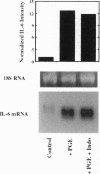
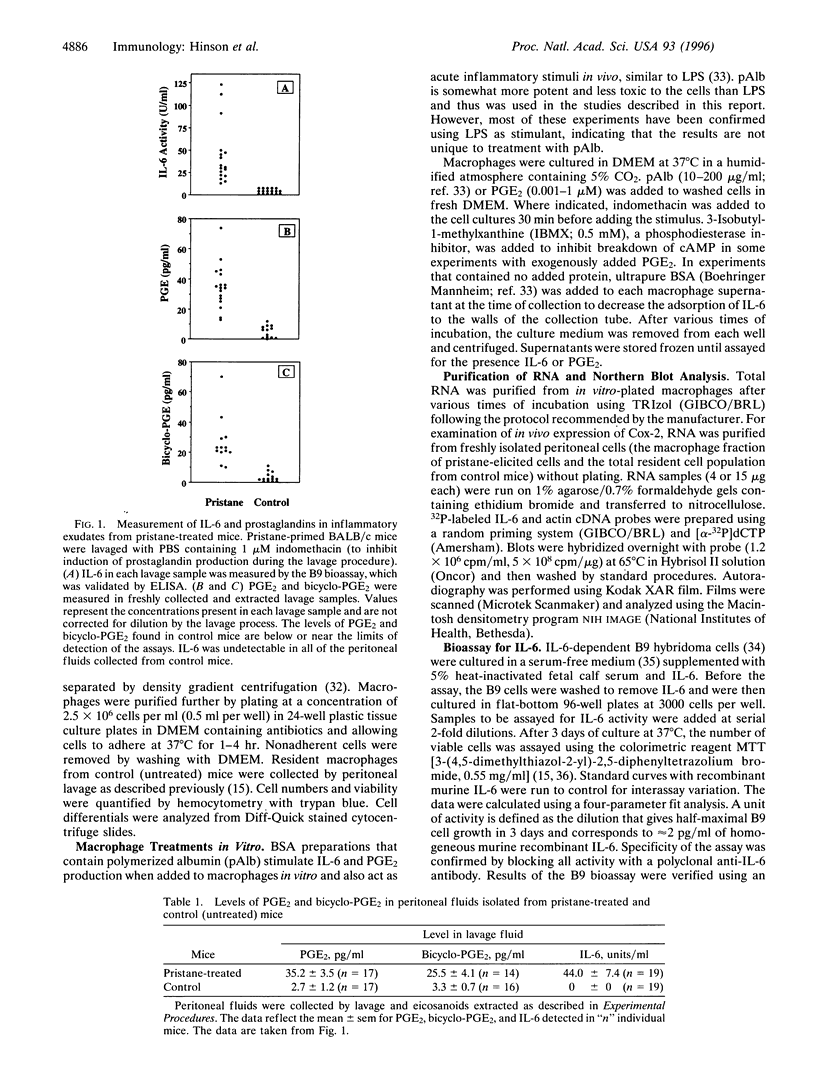
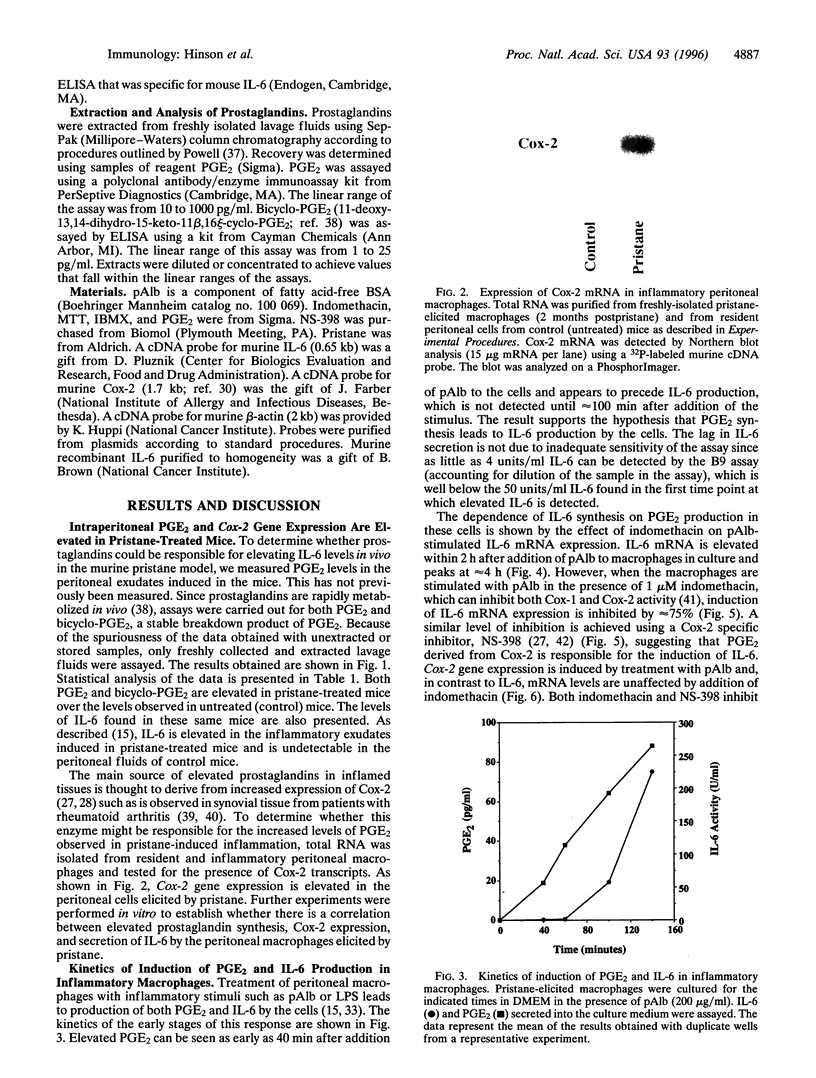
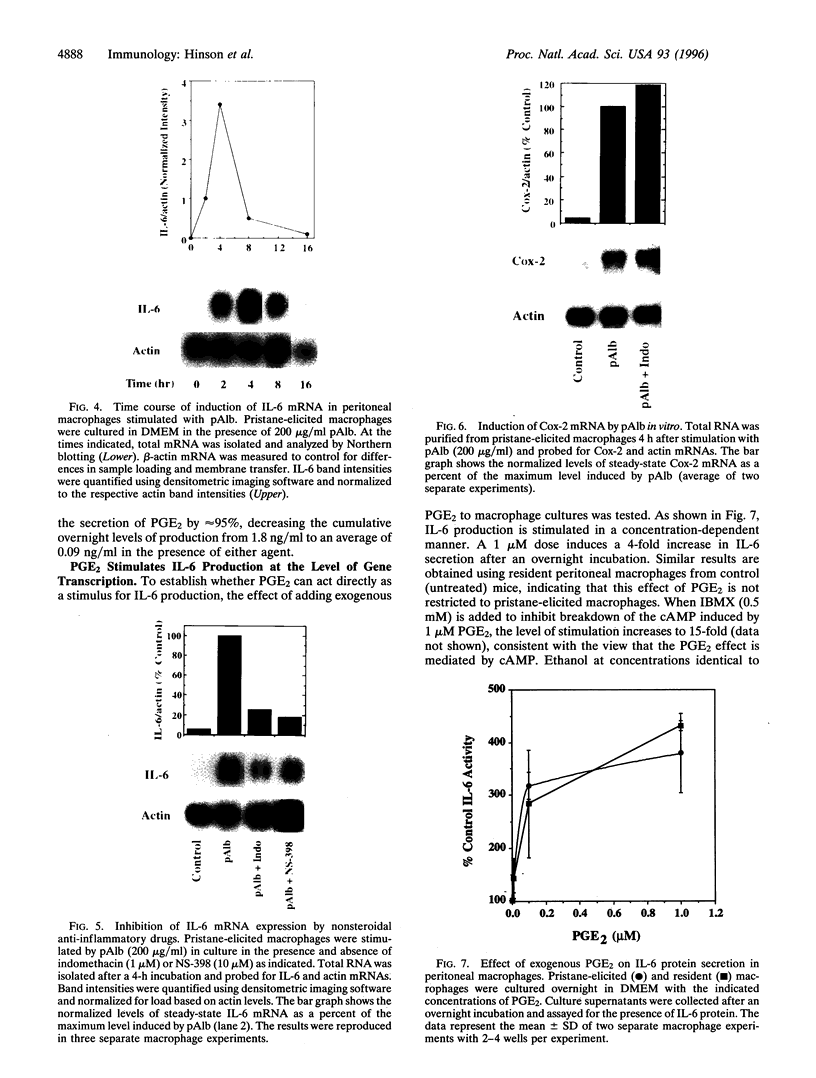
Abstract
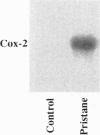
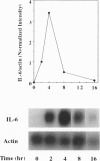
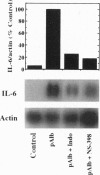
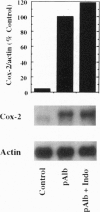
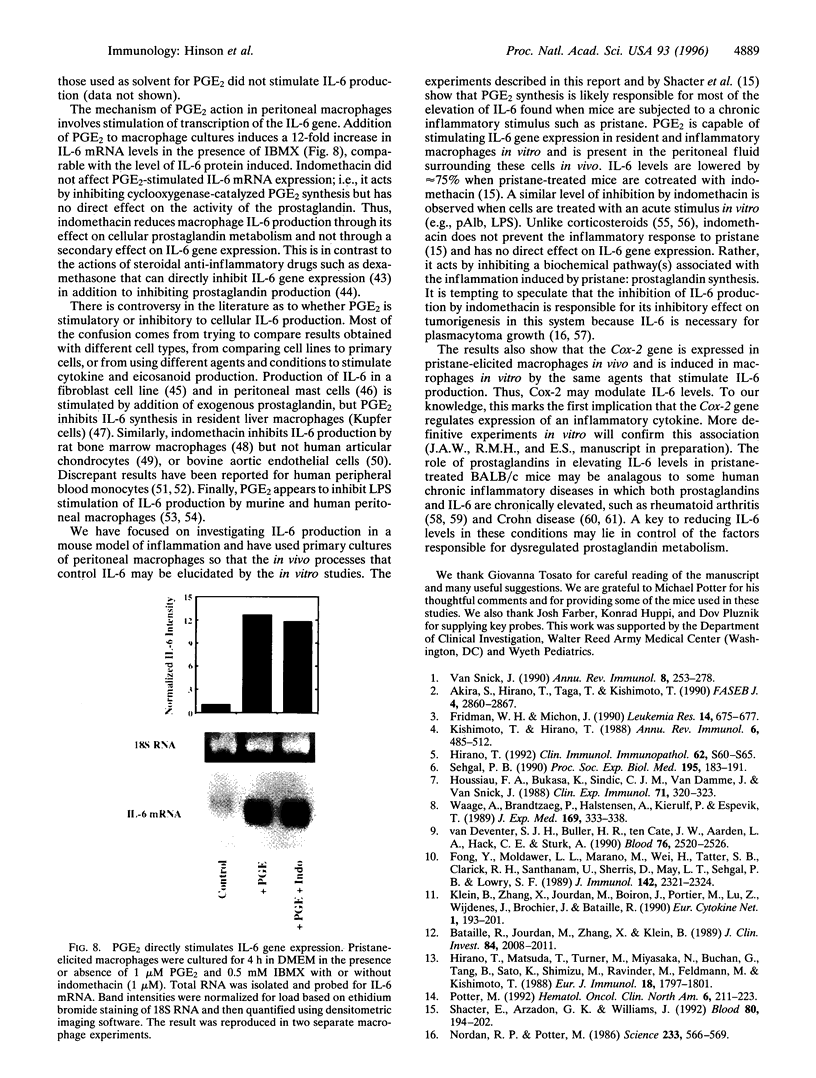
Injection of mineral oils such as pristane into the peritoneal cavities of BALB/c mice results in a chronic peritonitis associated with high tissue levels of interleukin 6 (IL-6). Here we show that increased prostaglandin E2 (PGE2) synthesis causes induction of IL-6 and that expression of an inducible cyclooxygenase, Cox-2, may mediate this process. Levels of both PGE2 and IL-6 are elevated in inflammatory exudates from pristane-treated mice compared with lavage samples from untreated mice. The Cox-2 gene is induced in the peritoneal macrophage fraction isolated from the mice. A cause and effect relationship between increased macrophage PGE2 and IL-6 production is shown in vitro. When peritoneal macrophages are activated with an inflammatory stimulus (polymerized albumin), the Cox-2 gene is induced and secretion of PGE2 and IL-6 increases, with elevated PGE2 appearing before IL-6. Cotreatment with 1 microM indomethacin inhibits PGE2 production by the cells and reduces the induction of IL-6 mRNA but has no effect on Cox-2 mRNA, consistent with the fact that the drug inhibits catalytic activity of the cyclooxygenase but does not affect expression of the gene. Addition of exogenous PGE2 to macrophages induces IL-6 protein and mRNA synthesis, indicating that the eicosanoid stimulates IL-6 production at the level of gene expression. PGE2-stimulated IL-6 production is unaffected by addition of indomethacin. Taken together with the earlier finding that indomethacin diminishes the elevation of IL-6 in pristane-treated mice, the results show that PGE2 can induce IL-6 production in vivo and implicate expression of the Cox-2 gene in the regulation of this cytokine.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abramson S., Korchak H., Ludewig R., Edelson H., Haines K., Levin R. I., Herman R., Rider L., Kimmel S., Weissmann G. Modes of action of aspirin-like drugs. Proc Natl Acad Sci U S A. 1985 Nov;82(21):7227–7231. doi: 10.1073/pnas.82.21.7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akira S., Hirano T., Taga T., Kishimoto T. Biology of multifunctional cytokines: IL 6 and related molecules (IL 1 and TNF). FASEB J. 1990 Aug;4(11):2860–2867. [PubMed] [Google Scholar]
- Akira S., Taga T., Kishimoto T. Interleukin-6 in biology and medicine. Adv Immunol. 1993;54:1–78. doi: 10.1016/s0065-2776(08)60532-5. [DOI] [PubMed] [Google Scholar]
- Bataille R., Jourdan M., Zhang X. G., Klein B. Serum levels of interleukin 6, a potent myeloma cell growth factor, as a reflect of disease severity in plasma cell dyscrasias. J Clin Invest. 1989 Dec;84(6):2008–2011. doi: 10.1172/JCI114392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bothwell W., Verburg M., Wynalda M., Daniels E. G., Fitzpatrick F. A. A radioimmunoassay for the unstable pulmonary metabolites of prostaglandin E1 and E2: an indirect index of their in vivo disposition and pharmacokinetics. J Pharmacol Exp Ther. 1982 Feb;220(2):229–235. [PubMed] [Google Scholar]
- Bunning R. A., Russell R. G., Van Damme J. Independent induction of interleukin 6 and prostaglandin E2 by interleukin 1 in human articular chondrocytes. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1163–1170. doi: 10.1016/0006-291x(90)90988-y. [DOI] [PubMed] [Google Scholar]
- Cancro M., Potter M. The requirement of an adherent cell substratum for the growth of developing plasmacytoma cells in vivo. J Exp Med. 1976 Dec 1;144(6):1554–1567. doi: 10.1084/jem.144.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofford L. J., Wilder R. L., Ristimäki A. P., Sano H., Remmers E. F., Epps H. R., Hla T. Cyclooxygenase-1 and -2 expression in rheumatoid synovial tissues. Effects of interleukin-1 beta, phorbol ester, and corticosteroids. J Clin Invest. 1994 Mar;93(3):1095–1101. doi: 10.1172/JCI117060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farber J. M. A collection of mRNA species that are inducible in the RAW 264.7 mouse macrophage cell line by gamma interferon and other agents. Mol Cell Biol. 1992 Apr;12(4):1535–1545. doi: 10.1128/mcb.12.4.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong Y., Moldawer L. L., Marano M., Wei H., Tatter S. B., Clarick R. H., Santhanam U., Sherris D., May L. T., Sehgal P. B. Endotoxemia elicits increased circulating beta 2-IFN/IL-6 in man. J Immunol. 1989 Apr 1;142(7):2321–2324. [PubMed] [Google Scholar]
- Fridman W. H., Michon J. Pathophysiology of cytokines. Leuk Res. 1990;14(8):675–677. doi: 10.1016/0145-2126(90)90092-n. [DOI] [PubMed] [Google Scholar]
- Fu J. Y., Masferrer J. L., Seibert K., Raz A., Needleman P. The induction and suppression of prostaglandin H2 synthase (cyclooxygenase) in human monocytes. J Biol Chem. 1990 Oct 5;265(28):16737–16740. [PubMed] [Google Scholar]
- Futaki N., Takahashi S., Yokoyama M., Arai I., Higuchi S., Otomo S. NS-398, a new anti-inflammatory agent, selectively inhibits prostaglandin G/H synthase/cyclooxygenase (COX-2) activity in vitro. Prostaglandins. 1994 Jan;47(1):55–59. doi: 10.1016/0090-6980(94)90074-4. [DOI] [PubMed] [Google Scholar]
- Goode H. F., Rathbone B. J., Kelleher J., Walker B. E. Monocyte zinc and in vitro prostaglandin E2 and interleukin-1 beta production by cultured peripheral blood monocytes in patients with Crohn's disease. Dig Dis Sci. 1991 May;36(5):627–633. doi: 10.1007/BF01297030. [DOI] [PubMed] [Google Scholar]
- Goss J. A., Mangino M. J., Callery M. P., Flye M. W. Prostaglandin E2 downregulates Kupffer cell production of IL-1 and IL-6 during hepatic regeneration. Am J Physiol. 1993 Apr;264(4 Pt 1):G601–G608. doi: 10.1152/ajpgi.1993.264.4.G601. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Hirano T. Interleukin-6 and its relation to inflammation and disease. Clin Immunol Immunopathol. 1992 Jan;62(1 Pt 2):S60–S65. doi: 10.1016/0090-1229(92)90042-m. [DOI] [PubMed] [Google Scholar]
- Hirano T., Matsuda T., Turner M., Miyasaka N., Buchan G., Tang B., Sato K., Shimizu M., Maini R., Feldmann M. Excessive production of interleukin 6/B cell stimulatory factor-2 in rheumatoid arthritis. Eur J Immunol. 1988 Nov;18(11):1797–1801. doi: 10.1002/eji.1830181122. [DOI] [PubMed] [Google Scholar]
- Hla T., Neilson K. Human cyclooxygenase-2 cDNA. Proc Natl Acad Sci U S A. 1992 Aug 15;89(16):7384–7388. doi: 10.1073/pnas.89.16.7384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houssiau F. A., Bukasa K., Sindic C. J., Van Damme J., Van Snick J. Elevated levels of the 26K human hybridoma growth factor (interleukin 6) in cerebrospinal fluid of patients with acute infection of the central nervous system. Clin Exp Immunol. 1988 Feb;71(2):320–323. [PMC free article] [PubMed] [Google Scholar]
- Houssiau F. A., Devogelaer J. P., Van Damme J., de Deuxchaisnes C. N., Van Snick J. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum. 1988 Jun;31(6):784–788. doi: 10.1002/art.1780310614. [DOI] [PubMed] [Google Scholar]
- Kantrowitz F., Robinson D. R., McGuire M. B., Levine L. Corticosteroids inhibit prostaglandin production by rheumatiod synovia. Nature. 1975 Dec 25;258(5537):737–739. doi: 10.1038/258737a0. [DOI] [PubMed] [Google Scholar]
- Kishimoto T., Hirano T. Molecular regulation of B lymphocyte response. Annu Rev Immunol. 1988;6:485–512. doi: 10.1146/annurev.iy.06.040188.002413. [DOI] [PubMed] [Google Scholar]
- Klein B., Zhang X. G., Jourdan M., Boiron J. M., Portier M., Lu Z. Y., Wijdenes J., Brochier J., Bataille R. Interleukin-6 is the central tumor growth factor in vitro and in vivo in multiple myeloma. Eur Cytokine Netw. 1990 Oct-Nov;1(4):193–201. [PubMed] [Google Scholar]
- Komatsu H., Yaju H., Chiba K., Okumoto T. Inhibition by cyclo-oxygenase inhibitors of interleukin-6 production by human peripheral blood mononuclear cells. Int J Immunopharmacol. 1991;13(8):1137–1146. doi: 10.1016/0192-0561(91)90165-4. [DOI] [PubMed] [Google Scholar]
- Leal-Berumen I., O'Byrne P., Gupta A., Richards C. D., Marshall J. S. Prostanoid enhancement of interleukin-6 production by rat peritoneal mast cells. J Immunol. 1995 May 1;154(9):4759–4767. [PubMed] [Google Scholar]
- Lee S. H., Soyoola E., Chanmugam P., Hart S., Sun W., Zhong H., Liou S., Simmons D., Hwang D. Selective expression of mitogen-inducible cyclooxygenase in macrophages stimulated with lipopolysaccharide. J Biol Chem. 1992 Dec 25;267(36):25934–25938. [PubMed] [Google Scholar]
- Mahida Y. R., Kurlac L., Gallagher A., Hawkey C. J. High circulating concentrations of interleukin-6 in active Crohn's disease but not ulcerative colitis. Gut. 1991 Dec;32(12):1531–1534. doi: 10.1136/gut.32.12.1531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masferrer J. L., Zweifel B. S., Manning P. T., Hauser S. D., Leahy K. M., Smith W. G., Isakson P. C., Seibert K. Selective inhibition of inducible cyclooxygenase 2 in vivo is antiinflammatory and nonulcerogenic. Proc Natl Acad Sci U S A. 1994 Apr 12;91(8):3228–3232. doi: 10.1073/pnas.91.8.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meade E. A., Smith W. L., DeWitt D. L. Differential inhibition of prostaglandin endoperoxide synthase (cyclooxygenase) isozymes by aspirin and other non-steroidal anti-inflammatory drugs. J Biol Chem. 1993 Mar 25;268(9):6610–6614. [PubMed] [Google Scholar]
- Modat G., Dornand J., Bernad N., Junquero D., Mary A., Muller A., Bonne C. LPS-stimulated bovine aortic endothelial cells produce IL-1 and IL-6 like activities. Agents Actions. 1990 Jun;30(3-4):403–411. doi: 10.1007/BF01966305. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Nordan R. P., Potter M. A macrophage-derived factor required by plasmacytomas for survival and proliferation in vitro. Science. 1986 Aug 1;233(4763):566–569. doi: 10.1126/science.3726549. [DOI] [PubMed] [Google Scholar]
- Ogle C. K., Guo X., Szczur K., Hartmann S., Ogle J. D. Production of tumor necrosis factor, interleukin-6 and prostaglandin E2 by LPS-stimulated rat bone marrow macrophages after thermal injury: effect of indomethacin. Inflammation. 1994 Apr;18(2):175–185. doi: 10.1007/BF01534558. [DOI] [PubMed] [Google Scholar]
- Potter M. Perspectives on the origins of multiple myeloma and plasmacytomas in mice. Hematol Oncol Clin North Am. 1992 Apr;6(2):211–223. [PubMed] [Google Scholar]
- Potter M., Wax J. S., Anderson A. O., Nordan R. P. Inhibition of plasmacytoma development in BALB/c mice by indomethacin. J Exp Med. 1985 May 1;161(5):996–1012. doi: 10.1084/jem.161.5.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter M., Wax J. S. Genetics of susceptibility to pristane-induced plasmacytomas in BALB/cAn: reduced susceptibility in BALB/cJ with a brief description of pristane-induced arthritis. J Immunol. 1981 Oct;127(4):1591–1595. [PubMed] [Google Scholar]
- Powell W. S. Rapid extraction of arachidonic acid metabolites from biological samples using octadecylsilyl silica. Methods Enzymol. 1982;86:467–477. doi: 10.1016/0076-6879(82)86218-6. [DOI] [PubMed] [Google Scholar]
- Pruimboom W. M., van Dijk J. A., Tak C. J., Garrelds I., Bonta I. L., Wilson P. J., Zijlstra F. J. Interactions between cytokines and eicosanoids: a study using human peritoneal macrophages. Immunol Lett. 1994 Jul;41(2-3):255–260. doi: 10.1016/0165-2478(94)90142-2. [DOI] [PubMed] [Google Scholar]
- Rosen G. D., Birkenmeier T. M., Raz A., Holtzman M. J. Identification of a cyclooxygenase-related gene and its potential role in prostaglandin formation. Biochem Biophys Res Commun. 1989 Nov 15;164(3):1358–1365. doi: 10.1016/0006-291x(89)91819-6. [DOI] [PubMed] [Google Scholar]
- Sano H., Hla T., Maier J. A., Crofford L. J., Case J. P., Maciag T., Wilder R. L. In vivo cyclooxygenase expression in synovial tissues of patients with rheumatoid arthritis and osteoarthritis and rats with adjuvant and streptococcal cell wall arthritis. J Clin Invest. 1992 Jan;89(1):97–108. doi: 10.1172/JCI115591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sehgal P. B. Interleukin 6 in infection and cancer. Proc Soc Exp Biol Med. 1990 Nov;195(2):183–191. doi: 10.3181/00379727-195-43129d. [DOI] [PubMed] [Google Scholar]
- Seibert K., Masferrer J., Zhang Y., Leahy K., Hauser S., Gierse J., Koboldt C., Anderson G., Bremer M., Gregory S. Expression and selective inhibition of constitutive and inducible forms of cyclooxygenase. Adv Prostaglandin Thromboxane Leukot Res. 1995;23:125–127. [PubMed] [Google Scholar]
- Seibert K., Zhang Y., Leahy K., Hauser S., Masferrer J., Perkins W., Lee L., Isakson P. Pharmacological and biochemical demonstration of the role of cyclooxygenase 2 in inflammation and pain. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):12013–12017. doi: 10.1073/pnas.91.25.12013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shacter E., Arzadon G. K., Williams J. A. Stimulation of interleukin-6 and prostaglandin E2 secretion from peritoneal macrophages by polymers of albumin. Blood. 1993 Nov 1;82(9):2853–2864. [PubMed] [Google Scholar]
- Shacter E., Arzadon G. K., Williams J. Elevation of interleukin-6 in response to a chronic inflammatory stimulus in mice: inhibition by indomethacin. Blood. 1992 Jul 1;80(1):194–202. [PubMed] [Google Scholar]
- Shacter E., Beecham E. J., Covey J. M., Kohn K. W., Potter M. Activated neutrophils induce prolonged DNA damage in neighboring cells. Carcinogenesis. 1988 Dec;9(12):2297–2304. doi: 10.1093/carcin/9.12.2297. [DOI] [PubMed] [Google Scholar]
- Shacter E., Lopez R. L., Pati S. Inhibition of the myeloperoxidase-H2O2-Cl- system of neutrophils by indomethacin and other non-steroidal anti-inflammatory drugs. 1991 Mar 15-Apr 1Biochem Pharmacol. 41(6-7):975–984. doi: 10.1016/0006-2952(91)90204-i. [DOI] [PubMed] [Google Scholar]
- Shacter E. Serum-free medium for growth factor-dependent and -independent plasmacytomas and hybridomas. J Immunol Methods. 1987 May 20;99(2):259–270. doi: 10.1016/0022-1759(87)90136-0. [DOI] [PubMed] [Google Scholar]
- Strassmann G., Patil-Koota V., Finkelman F., Fong M., Kambayashi T. Evidence for the involvement of interleukin 10 in the differential deactivation of murine peritoneal macrophages by prostaglandin E2. J Exp Med. 1994 Dec 1;180(6):2365–2370. doi: 10.1084/jem.180.6.2365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takakura K., Mason W. B., Hollander V. P. Studies on the pathogenesis of plasma cell tumors. I. Effect of cortisol on development of plasma cell tumors. Cancer Res. 1966 Apr;26(4):596–599. [PubMed] [Google Scholar]
- Taupin V., Gogusev J., Descamps-Latscha B., Zavala F. Modulation of tumor necrosis factor-alpha, interleukin-1 beta, interleukin-6, interleukin-8, and granulocyte/macrophage colony-stimulating factor expression in human monocytes by an endogenous anxiogenic benzodiazepine ligand, triakontatetraneuropeptide: evidence for a role of prostaglandins. Mol Pharmacol. 1993 Jan;43(1):64–69. [PubMed] [Google Scholar]
- Van Snick J. Interleukin-6: an overview. Annu Rev Immunol. 1990;8:253–278. doi: 10.1146/annurev.iy.08.040190.001345. [DOI] [PubMed] [Google Scholar]
- Vink A., Coulie P., Warnier G., Renauld J. C., Stevens M., Donckers D., Van Snick J. Mouse plasmacytoma growth in vivo: enhancement by interleukin 6 (IL-6) and inhibition by antibodies directed against IL-6 or its receptor. J Exp Med. 1990 Sep 1;172(3):997–1000. doi: 10.1084/jem.172.3.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waage A., Brandtzaeg P., Halstensen A., Kierulf P., Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989 Jan 1;169(1):333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu C., Mathews K. P. Indomethacin inhibition of glutathione S-transferases. Biochem Biophys Res Commun. 1983 May 16;112(3):980–985. doi: 10.1016/0006-291x(83)91714-x. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Lin J. X., Vilcek J. Synthesis of interleukin 6 (interferon-beta 2/B cell stimulatory factor 2) in human fibroblasts is triggered by an increase in intracellular cyclic AMP. J Biol Chem. 1988 May 5;263(13):6177–6182. [PubMed] [Google Scholar]
- van Deventer S. J., Büller H. R., ten Cate J. W., Aarden L. A., Hack C. E., Sturk A. Experimental endotoxemia in humans: analysis of cytokine release and coagulation, fibrinolytic, and complement pathways. Blood. 1990 Dec 15;76(12):2520–2526. [PubMed] [Google Scholar]