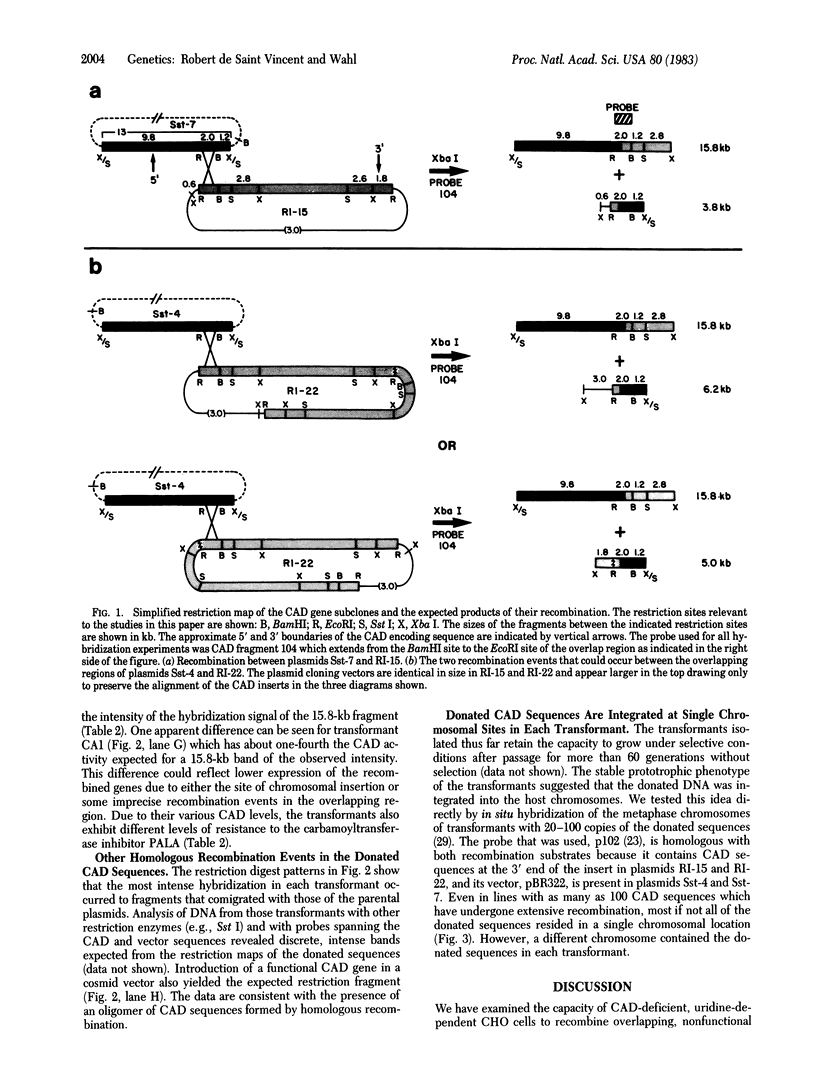
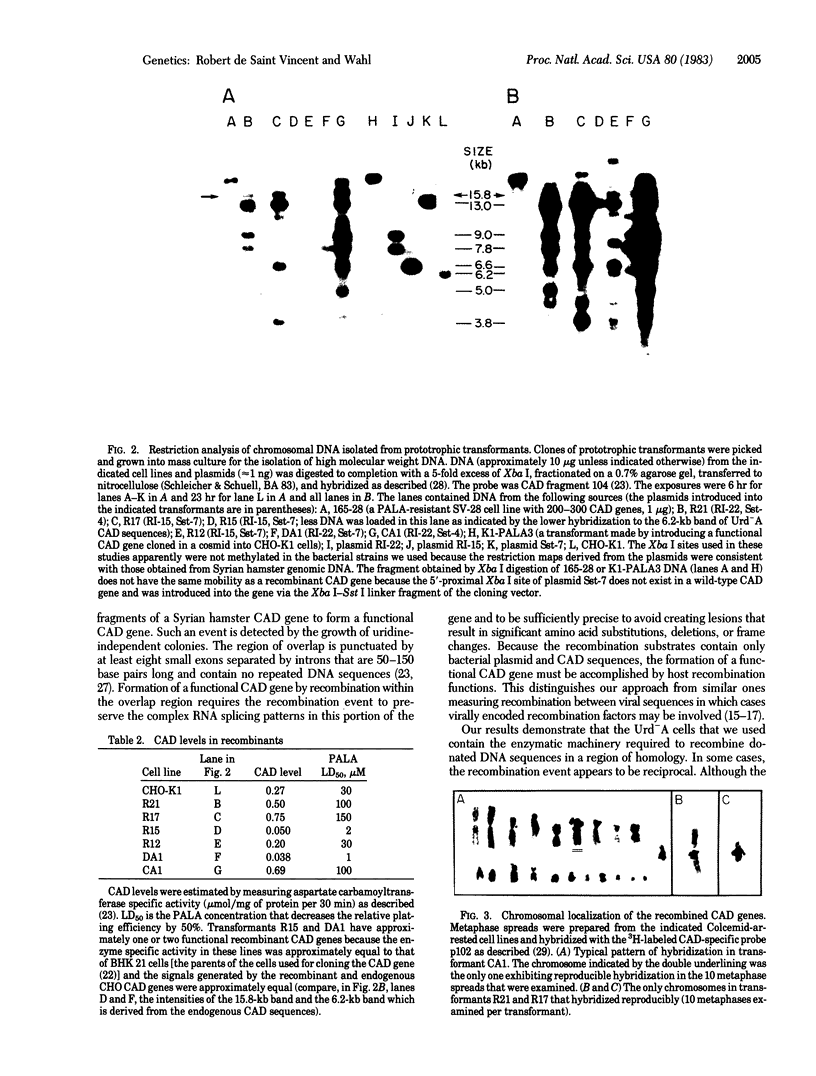
Abstract
Chinese hamster cells with a lesion in the CAD gene (cell line Urd-A) require exogenous uridine to survive. Uridine prototrophs could be isolated after introducing two recombinant plasmids containing overlapping fragments of a cloned Syrian hamster CAD gene. In contrast, no uridine prototrophs were obtained after introducing a plasmid containing only one of the two overlapping fragments. DNA restriction analysis showed that the prototrophic transformants contain a functional CAD gene which was formed by a recombination event in the overlapping region of the two clones. Most of the recombination events involved homologous exchanges, and some of them apparently were reciprocal. In situ hybridization analysis revealed that the donated sequences were integrated at a single chromosomal site which was different in each transformant. These results demonstrate the existence of a recombination system(s) in mammalian cells that can catalyze homologous exchanges. Recombination between donated sequences is a means by which this system can be characterized and also utilized for the production of novel gene fusions.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. A., Krakauer T., Camerini-Otero R. D. DNA-mediated gene transfer: recombination between cotransferred DNA sequences and recovery of recombinants in a plasmid. Proc Natl Acad Sci U S A. 1982 May;79(9):2748–2752. doi: 10.1073/pnas.79.9.2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell C. E., Worton R. G. Segregation of recessive phenotypes in somatic cell hybrids: role of mitotic recombination, gene inactivation, and chromosome nondisjunction. Mol Cell Biol. 1981 Apr;1(4):336–346. doi: 10.1128/mcb.1.4.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaganti R. S., Schonberg S., German J. A manyfold increase in sister chromatid exchanges in Bloom's syndrome lymphocytes. Proc Natl Acad Sci U S A. 1974 Nov;71(11):4508–4512. doi: 10.1073/pnas.71.11.4508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang A. C., Cohen S. N. Construction and characterization of amplifiable multicopy DNA cloning vehicles derived from the P15A cryptic miniplasmid. J Bacteriol. 1978 Jun;134(3):1141–1156. doi: 10.1128/jb.134.3.1141-1156.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. E., Esposito M. S. Genetic recombination and commitment to meiosis in Saccharomyces. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3172–3176. doi: 10.1073/pnas.71.8.3172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folger K. R., Wong E. A., Wahl G., Capecchi M. R. Patterns of integration of DNA microinjected into cultured mammalian cells: evidence for homologous recombination between injected plasmid DNA molecules. Mol Cell Biol. 1982 Nov;2(11):1372–1387. doi: 10.1128/mcb.2.11.1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frischauf A. M., Garoff H., Lehrach H. A subcloning strategy for DNA sequence analysis. Nucleic Acids Res. 1980 Dec 11;8(23):5541–5549. doi: 10.1093/nar/8.23.5541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GERMAN J. CYTOLOGICAL EVIDENCE FOR CROSSING-OVER IN VITRO IN HUMAN LYMPHOID CELLS. Science. 1964 Apr 17;144(3616):298–301. doi: 10.1126/science.144.3616.298. [DOI] [PubMed] [Google Scholar]
- Game J. C., Johnston L. H., von Borstel R. C. Enhanced mitotic recombination in a ligase-defective mutant of the yeast Saccharomyces cerevisiae. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4589–4592. doi: 10.1073/pnas.76.9.4589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- James A P, Lee-Whiting B. Radiation-Induced Genetic Segregations in Vegetative Cells of Diploid Yeast. Genetics. 1955 Nov;40(6):826–831. doi: 10.1093/genetics/40.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz E. R., Kao V. Evidence for mitotic recombination in the cellular slime mold Dictyostelium discoideum. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4025–4026. doi: 10.1073/pnas.71.10.4025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunz B. A., Haynes R. H. Phenomenology and genetic control of mitotic recombination in yeast. Annu Rev Genet. 1981;15:57–89. doi: 10.1146/annurev.ge.15.120181.000421. [DOI] [PubMed] [Google Scholar]
- Latt S. A. Sister chromatid exchanges, indices of human chromosome damage and repair: detection by fluorescence and induction by mitomycin C. Proc Natl Acad Sci U S A. 1974 Aug;71(8):3162–3166. doi: 10.1073/pnas.71.8.3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luria S. E., Delbrück M. Mutations of Bacteria from Virus Sensitivity to Virus Resistance. Genetics. 1943 Nov;28(6):491–511. doi: 10.1093/genetics/28.6.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell M. B. ABERRANT RECOMBINATION OF PYRIDOXINE MUTANTS OF Neurospora. Proc Natl Acad Sci U S A. 1955 Apr 15;41(4):215–220. doi: 10.1073/pnas.41.4.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore P. D., Holliday R. Evidence for the formation of hybrid DNA during mitotic recombination in Chinese hamster cells. Cell. 1976 Aug;8(4):573–579. doi: 10.1016/0092-8674(76)90225-7. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PONTECORVO G., KAFER E. Genetic analysis based on mitotic recombination. Adv Genet. 1958;9:71–104. [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Stark G. R. Properties of dispersed, highly repeated DNA within and near the hamster CAD gene. Mol Cell Biol. 1982 Mar;2(3):302–307. doi: 10.1128/mcb.2.3.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padgett R. A., Wahl G. M., Stark G. R. Structure of the gene for CAD, the multifunctional protein that initiates UMP synthesis in Syrian hamster cells. Mol Cell Biol. 1982 Mar;2(3):293–301. doi: 10.1128/mcb.2.3.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick M. A., Kasimos J. N., Game J. C., Braun R. J., Roth R. M. Changes in DNA during meiosis in a repair-deficient mutant (rad 52) of yeast. Science. 1981 May 1;212(4494):543–545. doi: 10.1126/science.7010606. [DOI] [PubMed] [Google Scholar]
- Rommelaere J., Miller-Faurès A. Detection by density equilibrium centrifugation of recombinant-like DNA molecules in somatic mammalian cells. J Mol Biol. 1975 Oct 15;98(1):195–218. doi: 10.1016/s0022-2836(75)80109-4. [DOI] [PubMed] [Google Scholar]
- Rosenstraus M. J., Chasin L. A. Separation of linked markers in Chinese hamster cell hybrids: mitotic recombination is not involved. Genetics. 1978 Dec;90(4):735–760. doi: 10.1093/genetics/90.4.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C. Somatic Crossing over and Segregation in Drosophila Melanogaster. Genetics. 1936 Nov;21(6):625–730. doi: 10.1093/genetics/21.6.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern H., Hotta Y. Biochemical controls of meiosis. Annu Rev Genet. 1973;7:37–66. doi: 10.1146/annurev.ge.07.120173.000345. [DOI] [PubMed] [Google Scholar]
- Stringer J. R. DNA sequence homology and chromosomal deletion at a site of SV40 DNA integration. Nature. 1982 Mar 25;296(5855):363–366. doi: 10.1038/296363a0. [DOI] [PubMed] [Google Scholar]
- Tarrant G. M., Holliday R. A search for allelic recombination in Chinese hamster cell hybrids. Mol Gen Genet. 1977 Nov 18;156(3):273–279. doi: 10.1007/BF00267182. [DOI] [PubMed] [Google Scholar]
- Taylor J H. Sister Chromatid Exchanges in Tritium-Labeled Chromosomes. Genetics. 1958 May;43(3):515–529. doi: 10.1093/genetics/43.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upcroft P., Carter B., Kidson C. Analysis of recombination in mammalian cells using SV40 genome segments having homologous overlapping termini. Nucleic Acids Res. 1980 Jun 25;8(12):2725–2736. doi: 10.1093/nar/8.12.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Stern M., Stark G. R. Efficient transfer of large DNA fragments from agarose gels to diazobenzyloxymethyl-paper and rapid hybridization by using dextran sulfate. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3683–3687. doi: 10.1073/pnas.76.8.3683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahl G. M., Vitto L., Padgett R. A., Stark G. R. Single-copy and amplified CAD genes in Syrian hamster chromosomes localized by a highly sensitive method for in situ hybridization. Mol Cell Biol. 1982 Mar;2(3):308–319. doi: 10.1128/mcb.2.3.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young C. S., Fisher P. B. Adenovirus recombination in normal and repair-deficient human fibroblasts. Virology. 1980 Jan 15;100(1):179–184. doi: 10.1016/0042-6822(80)90564-4. [DOI] [PubMed] [Google Scholar]
- de Saint Vincent B. R., Delbrück S., Eckhart W., Meinkoth J., Vitto L., Wahl G. The cloning and reintroduction into animal cells of a functional CAD gene, a dominant amplifiable genetic marker. Cell. 1981 Dec;27(2 Pt 1):267–277. doi: 10.1016/0092-8674(81)90410-4. [DOI] [PubMed] [Google Scholar]