Abstract
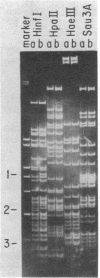
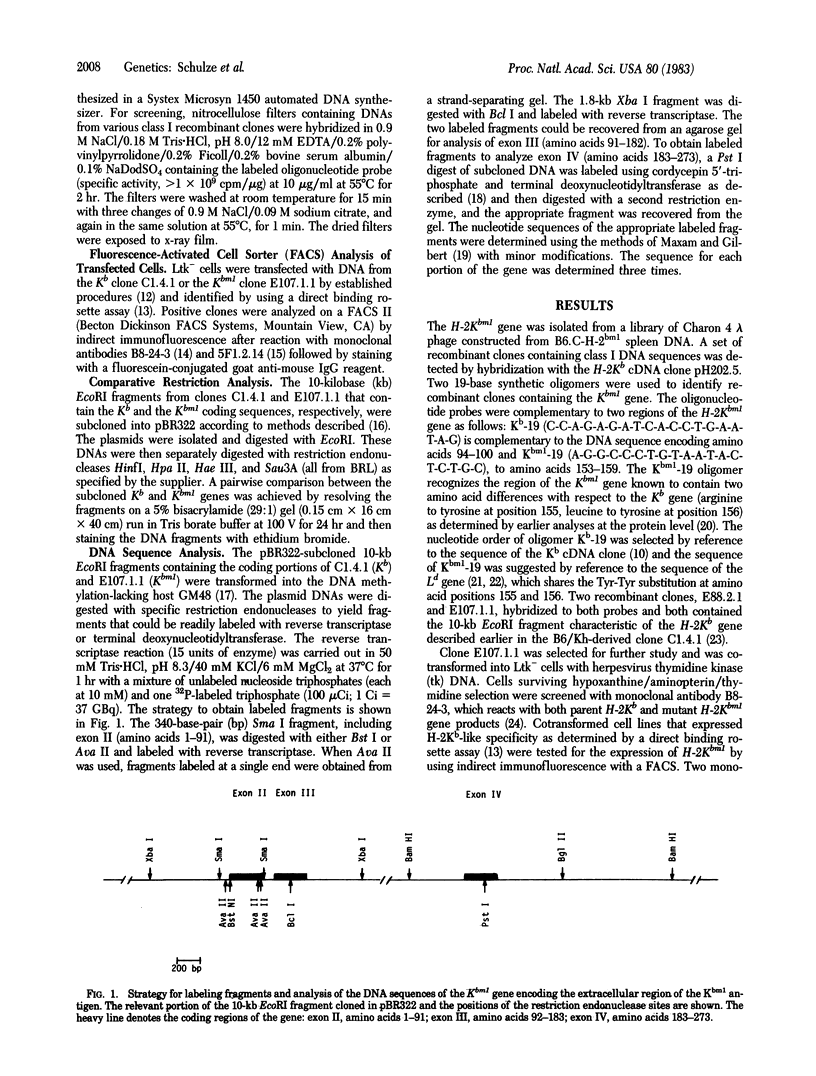
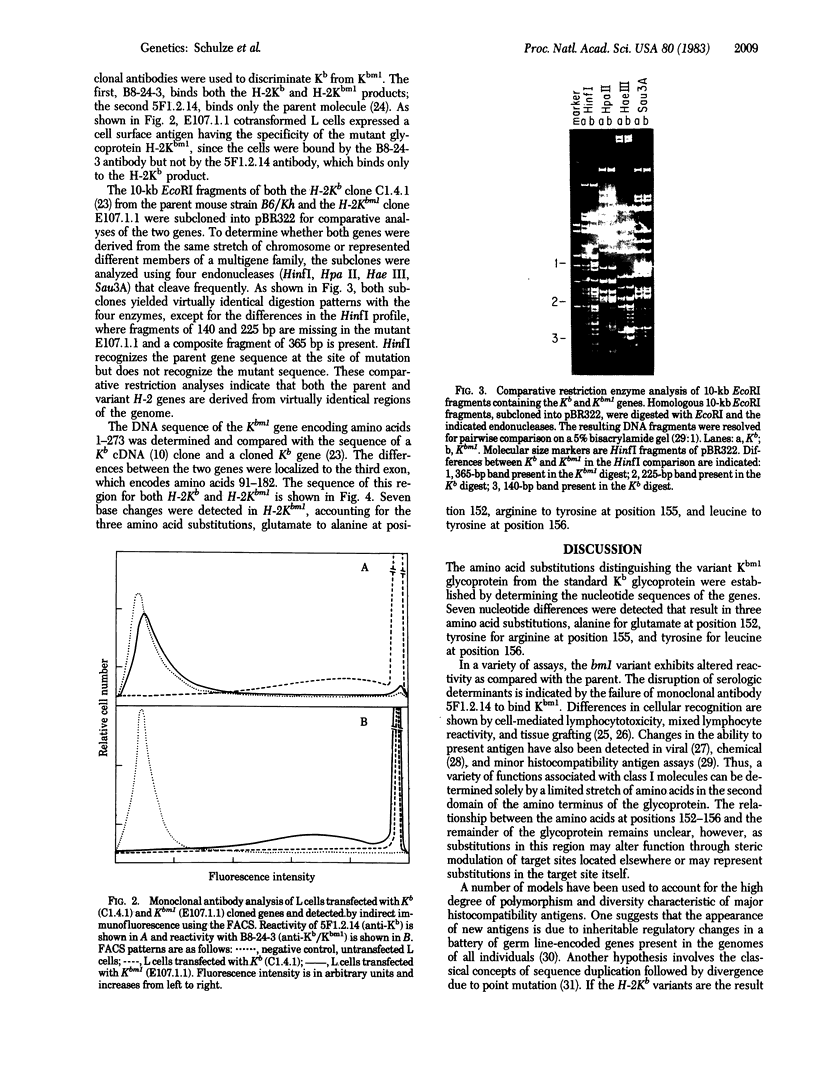
The gene for the H-2K class I antigen of the bm1 variant was cloned and analyzed at the DNA level and compared with the previously cloned parent B6/Kh gene. Sequence determination and comparative restriction endonuclease studies indicate that Kbm1 is derived from the Kb gene. Seven nucleotide changes within a 13-nucleotide stretch distinguish the mutant from the parent gene and result in amino acid differences at positions 152, 155, and 156 in the antigen. The data confirm previously reported changes at amino acid positions 155 and 156 (arginine to tyrosine and leucine to tyrosine, respectively) and extend the altered region to include two nucleotides encoding a glutamate to alanine substitution at amino acid 152, a change not detected by the protein studies because of limitations of the methods used. The DNA sequence encoding this region of the Kbm1 glycoprotein is identical to the DNA sequence of at least one other known class I gene in the mouse, a finding consistent with the hypothesis that the mutation was not a random event but may be the result of a block transfer of information by a copy mechanism analogous to gene conversion. As the sequence analysis of the coding region for the first 273 amino acid residues shows identity between parent and mutant except for the seven nucleotide changes, all variant-parent functional differences must depend only on the cluster of three amino acid differences in the second domain of the Kb glycoprotein.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albino A. P., Lloyd K. O., Houghton A. N., Oettgen H. F., Old L. J. Heterogeneity in surface antigen and glycoprotein expression of cell lines derived from different melanoma metastases of the same patient. Implications for the study of tumor antigens. J Exp Med. 1981 Dec 1;154(6):1764–1778. doi: 10.1084/jem.154.6.1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backman K. A cautionary note on the use of certain restriction endonucleases with methylated substrates. Gene. 1980 Oct;11(1-2):169–171. doi: 10.1016/0378-1119(80)90097-9. [DOI] [PubMed] [Google Scholar]
- Bodmer W. F. New genetic model for allelism at histocompatibility and other complex loci: polymorphism for control of gene expression. Transplant Proc. 1973 Dec;5(4):1471–1475. [PubMed] [Google Scholar]
- Evans G. A., Margulies D. H., Camerini-Otero R. D., Ozato K., Seidman J. G. Structure and expression of a mouse major histocompatibility antigen gene, H-2Ld. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1994–1998. doi: 10.1073/pnas.79.6.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hämmerling G. J., Rüsch E., Tada N., Kimura S., Hämmerling U. Localization of allodeterminants on H-2Kb antigens determined with monoclonal antibodies and H-2 mutant mice. Proc Natl Acad Sci U S A. 1982 Aug;79(15):4737–4741. doi: 10.1073/pnas.79.15.4737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein J., Figueroa F. Polymorphism of the mouse H-2 loci. Immunol Rev. 1981;60:23–57. doi: 10.1111/j.1600-065x.1981.tb00361.x. [DOI] [PubMed] [Google Scholar]
- Klein J. H-2 mutations: their genetics and effect on immune functions. Adv Immunol. 1978;26:55–146. doi: 10.1016/s0065-2776(08)60229-1. [DOI] [PubMed] [Google Scholar]
- Kössel H., Roychoudhury R., Fischer D., Otto A. 3' End-group labeling and partial sequence determination of oligodeoxynucleotides. Methods Enzymol. 1974;29:322–341. doi: 10.1016/0076-6879(74)29030-x. [DOI] [PubMed] [Google Scholar]
- Lalanne J. L., Bregegere F., Delarbre C., Abastado J. P., Gachelin G., Kourilsky P. Comparison of nucleotide sequences of mRNAs belonging to the mouse H-2 multigene family. Nucleic Acids Res. 1982 Feb 11;10(3):1039–1049. doi: 10.1093/nar/10.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy R. B., Shearer G. M. Cell-mediated lympholysis responses against autologous cells modified with haptenic sulfhydryl reagents. IV. Self-determinants recognized by wild-type anti-H-2Kb and H-2Db-restricted cytotoxic T cells specific for sulfhydryl and amino-reactive haptens are absent in certain H-2 mutant strains. J Immunol. 1982 Oct;129(4):1525–1529. [PubMed] [Google Scholar]
- López de Castro J. A., Strominger J. L., Strong D. M., Orr H. T. Structure of crossreactive human histocompatibility antigens HLA-A28 and HLA-A2: possible implications for the generation of HLA polymorphism. Proc Natl Acad Sci U S A. 1982 Jun;79(12):3813–3817. doi: 10.1073/pnas.79.12.3813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Melief C. J., de Waal L. P., van der Meulen M. Y., Melvold R. W., Kohn H. I. Fine specificity of alloimmune cytotoxic T lymphocytes directed against H-2K. A study with Kb mutants. J Exp Med. 1980 May 1;151(5):993–1013. doi: 10.1084/jem.151.5.993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. W., Sher B. T., Sun Y. H., Eakle K. A., Hood L. DNA sequence of a gene encoding a BALB/c mouse Ld transplantation antigen. Science. 1982 Feb 5;215(4533):679–682. doi: 10.1126/science.7058332. [DOI] [PubMed] [Google Scholar]
- Nairn R., Yamaga K., Nathenson S. G. Biochemistry of the gene products from murine MHC mutants. Annu Rev Genet. 1980;14:241–277. doi: 10.1146/annurev.ge.14.120180.001325. [DOI] [PubMed] [Google Scholar]
- Nathenson S. G., Uehara H., Ewenstein B. M., Kindt T. J., Coligan J. E. Primary structural: analysis of the transplantation antigens of the murine H-2 major histocompatibility complex. Annu Rev Biochem. 1981;50:1025–1052. doi: 10.1146/annurev.bi.50.070181.005113. [DOI] [PubMed] [Google Scholar]
- Nisizawa T., Ewenstein B. M., Uehara H., McGovern D., Nathenson S. G. Biochemical studies on the H-2K antigens of the MHC mutant bml. Immunogenetics. 1981;12(1-2):33–44. doi: 10.1007/BF01561649. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Nathenson S. G., Leinwand L. A. Mapping class I gene sequences in the major histocompatibility complex. Nature. 1982 Jul 22;298(5872):382–385. doi: 10.1038/298382a0. [DOI] [PubMed] [Google Scholar]
- Pease L. R., Schulze D. H., Pfaffenbach G. M., Nathenson S. G. Spontaneous H-2 mutants provide evidence that a copy mechanism analogous to gene conversion generates polymorphism in the major histocompatibility complex. Proc Natl Acad Sci U S A. 1983 Jan;80(1):242–246. doi: 10.1073/pnas.80.1.242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes A. A., Schöld M., Itakura K., Wallace R. B. Isolation of a cDNA clone for the murine transplantation antigen H-2Kb. Proc Natl Acad Sci U S A. 1982 May;79(10):3270–3274. doi: 10.1073/pnas.79.10.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L. A., Randolph C. P. Monoclonal anti-H-2Kb antibodies detect serological differences between H-2Kb mutants. Immunogenetics. 1981;12(1-2):183–186. doi: 10.1007/BF01561661. [DOI] [PubMed] [Google Scholar]
- Shine J., Seeburg P. H., Martial J. A., Baxter J. D., Goodman H. M. Construction and analysis of recombinant DNA for human chorionic somatomammotropin. Nature. 1977 Dec 8;270(5637):494–499. doi: 10.1038/270494a0. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Moore K. W., Frelinger J. G., Sher B. T., Shen F. W., Boyse E. A., Hood L. A pseudogene homologous to mouse transplantation antigens: transplantation antigens are encoded by eight exons that correlate with protein domains. Cell. 1981 Sep;25(3):683–692. doi: 10.1016/0092-8674(81)90175-6. [DOI] [PubMed] [Google Scholar]
- Steinmetz M., Winoto A., Minard K., Hood L. Clusters of genes encoding mouse transplantation antigens. Cell. 1982 Mar;28(3):489–498. doi: 10.1016/0092-8674(82)90203-3. [DOI] [PubMed] [Google Scholar]
- Wettstein P. J. H-2 effects on cell-cell interactions in the response to single non-H-2 alloantigens. V. Effects of H-2Kb mutations on presentation of H-4 and H-3 alloantigens. J Immunol. 1982 Jun;128(6):2629–2633. [PubMed] [Google Scholar]
- Wigler M., Pellicer A., Silverstein S., Axel R., Urlaub G., Chasin L. DNA-mediated transfer of the adenine phosphoribosyltransferase locus into mammalian cells. Proc Natl Acad Sci U S A. 1979 Mar;76(3):1373–1376. doi: 10.1073/pnas.76.3.1373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinkernagel R. M. H-2 compatibility requirement for virus-specific T-cell-mediated cytolysis. The H-2K structure involved is coded by a single cistron defined by H-2Kb mutant mice. J Exp Med. 1976 Feb 1;143(2):437–443. doi: 10.1084/jem.143.2.437. [DOI] [PMC free article] [PubMed] [Google Scholar]