patients with and without metabolic syndrome: clinical and functional differences. Intern Emerg Med [Epub ahead of print] [DOI] [PubMed]
- 41. Clini EM, Beghé B, Fabbri LM (2013) Chronic obstructive pulmonary disease is just one component of the complex multimorbidities in patients with COPD. Am J Respir Crit Care Med 187: 668–71. [DOI] [PubMed] [Google Scholar]
- 42. Almagro P, Cabrera FJ, Diez J, Boixeda R, Alonso-Ortiz B, et al. (2012) Comorbidities and short-term prognosis in patients hospitalized for acute exacerbation of COPD. The ESMI study. Chest 142: 1126–33. [DOI] [PubMed] [Google Scholar]
- 43. Vanfleteren LE, Spruit MA, Groenen M, Gaffron S, van Empel VP, et al. (2013) Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 187: 728–35. [DOI] [PubMed] [Google Scholar]
- 44. Müllerova H, Agusti A, Erqou S, Mapel DW (2013) Cardiovascular comorbidity in chronic obstructive pulmonary disease: systematic literature review. Chest 144: 1163–78. [DOI] [PubMed] [Google Scholar]
- 45.Yu D, Simmons D (2013) Association between lung capacity measurements and abnormal glucose metabolism: findings from the Crossroads study. Diabet Med [Epub ahead of print] [DOI] [PubMed]
- 46. Nourizadeh M, Ghelich Y, Amin A, Eidani E, Gholampoor Y, et al. (2013) Study the mechanical pulmonary changes in patients with congestive heart failure (CHF) by impulse oscillometry. J Cardiovasc Dis Res 4: 130–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Moayyeri A, Bingham SA, Luben RN, Wareham NJ, Khaw KT (2009) Respiratory function as a marker of bone health and fracture risk in an older population. J Bone Miner Res 24: 956–63. [DOI] [PubMed] [Google Scholar]
- 48.Johnson LS, Juhlin T, Engström G, Nilsson PM (2013) Reduced forced expiratory volume is associated with increased incidence of atrial fibrillation: the Malmo Preventive Project. Europace [Epub ahead of print] [DOI] [PubMed]
- 49. Baughman P, Marott JL, Lange P, Martin CJ, Shankar A, et al. (2012) Combined effect of lung function level and decline increases morbidity and mortality risks. Eur J Epidemiol 27: 933–43. [DOI] [PubMed] [Google Scholar]
- 50. Miravitlles M, Soriano JB, García-Río F, Muñoz L, Duran-Tauleria E, et al. (2009) Prevalence of COPD in Spain: Impact of undiagnosed COPD on quality of life and daily life activities. Thorax 64: 863–868. [DOI] [PubMed] [Google Scholar]