Abstract
Every year, a huge quantity of fishery wastes and by-products are generated by fish processing industries. These wastes are either underutilized to produce low market value products or dumped leading to environmental issues. Complete utilization of fishery wastes for recovering value added products would be beneficial to the society and individual. The fish protein hydrolysates and derived peptides of fishery resources are widely used as nutritional supplements, functional ingredients, and flavor enhancers in food, beverage and pharmaceutical industries. Antioxidants from fishery resources have attracted the attention of researchers as they are cheaper in cost, easy to derive, and do not have side effects. Thus the present investigation was designed to produce protein hydrolysate by pepsin and papain digestion from the backbones of Rastrelliger kanagurta (Indian mackerel) and evaluate its antioxidant properties through various in vitro assays. The results reveal that both hydrolysates are potent antioxidants, capable of scavenging 46% and 36% of DPPH (1,1-diphenyl-2 picrylhydrazyl) and 58.5% and 37.54% of superoxide radicals respectively. The hydrolysates exhibit significant (p < 0.05) reducing power and lipid peroxidation inhibition. Among the two hydrolysates produced, pepsin derived fraction is superior than papain derived fraction in terms of yield, DH (Degree of hydrolysis), and antioxidant activity.
Keywords: Peptides, Antioxidants, Protein recovery, Fishery waste, Free radicals
1. Introduction
According to the report of food and agricultural organization of United Nations, India is third among top fishing countries behind China and Indonesia in terms of quantity produced. Despite major proportion is being used for human consumption, a considerable quantity is wasted or directed toward non-food products from the global fish production (FAO yearbook, 2010). Every year, a huge quantity of fishery wastes and by-products are generated by fish processing industries. Either these marine wastes are underutilized to produce low market value products such as fish meal, fish oil, fertilizers or simply dumped leading to environmental issues (Quaglia and Orban, 1987; Gildberg, 1993). The major quantity of solid wastes discarded from seafood processing plants are in the form of fish head, viscera, skin, bones, frames, and some muscle tissue (Awarenet, 2004). Complete utilization of fishery wastes for recovering high-end products would be the fruitful strategy to overcome the issue and increase the economic gain. Fishery wastes and by-products are valuable sources of raw material for recovery of bioactive compounds. The discarded wastes are rich sources of protein that can be made use in various commercial and industrial applications. The fishery wastes converted by proteolytic hydrolysis into a more marketable and functional form are called as fish protein hydrolysates. Production of fish protein hydrolysates by enzymatic degradation is a significant research arena of recent past. The fish protein hydrolysates thus produced are widely used as nutritional supplements, functional ingredients, and flavor enhancers in food, beverage and pharmaceutical industries (Je et al., 2008).
Formation of free radicals is an unavoidable consequence in aerobic organisms. Free radicals are highly reactive oxygen species (ROS) and among them, superoxide (), hydroxyl (OH•), peroxyl (ROO•), peroxynitrite (•ONOO−), and nitric oxide (NO•) radicals are the important ones (Atta-ur-Rahman and Choudhary, 2001). These radicals are very unstable and react rapidly with other substances in the body, leading to cell or tissue injury (Wang et al., 2008). The human body possesses many defense mechanisms against oxidative stress, including antioxidant enzymes and non-enzymatic compounds. However, various pathological conditions arise due to the inappropriate balance between generation and elimination of ROS and result in the oxidative modification of cellular macromolecules like DNA, membrane lipids, and proteins in various health disorders such as diabetes mellitus, cancer, neurodegenerative and inflammatory diseases (Pryor and Ann, 1982; Butterfield et al., 2002). Antioxidants could be used to overcome the deleterious effect of these free radical induced damages. Antioxidants could be any substance that, when present at low concentrations compared to those of an oxidizable substrate (Halliwell and Gutteridge, 1989). However, the safety and negative consumer perception of synthetic antioxidants such as butylated hydroxyl anisole (BHA) and butylated hydroxyl toluene (BHT) restrict their applications in food products (Park et al., 2001). Hence, use of dietary antioxidants to promote human health by increasing the body’s antioxidant load has been recognized as a feasible and potentially effective measure. Therefore, it is important to find potential antioxidants, especially food-derived natural antioxidants to encounter the radical mediated damage. Despite the availability of numerous dietary antioxidants, the antioxidants derived from marine resources are easy to obtain, cheap and safe without any adverse effects in comparison to their synthetic counterparts. These marine derived antioxidants are capable of sequestering oxygen radicals, chelating prooxidant metal ions and inhibiting lipid peroxidation in food systems (You et al., 2010). The antioxidant protein hydrolysates derived from various fish proteins have potential for nutritional, pharmaceutical, cosmetic and nutraceutical applications as a functional ingredient due to their health promoting effects (Chalamaiah et al., 2012).
Antioxidants from marine resources have attracted the attention of researchers as they are extracted from by-products of marine processing and do not have side effects. Fish protein hydrolysates from different species such as, yellow stripe trevally (Klompong et al., 2007), yellowfin sole frame (Jun et al., 2004), herring (Sathivel et al., 2003), mackerel (Wu et al., 2003), muscles of Nemipterus japonicus and Exocoetus volitans (Shabeena and Nazeer, 2010), backbones of Exocoetus volitans (Shabeena and Nazeer, 2011), backbones of Sphyraena barracuda and Lepturacanthus savala (Nazeer et al., 2011) have been found to possess antioxidant activity. Rastrelliger kanagurta (Indian mackerel) is a pelagic shoaling fish, widely distributed in the tropical Indo-Pacific region and is an important revenue yielding variety in India. Despite the huge catch a larger amount is wasted and underutilized (Doiphode, 1974; Choudhury et al., 2008). Reports have indicated that fish backbone is one of the major fractions of fish wastes, and it contains around 30% protein (Je et al., 2007). This protein could be good candidate as nutraceuticals. Therefore, the objective of this study was to synthesize fish protein hydrolysate from Rastrelliger kanagurta (Indian mackerel) backbones by enzymatic hydrolysis and to evaluate its antioxidant properties using different in vitro systems.
2. Materials and methods
2.1. Fish sample
Enough quantity of the Marine fish, Rastrelliger kanagurta (Indian mackerel) was collected from the coast of Chennai, Tamilnadu, India, and backbones were separated from the fish, minced for uniformity and stored in plastic bags at −20 °C until used.
2.2. Chemicals and reagents
The enzymes pepsin and papain for proteolytic digestion were purchased from Sigma Chemical Co. (St. Louis, MO, USA). The chemicals such as linoleic acid, DPPH (1,1-diphenyl-2 picrylhydrazyl), Ferrous Chloride, hydrogen peroxide, Ammonium thiocyanate, Ferric Chloride, Butylated hydroxytoluene (BHT), α-tocopherol, thiobarbituric acid (TBA), potassium ferricyanide, trichloroacetic acid (TCA), phenazine methosulfate (PMS), Nicotinamide adenine dinucleotide (NADH), Nitroblue tetrazolium (NBT), methanol, phosphate buffer, and all other chemicals and reagents (acids, bases, solvents and salts) used were of analytical grade obtained from Sisco Research Laboratories Pvt. Ltd., Mumbai, India and Glaxo Laboratories, CDH division, Mumbai, India.
2.3. Preparation of fish protein hydrolysate
The proteolytic digestion of Rastrelliger kanagurta was performed according to the method described by Je et al. (2007). To produce antioxidant peptides from fish backbone, enzymatic hydrolysis was carried out separately with the enzymes pepsin (0.1 M Glycine–HCl buffer, pH 2.0 temperature 37 °C), and Papain (0.1 M Na2HPO4–NaH2PO4 buffer, pH 6.0, temperature 37 °C) at enzyme/substrate ratio (1/100 w/w). The minced backbone fraction of Rastrelliger kanagurta was homogenized with blender and then thoroughly mixed with enzymes. The mixture was incubated for 6 h with continuous stirring and then heated in a boiling water bath at 100 °C for 10 min to inactivate enzyme activity. The contents were then centrifuged at 10,000 rpm for 15 min and supernatant obtained was the fish protein hydrolysate. The hydrolysates were lyophilized to get a powdered sample and were stored at −20 °C.
2.4. Degree of hydrolysis
The extent of hydrolysis was determined by adapting the procedure described by Tang et al. (2009). Briefly, the sample was mixed with pepsin and papain enzymes with different enzyme/substrate ratios (1/100, 2/100, 4/200 w/w) and the reaction was conducted at optimal conditions of the respective enzymes, for 0.5, 1, 2, 3, 4, 5 and 6 h. The pH of the mixture was maintained constant during hydrolysis using 2 M NaOH. After hydrolysis, the pH of the broths was brought to 7.0, and the solutions were then heated at 100 °C for 10 min to inactivate the enzymes. The hydrolysates were centrifuged at 10,000g for 15 min, and the supernatants were lyophilized to get a powdered sample and were stored at −20 °C. The degree of hydrolysis (DH) is defined as the ratio between the number of broken peptide bonds (h) and the total number of peptide bonds per mass unit (htot).
The degree of hydrolysis (DH) of hydrolyzed protein was determined by measuring the amount of free α-amino groups based on the reaction between Sanger’s reagent of fluorodinitrobenzene (FDNB) and the amino groups in the amino acids which resulted a yellow complex of amino acids (Goodwin, 1968). The absorbance was measured spectrophotometrically at 410 nm.
2.5. Nitrogen recovery
The nitrogen recovery (NR) in the soluble fraction was calculated using the method of Benjakul and Morrissey (1997) by the following formula.
2.5.1. Determination of DPPH radical scavenging activity
DPPH radical scavenging activity of the hydrolysate was determined according to the method of Burits and Bucar (2000). Aliquots of sample at concentrations 0.5–3.0 mg were taken in different test tubes and were dissolved in 1 ml of ethanol followed by 4 ml of 0.004% of DPPH solution in methanol solution in each test tube. The test tubes were incubated at room temperature for 30 min and absorbance was read at 517 nm.
where A is Absorbance at 517 nm of sample and B is Absorbance at 517 nm of the blank.
2.6. Superoxide radical scavenging activity
Superoxide anion radical scavenging activity of hydrolysate was determined using the method described by Hseu et al. (2008). Superoxide anion radicals are generated in PMS-NADH systems by oxidation of NADH and assayed by the reduction of NBT. In this experiment, superoxide radicals were generated in 3 mL of Tris–HCl buffer (16 mM, pH 8.0) containing 0.5 mL of 300 μM nitroblue tetrazolium solution, 0.5 mL of 936 μM NADH solution, after which different concentrations of the hydrolysate (0.5–3 mg) were added in different test tubes. The reaction was started by the addition of 0.5 mL of 120 μM phenazine methosulfate solution. The reaction mixture was later incubated at 25 °C for 5 min, and the absorbance was read at 560 nm.
where A is Absorbance at 560 nm of sample and B is Absorbance at 560 nm of the blank.
2.7. Reducing power assay
The reducing power ability of protein hydrolysates produced from the backbones of Indian mackerel was determined by using the method of Yildirim et al. (2001). Different concentrations of the hydrolysates ranging 0.5–3.0 mg were taken in different test tubes and were dissolved in 1 ml of methanol. 2.5 ml of phosphate buffer and 2.5 ml of 1% potassium ferricyanide were added to each test tube and incubated for 20 min at 50 °C. After incubation 2.5 ml of 10% trichloroacetic acid was added to each test tube and centrifuged at 3,000 rpm for 10 min. 2.5 ml of the upper layer was mixed with 2.5 ml of distilled water and 0.5 ml of 0.1% ferric chloride and the absorbance was read at 700 nm.
2.8. Lipid peroxidation inhibition assay
Lipid peroxidation inhibition (LPO) assay of the protein hydrolysates was determined by the methods of Osawa and Namiki (1985). Briefly, 1 mg of the fish protein hydrolysate was dissolved in 5 ml of 50 mM phosphate buffer (pH 7.0). 65 μl of linoleic acid was added to 5 ml of 99.5% ethanol and this linoleic acid solution was mixed with the above solution and up to 12.5 ml using distilled water. This solution was incubated in the dark at 45 °C for 7 days. The degree of oxidation of linoleic acid was measured using the ferric thiocyanate method of Mitsuda et al. (1996). To 100 μl of the incubated sample 4.7 ml of 75% ethanol is added followed by 100 μl of 30% ammonium thiocyanate and mixed well. 100 μl of 20 mM ferrous chloride was added. As soon as ferrous chloride is added, color formation occurs and the intensity of the color is measured at 500 nm.
2.9. Statistical analysis
The statistical analysis of data was performed using SPSS 16 for windows. The results were expressed as mean of triplicates ± SD (Table 1). For degree of hydrolysis, yield, and LPO inhibition repeated measures ANOVA and post hoc analysis were done by Least Significant Difference (LSD), with statistical significance at p < 0.05. The remaining experiments were analyzed through factorial ANOVA and post hoc analysis by Dunnet test with a statistical significance at p < 0.05.
Table 1.
Effect of different enzyme/substrate ratios in DH of protein hydrolysate derived by pepsin and papain from backbones of Indian mackerel.
| DH (%)a |
||||||
|---|---|---|---|---|---|---|
| 60 min | 120 min | 180 min | 240 min | 300 min | 360 min | |
| Pepsin | ||||||
| E/S = 1:100 w/w | 14.3 ± 0.41 | 18.4 ± 0.60 | 21.3 ± 0.80 | 22.1 ± 0.90 | 22.8 ± 1.20 | 24.7 ± 1.40 |
| E/S = 2:100 w/w | 15.8 ± 0.61 | 19.6 ± 0.65 | 21.7 ± 0.69 | 23.0 ± 0.81 | 23.8 ± 0.88 | 25.6 ± 0.91 |
| E/S = 4:100 w/w | 17.3 ± 0.14 | 20.9 ± 0.19 | 22.2 ± 0.21 | 23.9 ± 0.17 | 24.7 ± 0.14 | 25.9 ± 0.16 |
| Papain | ||||||
| E/S = 1:100 w/w | 11.8 ± 0.18 | 12.6 ± 0.14 | 14.8 ± 0.17 | 16.1 ± 1.20 | 17.7 ± 1.11 | 18.1 ± 0.78 |
| E/S = 2:100 w/w | 11.1 ± 0.12 | 13.7 ± 0.14 | 15.5 ± 0.16 | 17.0 ± 0.19 | 18.12 ± 1.0 | 19.6 ± 0.13 |
| E/S = 4:100 w/w | 12.4 ± 0.11 | 14.0 ± 0.16 | 15.9 ± 0.15 | 17.4 ± 1.10 | 18.90 ± 0.89 | 19.9 ± 0.67 |
The values of DH (%) are reported as mean of triplicates and ± SD.
3. Results and discussion
3.1. Degree of hydrolysis
DH estimates the change of peptide content in a hydrolytic reaction. It is generally used as a proteolysis monitoring parameter and an important factor highly related with the hydrolytic process yield (Adler-Nissen, 1986). DH actually measures the extent of protein degradation by the respective enzymes in different proportions with that of the recovered protein hydrolysate. Higher the DH, more number of peptides would be produced in the solution. The increased production of peptides during hydrolytic reaction will result in the increased solubility of the protein and will increase the possibility of recovered protein to be used as food grade protein additive or replacer in various food preparations. Moreover, the cleavage of recovered protein would yield peptides of different sizes that are proven to be bioactive and antioxidant in nature (Sampath Kumar et al., 2011). Many investigators have isolated peptides with antioxidant properties from the protein hydrolysates prepared from various parts of different fish species (Najafian and Babji, 2012). For example Nalinanon et al. (2011) have isolated an antioxidant peptide from the protein hydrolysate prepared with the muscle of ornate threadfin bream by skipjack tuna pepsin with 20% DH. The results of the DH of current investigation are presented in Fig. 1. The measured values for DH at the end of 6 h were, 24.7% and 18.1% for pepsin and papain, respectively. The higher level of breakdown 14.3% for pepsin and 11.8% for papain, was achieved during the first hour of digestion. After that, hydrolytic reaction proceeded gradually and steadied toward the end. This typical pattern of hydrolytic curve was obtained in many investigations by different investigators earlier (Guerard et al., 2002; Sathivel et al., 2003; Kong et al., 2007). Among the two enzymes used pepsin showed higher degree of hydrolysis than papain. This finding matches with earlier report (Je et al., 2007).The higher (p < 0.05) level of DH by pepsin treatment suggested that pepsin has higher affinity and, therefore, is a more efficient enzyme choice than papain for preparing Indian mackerel backbone protein hydrolysates.
Figure 1.
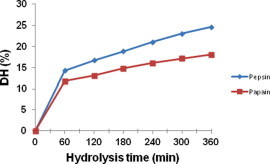
Percentage DH of backbones of Indian mackerel by pepsin and papain at various time intervals. The data were expressed as mean of triplicates ± SD measurements.
The DH changes during enzymatic hydrolysis of Indian mackerel backbones using pepsin and papain with E/S ratios of 1:100, 2:100 and 4:100 (w/w), respectively, were monitored up to 360 min, as shown in Table 1. As expected, the DH for both the enzymes increased with hydrolysis time, indicating a gradual release of peptide fragments during the hydrolysis. The rate of the hydrolysis or the release of peptide fragments was fast during initial hydrolysis (e.g., <60 min) and gradually decreased with the hydrolysis time increasing. The varied pattern of DH was closely reliant upon applied enzyme concentration, namely the E/S ratio. With E/S ratio increasing (from 1:100, 2:100 and 4:100 w/w), the rate of DH increases during initial hydrolysis process. However, during final process (e.g., at a hydrolysis time >360 min), the rate of DH increase at different E/S ratios became similar. Pepsin exhibited statistically significant (p < 0.05) higher degree of hydrolysis than papain in all tested E/S ratios and confirms our previous observation.
3.2. Protein recovery
The degree of hydrolysis has been used as an indicator of the cleavage of peptide bond, whereas nitrogen recovery reflects the yield of proteins that can be recovered from the hydrolysis process. Enzymatic digestion of protein results in the release of peptides during hydrolysis. Fig. 2 describes the recovery of protein in terms of yield % with respect to incubation time. It is common that yield of protein increased with an increase in the time of hydrolysis. At 6 h of incubation, pepsin yielded a maximum of 76.2% and papain produced 54.1% protein, respectively. Both enzymes recovered a good percent of protein from the backbones of Indian mackerel. The higher % protein recovery by pepsin in different fish species was earlier reported by Je et al. (2007, 2008). Our findings coincide with the above report and suggest that pepsin could be a better choice over papain for producing protein hydrolysate from the backbones of Indian mackerel.
Figure 2.
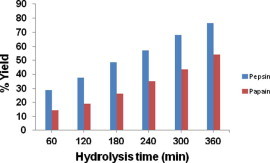
Percentage yield of protein from backbones of Indian mackerel by pepsin and papain at various time intervals. The data were expressed as mean of triplicates ± SD measurements.
3.3. Antioxidant analysis
The backbone of Rastrelliger kanagurta (Indian mackerel) was hydrolyzed with commercial enzymes pepsin and papain. The antioxidant potential of hydrolysates was evaluated through DPPH radical scavenging assay, superoxide scavenging activity, reducing power assay and lipid peroxidation inhibition ability. The results were compared with a natural antioxidant α-tocopherol and synthetic antioxidant BHT.
3.3.1. DPPH radical scavenging activity
Antioxidant feature of protein hydrolysate can be determined from its ability to scavenge DPPH radicals (Wang et al., 2007). At an appropriate environment DPPH is a stable free radical that shows a maximum absorbance at 517 nm and when encountering a proton donating substrate like fish protein hydrolysate, the radicals get scavenged and the absorbance is reduced (Mosquera et al., 2007). The reduction in absorbance at 517 nm is taken as a measure for radical-scavenging capacity of the fish protein hydrolysate derived from the back bone of Indian mackerel by pepsin and papain.
Fig. 3 depicts the radical scavenging ability of pepsin and papain derived protein hydrolysates of Indian mackerel and it was found to be a maximum of 46% and 36%, respectively. The radical scavenging ability increased with an increase in the concentration of hydrolysate. These values were well above in case of pepsin and equal for papain to that of synthetic antioxidant BHT in comparison. Similar findings were earlier reported in frog skin (Qian et al., 2008), Nemipterus japonicus and Exocoetus volitans muscle (Shabeena and Nazeer, 2010). The peptic fraction exhibited higher scavenging activity p < 0.05 over papain fraction, which was also observed in the backbones of tuna and bigeye (Thunnus obesus) tuna muscle by Je et al. (2007, 2008).
Figure 3.
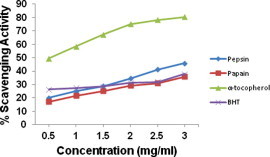
DPPH radical scavenging activity of protein hydrolysate derived by pepsin and papain from backbones of Indian mackerel. The data were expressed as mean of triplicates ± SD measurements.
3.3.2. Superoxide radical scavenging activity
Superoxide is generated in vivo by several oxidative enzymes, including xanthine oxidase. In the PMS-NADH-NBT system, superoxide anion derived from dissolved oxygen by PMS-NADH coupling reaction reduces NBT (Gulcin et al., 2005). The decrease of absorbance at 560 nm with antioxidants such as fish protein hydrolysate indicates the consumption of superoxide anion in the reaction mixture. Fig. 4 indicates superoxide radical scavenging ability of protein hydrolysate derived from Indian mackerel. Both hydrolysates scavenge superoxide anion efficiently in comparison with that of reference antioxidants. With the increase in sample concentration, scavenging activity increased and showed a maximum of 58.5% and 37.54% for 3 mg/ml of hydrolysate of pepsin and papain, respectively. Several reports have suggested that phenolic hydroxyl groups present in aromatic amino acids acting as electron donors are responsible for scavenging free radicals (Suetsuna et al., 2000). In addition, some other amino acids such as histidine, proline, alanine, and leucine have been reported to contribute to the scavenging of free radicals (Kim et al., 2001). Thus the free radical scavenging ability of the derived protein hydrolysates could be attributed to the peptides with higher contents of aromatic amino acids in the hydrolysate.
Figure 4.
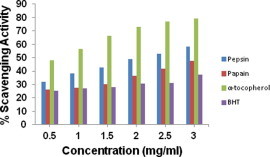
Superoxide radical scavenging activity of protein hydrolysate derived by pepsin and papain from backbones of Indian mackerel. The data were expressed as mean of triplicates ± SD measurements.
3.3.3. Reducing power
Measuring the reducing power ability has direct correlation (Duh et al., 1999) in testing the antioxidant activity of protein hydrolysate. Antioxidants with reducing power can reduce Fe3+/ferricyanide complex to the ferrous form (Bougatef et al., 2009). Fish protein hydrolysates produced from the backbones of Indian Mackerel by pepsin and papain digestion showed a marked reducing activity as shown in Fig. 5. The reducing power of pepsin fraction was similar to that of α-tocopherol and papain fraction exhibited similar reducing potential to that of BHT. Our results suggest that the peptide content of the hydrolysate could have functioned as an electron donor to react with free radicals to form more stable products. This indicates the ability of hydrolysate to bind with iron and chelate proxidative iron to result in decreased oxidation (Zhu et al., 2006). The significant reducing potential p > 0.05 observed for pepsin fraction over papain fraction in our study was in par with the earlier reports of Je et al. (2007, 2008) and confirms the antioxidant ability of the fish protein hydrolysate.
Figure 5.
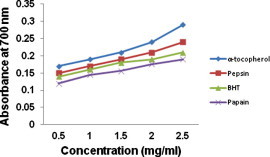
Reducing ability of protein hydrolysate derived by pepsin and papain from backbones of Indian mackerel. The data were expressed as mean of triplicates ± SD measurements.
3.3.4. Lipid peroxidation inhibition
Linoleic acid, an unsaturated fatty acid, served as an efficient model compound for lipid oxidation and antioxidant studies in the emulsion system associated with protein hydrolysates produced by different enzymatic sources (Zhu et al., 2006). Formation of peroxides in the linoleic acid model system results in increased absorption at 500 nm (Yen and Chen, 1995). These hydroperoxides are highly unstable and react readily with ferrous ions to produce ferric ions. The resulting ferric ions are detected using thiocyanate ion as chromogen, with an optimum wavelength at 500 nm (Mihaljevic et al., 1996). Lipid peroxidation is thought to proceed via radical-mediated abstraction of hydrogen from methylene carbons in polyunsaturated fatty acids (Rajapakse et al., 2005). Antioxidant peptides derived from different sources have exhibited varying potencies to scavenge the free radicals and inhibit lipid peroxidation (Jun et al., 2004; Kim et al., 2007; Bougatef et al., 2009; Shabeena and Nazeer, 2010, 2011).
Lipid peroxidation inhibition activity of fish protein hydrolysates prepared from the backbones of Indian mackerel was observed for 7 days at controlled temperature and light conditions. The pepsin hydrolysate possesses strong antioxidant activity when compared to that of papain fraction, BHT and α-tocopherol as represented in Fig. 6. Similar observations were made earlier from the hydrolysate of yellowfin sole frame (Jun et al., 2004) and Alaska pollack frame (Je et al., 2005) backbone of Seela and Ribbon Fish (Nazeer et al., 2011). The ability to inhibit lipid peroxidation by the hydrolysate depends on its hydrophobicity (Wu et al., 2003; Qian et al., 2008). Thus it can be suggestive that, antioxidant property of protein hydrolysate derived from the backbones of Indian mackerel might be due to its peptide content with higher hydrophobic amino acids that successfully defend lipid peroxidation.
Figure 6.
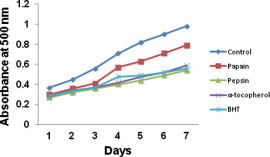
Lipid peroxidation inhibition of protein hydrolysate derived by pepsin and papain from backbones of Indian mackerel. The data were expressed as mean of triplicates ± SD measurements.
4. Conclusions
The present investigation was carried out to synthesize and evaluate antioxidant properties of protein hydrolysate recovered from the backbones of Rastrelliger kanagurta (Indian mackerel) by pepsin and papain digestion. The results of the study revealed that, the produced hydrolysates have yielded significant quantity of protein (76.2% and 54.1%) and they have undergone good extent of degradation (24.7% and 18.1%) by pepsin and papain mediated proteolysis, respectively. The peptides produced in solution exhibited potent antioxidant properties in all tested in vitro systems. The observations of the present study suggest that pepsin would be an ideal choice for protein recovery than papain. The antioxidant property may be due to size and aromatic amino acid content of the peptides produced during digestion. Further purification and characterization would be a future direction.
Footnotes
Peer review under responsibility of King Saud University.
References
- Adler-Nissen J. Elsevier Applied Science Publishers; New York: 1986. Enzymatic Hydrolysis of Food Proteins. [Google Scholar]
- Atta-ur-Rahman, Choudhary M.I. Bioactive natural products as potential source of new pharmacophores. A theory of memory. Pure Appl. Chem. 2001;73:555–560. [Google Scholar]
- Awarenet . Grow Programme; European Commission: 2004. Agro-food waste minimization and reduction network (AWARENET) pp. 1–7. [Google Scholar]
- Benjakul S., Morrissey M. Protein hydrolysates from Pacific whiting solid wastes. J. Agric. Food Chem. 1997;45:3423–3430. [Google Scholar]
- Bougatef A., Hajji M., Balti R., Lassoued I., Triki-Ellouz Y., Nasri M. Antioxidant and free radical-scavenging activities of smooth hound (Mustelus mustelus) muscle protein hydrolysates obtained by gastrointestinal proteases. Food Chem. 2009;114:1198–1205. [Google Scholar]
- Burits M., Bucar F. Antioxidant activity of Nigella sativa essential oil. Phytother. Res. 2000;14:323–328. doi: 10.1002/1099-1573(200008)14:5<323::aid-ptr621>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Butterfield D.A., Castenga A., Pocernich C.B., Drake J., Scapagnini G., Calabrese V. Nutritional approaches to combat oxidative stress in Alzheimer’s disease. J. Nutr. Biochem. 2002;13:444–461. doi: 10.1016/s0955-2863(02)00205-x. [DOI] [PubMed] [Google Scholar]
- Chalamaiah M., Dinesh Kumar B., Hemalatha R., Jyothirmayi T. Fish protein hydrolysates: proximate composition, amino acid composition, antioxidant activities and applications: a review. Food Chem. 2012;135:3020–3038. doi: 10.1016/j.foodchem.2012.06.100. [DOI] [PubMed] [Google Scholar]
- Choudhury M., Sahu M.K., Sivakumar K., Thangaradjou T., Kannan L. Inhibition of actinomycetes to Histamine producing bacteria associated with Indian Makerel fish (Rastrelliger kanagurta, cuvier, 1816) J. Fish Aquat. Sci. 2008;3:126–136. [Google Scholar]
- Doiphode P.V. Observations on the Indian mackerel. Rastrelliger kanagurta (Cuv) from purse seine catches along Goa coast. Indian J. Fish. 1974;21:85–88. [Google Scholar]
- Duh P.D., Du P.C., Yen G.C. Action of methanolic extract of mung bean hulls as inhibitors of lipid peroxidation and non-lipid oxidative damage. Food Chem. Toxicol. 1999;37:1055–1061. doi: 10.1016/s0278-6915(99)00096-4. [DOI] [PubMed] [Google Scholar]
- FAO Year book . FAO; Rome: 2010. Statistics and Information Service of the Fisheries and Aquaculture Department Fishery and Aquaculture Statistics. p. 78. [Google Scholar]
- Gildberg A. Enzymatic processing of marine raw materials. Process Biochem. 1993;28:1–15. [Google Scholar]
- Goodwin J.F. The colorimetric estimation of plasma amino nitrogen with DNFB. Clin. Chem. 1968;14:1080–1090. [PubMed] [Google Scholar]
- Guerard F., Guimas L., Binet A. Production of tuna waste hydrolysates by a commercial neutral protease preparation. J. Mol. Catal. B: Enzym. 2002;19–20:489–498. [Google Scholar]
- Gulcin I., Alici H.A., Cesur M. Determination of in vitro antioxidant and radical scavenging activities of propofol. Chem. Pharm. Bull. 2005;53:281–285. doi: 10.1248/cpb.53.281. [DOI] [PubMed] [Google Scholar]
- Halliwell B., Gutteridge J.M.C. second ed. Clarendon Press; Oxford: 1989. Free Radicals in Biology and Medicine. pp. 1–81. [Google Scholar]
- Hseu Y.C., Chang W.H., Chen C.S., Liao J.W., Huang C.J., Lu F.J., Chia Y.C., Hsu H.K., Wu J.J., Yang H.L. Antioxidant activities of Toona sinensis leaves extracts using different antioxidant models. Food Chem. Toxicol. 2008;46:105–114. doi: 10.1016/j.fct.2007.07.003. [DOI] [PubMed] [Google Scholar]
- Je J.Y., Park P.J., Kim S.K. Antioxidant activity of a peptide isolated from Alaska pollack (Theragra chalcogramma) frame protein hydrolysate. Food Res. Int. 2005;38:45–50. [Google Scholar]
- Je J.Y., Qian Z., Byun H.G., Kim S.K. Purification and characterization of an antioxidant peptide obtained from tuna backbone protein by enzymatic hydrolysis. Process Biochem. 2007;42:846–849. [Google Scholar]
- Je J.Y., Qian Z.I., Lee S.H., Byun H.G., Kim S.K. Purification and antioxidant properties of Bigeye Tuna (Thunnus obesus) dark muscle peptide on free radical-mediated oxidative systems. J. Med. Food. 2008;11:629–637. doi: 10.1089/jmf.2007.0114. [DOI] [PubMed] [Google Scholar]
- Jun S.Y., Park P.J., Jung W.K., Kim S.K. Purification and characterization of an antioxidative peptide from enzymatic hydrolysate of yellowfin sole (Limanda aspera) frame protein. Eur. Food Res. Technol. 2004;219:20–26. [Google Scholar]
- Kim S.K., Kim Y.T., Byun H.G., Nam K.S., Joo D.S., Shahidi F. Isolation and characterization of antioxidative peptides from gelatin hydrolysate of Alaska pollack skin. J. Agric. Food Chem. 2001;49:1984–1989. doi: 10.1021/jf000494j. [DOI] [PubMed] [Google Scholar]
- Kim S.Y., Je J.Y., Kim S.K. Purification and characterization of antioxidant peptide from hoki (Johnius belengerii) frame protein by gastrointestinal digestion. J. Nutr. Biochem. 2007;18:31–38. doi: 10.1016/j.jnutbio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- Klompong V., Benjakul S., Kantachote D., Shahidi F. Antioxidative activity and functional properties of protein hydrolysate of yellow stripe trevally (Selaroides leptolepis) as influenced by the degree of hydrolysis and enzyme type. Food Chem. 2007;102:1317–1327. [Google Scholar]
- Kong X., Zhou H., Qian H. Enzymatic hydrolysis of wheat gluten by proteases and properties of the resulting hydrolysates. Food Chem. 2007;102:759–763. [Google Scholar]
- Mihaljevic B., Katusin-Razem B., Razem D. The reevaluation of the ferric thiocyanate assay for lipid hydroperoxides with special considerations of the mechanistic aspects of the response. Free Radical Biol. Med. 1996;21:53–63. doi: 10.1016/0891-5849(95)02224-4. [DOI] [PubMed] [Google Scholar]
- Mitsuda H.K., Yasumoto K., Iwami E. Antioxidative action of indole compounds during the autoxidation of linoleic acid. Eiyo. Shokuryo. 1996;19:210–214. [Google Scholar]
- Mosquera O.M., Correa Y.M., Buitrago D.C., Nio J. Antioxidant activity of twenty five plants from Colombian biodiversity. Mem. Inst. Oswaldo Cruz. 2007;102:631–634. doi: 10.1590/s0074-02762007005000066. [DOI] [PubMed] [Google Scholar]
- Najafian L., Babji A.S. A review of fish derived antioxidant and antimicrobial peptides: their production, assessment, and applications. Peptides. 2012;33:178–185. doi: 10.1016/j.peptides.2011.11.013. [DOI] [PubMed] [Google Scholar]
- Nalinanon S., Benjakul S., Kishimura H., Shahidi F. Functionalities and antioxidant properties of protein hydrolysates from the muscle of ornate threadfin bream treated with pepsin from skipjack tuna. Food Chem. 2011;124:1354–1362. [Google Scholar]
- Nazeer R.A., Deeptha R., Jaiganesh R., Sampathkumar N.S., Shabeena Y.N. Radical scavenging activity of seela (Sphyraena barracuda) and ribbon fish (Lepturacanthus savala) backbone protein hydrolysates. Int. J. Pept. Res. Ther. 2011;17:209–216. [Google Scholar]
- Osawa T., Namiki M. Natural antioxidant isolated from eucalyptus leaf waxes. J. Agric. Food Chem. 1985;33:777–780. [Google Scholar]
- Park P.J., Jung W.K., Nam K.S., Shahidi F., Kim S.K. Purification and characterization of antioxidative peptides from protein hydrolysate of lecithin-free egg yolk. J. Am. Oil Chem. Soc. 2001;78:651–656. [Google Scholar]
- Pryor W.A., Ann N.Y. Free radical biology: xenobiotics, cancer, and aging. Acad. Sci. 1982;393:1–22. doi: 10.1111/j.1749-6632.1982.tb31228.x. [DOI] [PubMed] [Google Scholar]
- Qian Z.J., Jung W.K., Kim S.K. Free radical scavenging activity of a novel antioxidative peptide purified from hydrolysate of bull frog skin Rana catesbeiana Shaw. Bioresour. Technol. 2008;99:1690–1698. doi: 10.1016/j.biortech.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Quaglia G.B., Orban E. Enzymic solubilisation of proteins of sardine (Sardina pilchardus) by commercial proteases. J. Sci. Food Agric. 1987;38:263–269. [Google Scholar]
- Rajapakse N., Mendis E., Byun H.G., Kim S.K. Purification and in vitro antioxidative effects of giant squid muscle peptides on free radical-mediated oxidative systems. J. Nutr. Biochem. 2005;16:562–569. doi: 10.1016/j.jnutbio.2005.02.005. [DOI] [PubMed] [Google Scholar]
- Sampath Kumar N.S., Nazeer R.A., Jaiganesh R. Purification and biochemical characterization of antioxidant peptide from horse mackerel (Magalaspis cordyla) viscera protein. Peptides. 2011;32:1496–1501. doi: 10.1016/j.peptides.2011.05.020. [DOI] [PubMed] [Google Scholar]
- Sathivel S., Bechtel P.J., Babbitt J., Smiley S., Crapo C., Reppond K.D. Biochemical and functional properties of herring (Clupea harengus) by product hydrolysates. J. Food Sci. 2003;68:2196–2200. [Google Scholar]
- Shabeena Y.N., Nazeer R.A. Antioxidant activity of hydrolysates and peptide fractions of nemipterus japonicus and Exocoetus volitans muscle. J. Aquat. Food Prod. Technol. 2010;19:182–192. [Google Scholar]
- Shabeena Y.N., Nazeer R.A. Evaluation of bioactive properties of peptide isolated from Exocoetus volitans backbone. Int. J. Food Sci. Technol. 2011;46:37–43. [Google Scholar]
- Suetsuna K., Ukeda H., Ochi H. Isolation and characterization of free radical scavenging activities peptides derived from casein. J. Nutr. Biochem. 2000;11:128–131. doi: 10.1016/s0955-2863(99)00083-2. [DOI] [PubMed] [Google Scholar]
- Tang C.H., Peng J., Zhen D.W., Chen Z. Physicochemical and antioxidant properties of buckwheat (Fagopyrum esculentum Moench) protein hydrolysates. Food Chem. 2009;115:672–678. [Google Scholar]
- Wang J.S., Zhao M.M., Zhao Q.Z., Jiang Y.M. Antioxidant properties of papain hydrolysates of wheat gluten in different oxidation systems. Food Chem. 2007;101:1658–1663. [Google Scholar]
- Wang Y., Zhu F., Han F., Wang H. Purification and characterization of antioxidative peptides from salmon protamine hydrolysate. J. Food Biochem. 2008;32:654–671. [Google Scholar]
- Wu H.C., Chen H.M., Shiau C.Y. Free amino acids and peptides as related to antioxidant properties in protein hydrolysates of mackerel (Scomber austriasicus) Food Res. Int. 2003;36:949–957. [Google Scholar]
- Yen G.C., Chen H.Y. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J. Agric. Food Chem. 1995;43:27–32. [Google Scholar]
- Yildirim A., Mavi A., Kara A.A. Determination of antioxidant and antimicrobial activities of Rumex crispus L. extracts. J. Agric. Food Chem. 2001;49:4083–4089. doi: 10.1021/jf0103572. [DOI] [PubMed] [Google Scholar]
- You L., Zhao M., Regenstein J.M., Ren J. Purification and identification of antioxidative peptides from loach (Misgurnus anguillicaudatus) protein hydrolysate by consecutive chromatography and electrospray ionization-mass spectrometry. Food. Res. Int. 2010;43:1167–1173. [Google Scholar]
- Zhu K., Zhou H., Qian H. Antioxidant and free radical scavenging activities of wheat germ protein hydrolysates WGPH prepared with Alcalase. Process Biochem. 2006;41:1296–1302. [Google Scholar]



