Abstract
The effect of glutathione on the influences of heavy metals affecting rubisco and rubisco activase was studied in tobacco plants grown in vitro where the shoot explants of the tobacco plant cultured on MS medium under aseptic conditions and two explants were placed in the control, 0.1 mM GSH, 1 mM GSH, 0.2 mM Cd, 0.2 mM Cu, 0.2 mM Zn, and a mixture of Cd and GSH, Cu and GSH, Zn and GSH, respectively. The effect of GSH on the growth of the tobacco plant was minimal, but the heavy metals clearly retarded its growth. GSH recovered the growth retarded by heavy metals, and the concentration of GSH required to recover the growth differed depending on the heavy metals. The content of chlorophyll in the plant increased through GSH and Zn, and decreased through Cd and Cu. The chlorophyll content which decreased due to Cd and Cu was recovered by GSH, and the content which increased due to Zn was decreased by 1 mM GSH. The content of rubisco decreased due to GSH and heavy metals, and the content which decreased due to heavy metals was recovered by GSH, and when GSH was treated with Zn, the increased rate was maximum compared to other heavy metals. The activity of rubisco was increased due to GSH and heavy metals, and the activity increased by Cd and Zn decreased through GSH. In the case of Cu, the activity of GSH increased even more. There was no effect of GSH on the influences of heavy metals on the content and activity of rubisco activase. The activity of rubisco decreased by thiourea among six denaturing agents, and increased by l-cysteine, and in most cases the activity level was recorded as high. The activity of rubisco activase all decreased as a result of six denaturing agents, and the effect caused by EDTA and guanidine-HCl was the greatest, while the effect caused by l-cysteine and urea was minimal.
Keywords: Glutathione, Rubisco, Rubisco activase, Heavy metal, Tobacco
1. Introduction
Rubisco [ribulose-1,5-bisphosphate (RuBP) carboxylase/oxygenase; EC 4.1.1.39] is the most abundant protein in plants (Ellis, 1979), and is an enzyme that catalyzes the CO2 fixation reaction in photosynthesis forming phosphoglycerate (PGA) with the reaction of RuBP and CO2, and also catalyzes the photorespiration forming the phosphoglycolate and PGA with the reaction to O2 (Parry et al., 2003). The removal of a tightly bound inhibitor such as CA1P (2-carboxyarabinitol-1-phosphate) from the catalytic site of the carbamylated and decarbamylated forms of rubisco requires rubisco activase (Parry et al., 2008). Rubisco activase is one of a new type of chaperones, which functions to promote the catalytic activity of rubisco (Portis, 2003) in the presence of ATP and RuBP (Portis, 1990). The rubisco activation by rubisco activase is affected by environmental factors such as light intensity (Perchorowicz et al., 1981), content of O2 and CO2 (Sage et al., 1988; Schnyder et al., 1986), and temperature (Schnyder et al., 1984). ATP/ADP ratio affects the activation of rubisco activase (Robinson and Portis, 1989), which is regulated by the extension of C-terminal in the large isoform of rubisco activase (Shen et al., 1991).
Some of the heavy metals are used as essential trace elements in plants (Thormalley and Vasak, 1985), but their existence above a specific concentration in cells hinders the physiological metabolism in plants (Jarvis et al., 1976). In addition, the heavy metals accumulate either within vacuoles (Okorokov et al., 1980) or occur as cytoplasmic granules in plants (Christie and Costa, 1984). Among heavy metals cadmium (Cd), a soil pollutant with a strong toxicity (Waalkes, 2000), inhibits photosynthesis (Qian et al., 2009) and prevents the growth of roots and stem. Cd inactivates some enzymes by a strong affinity with the thiol group (Mendoza-Cozatl et al., 2005) and it forms the active oxygens such as hydrogen peroxide (H2O2), superoxide anion (), and hydroxyl radical (•OH) (Romero-Puertas et al., 2004) together with lipid peroxide, causing damage to biopolymer and cell membrane by inducing the oxidative stress (Heyno et al., 2008).
Copper (Cu) is absorbed mainly through roots and causes the physiological disturbances in plants (Påhlsson, 1989). The excessively-absorbed Cu not only decreases the biomass by inducing the chlorosis (Quartacci et al., 2000), but also interferes with the electron transport system of photosynthesis (Pätsikkä et al., 2002). Zinc (Zn), chemically similar to Cd, is one of the trace elements essential for the growth of plants. Zn interferes with the diverse and essential physiological processes such as the inhibition of plant growth and formation of the toxic lipid peroxide (Panda et al., 2003) in a high concentration.
Glutathione, belonging to non-protein thiol, is synthesized through two phases from glutamate, cysteine, and glycine (Hell and Bergmann, 1990). In plants, glutathione exists mostly in a reduced form (GSH), and also exists in a small proportion of an oxidized form (GSSG) (Hell, 1997). Glutathione is present at relatively high concentrations and occurs as an antioxidant in all plant cells (Dixon et al., 1998). It acts as an antioxidant, protects cell constituents against oxidation and eliminates the oxygen radicals formed by products of photosynthesis together with ascorbate (Mittler, 2002). In addition, glutathione has a protective function for the plant in forming conjugates with xenobiotics (Coleman et al., 1997), and acts as a precursor for the synthesis of phytochelatins, which are involved in the detoxification of heavy metals (Cobbett and Goldsbrough, 2002). Although there are many reports regarding the inhibitory effects of heavy metals in plants (Márquez-García et al., 2012), the study of glutathione on effect of heavy metals affecting the rubisco and rubisco activase which are the photosynthesis enzymes has not been reported yet. In this research work, the study on effectiveness of glutathione reducing the inhibitory effects of Cd, Cu, and Zn was carried out by measuring the growth, content of chlorophyll, content and activity of rubisco and rubisco activase, and activity of denaturing agent in tobacco plant cultured in vitro.
2. Materials and methods
2.1. Growth of tobacco plant
Tobacco (Nicotiana tabacum L.) seeds were germinated and grown aseptically in cell culture vessel containing MS (Murashige and Skoog, 1962) agar (0.8%) medium in the dark at 26 ± 1 °C. Four week-old shoots were cut into 3 cm segments and used as explants. Two explants were placed on an induction MS medium supplemented with control, 0.1 mM GSH, 1 mM GSH, Cd, Cd + 0.1 mM GSH, Cd + 1 mM GSH, Cu, Cu + 0.1 mM GSH, Cu + 1 mM GSH, Zn, Zn + 0.1 mM GSH, and Zn + 1 mM GSH using 0.2 mM CdCl2·2.5H2O, 0.2 mM CuSO4·5H2O, 0.2 mM ZnSO4·7H2O, and GSH (0.1 mM, 1 mM), respectively. The plants were maintained for 5 weeks on media at 26 ± 1 °C under a 16-h light (800 μM/m2/s PFD) and 8-h dark photoperiod (Roh et al., 1996).
Plant growth of each experiment was measured in terms of total fresh weight and leaves weight, and then compared. Fully expanded leaves from mature tobacco plants were used for rubisco and rubisco activase experiments. Three samples were used for each experiment and the data were analyzed statistically.
2.2. Chlorophyll content
Frozen leaves were transferred to DMF and stored at 5 °C in the dark. Extracts were centrifuged for 5 min at 8000g. Chlorophyll contents in the supernatants were measured spectrophotometrically using its specific absorption coefficients at 664.5 nm and 647 nm (Inskeep and Bloom, 1985).
2.3. Isolation of rubisco and rubisco activase
Rubisco was isolated from the leaves of tobacco grown in vitro (Wang et al., 1992). Frozen leaf tissue was pulverized in a mortar under liquid nitrogen and then extracted in the extraction buffer containing 50 mM BTP (pH 7.0), 10 mM NaHCO3, 10 mM MgCl2, 1 mM EDTA, 0.5 mM ATP, 10 mM DTT, 1 mM PMSF, 1 mM benzamidine, 0.01 mM leupeptin, 1.5% PVPP and 3 mM MBT. Solution filtered from the leaf slurry through cheesecloth and Miracloth was centrifuged at 16,000 rpm for 40 min. (NH4)2SO4 powder was slowly added into the supernatant to 35% saturation and stirred for 30 min. The supernatant and pellet were collected by centrifugation at 8000g for 8 min. The supernatant contains rubisco, and the resuspended pellet contains rubisco activase. The supernatant collected was brought to 55% saturation of (NH4)2SO4 by the addition of powder. The pellet collected by centrifugation at 8000 rpm for 8 min was resuspended in 5 ml of 50 mM Tricine (pH 8.0), 10 mM NaHCO3, 10 mM MgCl2, 10 mM DTT, and 2 mM MBT (buffer A), and 50% PEG-10K was added to a final concentration of 17%, stirred 5 min. The resulting precipitate was collected by centrifugation at 8000 rpm for 8 min and resuspended in buffer A. Resuspended solution was loaded onto a Q-Sepharose column equilibrated with 20 mM Tris–HCl (pH 7.5). The column was washed with the same buffer containing 0.1 M NaCl before starting elution with a linear gradient from 0.1 to 0.5 M NaCl at a flow rate of 1 ml/min. 3 ml fractions were pooled, and assayed for rubisco content and activity.
Above obtained pellet was resuspended in 20 mM BTP (pH 7.0), 10 mM MgCl2, 0.2 mM ATP, and 2 mM MBT (buffer B) and 50% (w/v) PEG-10 K was added to a final concentration of 18%, stirred 5 min, and centrifuged at 8,000 rpm for 8 min. The pellet was dissolved in 2.5 ml of buffer B. Solution was cleared by spinning at 10,000 rpm for 10 min. Pellet was resuspended again in 2.5 ml buffer B and the solution cleared again. The collected supernatants were loaded onto a 20 ml Q-Sepharose column equilibrated with 20 mM BTP (pH 7.0). The column was eluted with 40 ml of 20 mM BTP (pH 7.0) at a flow rate of 1 ml/min before continuing with 140 ml of a linear gradient from 0 to 0.5 M NaCl in 20 mM BTP (pH 7.0). 3 ml fractions were pooled, and assayed for rubisco activase content and activity.
Unless indicated otherwise, all purification processes were done at 4 °C.
2.4. Determination of rubisco and rubisco activase contents
Rubisco content was determined spectrophotometrically using A280 × 0.61 = mg/ml by the method of Wishnick and Lane (1971). Rubisco activase content was determined at 595 nm using bovine serum albumin as a standard by the method of Bradford (1976).
2.5. Assay of rubisco and rubisco activase activities
Rubisco activity was determined spectrophotometrically at 25 °C by monitoring NADH oxidation at 340 nm by the method of Racker (1962). The assay medium contained 1 M Tris–HCl (pH 7.8), 0.006 M NADH, 0.5% glyceraldehyde-3-phosphate dehydrogenase, 0.1 M GSH, 10 units/20 μl 3-phosphoglycerate kinase, 0.2 M ATP, 0.05% α-glycerophosphate dehydrogenase-triose phosphateisomerase, 0.025 M RuBP, 0.5 M MgCl2, 0.5 M KHCO3, and isolated rubisco solution in a final volume of 0.5 ml. One unit of enzyme was defined as the amount of enzyme producing 1 μM of RuBP per min. Rubisco activase activity was determined as the ability to produce ADP in an ATP-dependent reaction in absorption at 340 nm by the method of Robinson and Portis (1989). The isolated rubisco activase solution was added to a total volume of 0.4 ml of the activation reaction mixture containing 50 mM Tricine-KOH (pH 8.0), 20 mM KCl, 5 mM MgCl2, 2.5 mM ATP, 1 mM phosphoenolpyruvate, 0.3 mM NADH, 40 units/ml pyruvate kinase, and 40 units/ml l-lactic dehydrogenase. One unit was defined as the amount that catalyzed the cleavage of 1 μM ATP per min.
2.6. Assay of rubisco and rubisco activase activities by denaturing agents
10 mM of l-cysteine, EDTA, urea, thiourea, guanidine-HCl, and β-mercaptoethanol were used to determine the effects of denaturing agents on rubisco and rubisco activase activity. Results were calculated presuming the activity of denaturing agent untreated control as 100%.
2.7. Statistical analysis
Treatment means were compared by the analysis of variance using SPSS (SPSS ver. 21). Standard error between replicates was also calculated.
3. Results
3.1. Effects of GSH and heavy metals on the growth of tobacco plant
The growth was retarded in the presence of various heavy metals as expected, and there were no significant differences in the growth of tobacco plant in the control, 0.1 mM GSH and 1 mM GSH. Different concentrations of GSH were necessary to recover the growth of tobacco plant depending on heavy metals. Among the trials, the growth of tobacco plant was shown as the best in Cu + 1 mM GSH, and as the worst in Cu + 0.1 mM GSH (Fig. 1). In order to confirm the above results, both total fresh weight of tobacco plant and weight of only leaves were measured. As a result of measuring the total fresh weight, the growth of tobacco plant in the control was similar to that in 0.1 mM GSH, and was lower than that in 1 mM GSH. The growth of tobacco plant in heavy metals showed no significant differences among heavy metals, but in all cases, growth was lower than that in the control. The growth was recovered in 0.1 mM GSH among mixture of Cd and GSH, and was recovered in 1 mM GSH among mixture of Cu and GSH, and was recovered in both 0.1 mM and 1 mM GSH among mixture of Zn and GSH (Fig. 2). The fresh weight of leaves alone showed the same tendency as the fresh weight of plants (Fig. 3).
Figure 1.

In vitro induction of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
Figure 2.
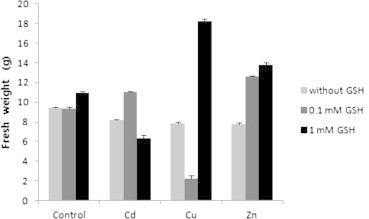
The fresh weight of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
Figure 3.
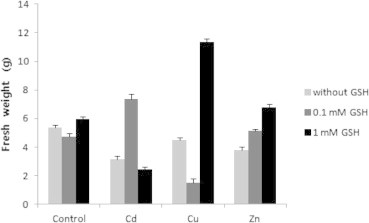
The fresh weight of leaves of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
3.2. Effects of GSH and heavy metals on chlorophyll content
In order to investigate the effects of GSH and heavy metals on chlorophyll content, total chlorophyll content was calculated. As a result, when GSH was treated, the content of chlorophyll increased more than that in the control, but was higher in 0.1 mM GSH, and the content in Cd and Cu among heavy metals decreased compared to the control and increased in Zn. In mixture of Cd and GSH, as the concentration of GSH became higher, the content increased, and in mixture of Cu and GSH, the content increased when GSH was treated, but the content in 0.1 mM GSH was higher. In mixture of Zn and GSH, the content in 0.1 mM GSH was highest, and lowest in 1 mM GSH, and that in 1 mM GSH was lower than that in the control (Fig. 4).
Figure 4.
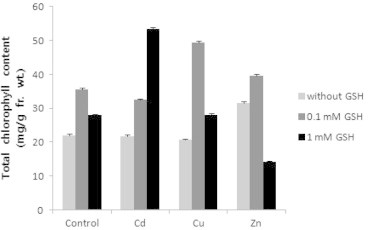
Effects of heavy metals and GSH on total chlorophyll content in leaves of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
3.3. Effects of GSH and heavy metals on the content and activity of rubisco
Rubisco content in all trials was remarkably low compared to the control. When GSH was treated in the control, as the concentration of GSH became higher, the content increased. When the heavy metals were treated, the content was lower than that in 0.1 mM GSH, the content was highest in Cu, and lowest in Zn. When GSH was treated in mixture of Cd and GSH, the content increased, but the content in 0.1 mM GSH was highest. When GSH was treated in mixture of Cu and GSH and Zn and GSH, the content increased as the concentration of GSH became higher (Fig. 5). When GSH was treated, rubisco activity increased on comparison to the control, but was highest in 0.1 mM GSH. When the heavy metals were treated, the activity was high in all cases in the control, and was highest in Zn, and was lowest in Cu. When GSH was treated in mixture of Cd and GSH and Zn and GSH, the activity decreased as the concentration of GSH became higher, but when GSH was treated in mixture of Cu and GSH, the activity increased as the concentration became higher (Fig. 6).
Figure 5.
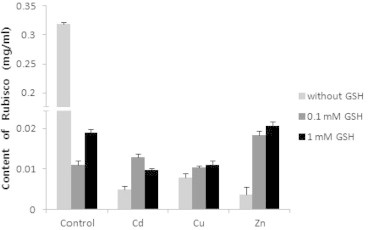
Effects of heavy metals and GSH on content of rubisco in leaves of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
Figure 6.
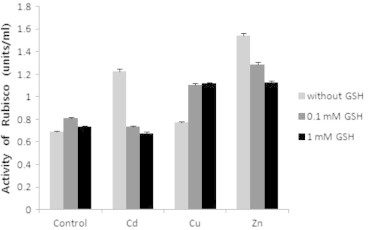
Effects of heavy metals and GSH on activity of rubisco in leaves of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
3.4. Effects of GSH and heavy metals on the content and activity of rubisco activase
When GSH and heavy metals were treated, the content of rubisco activase increased compared to the control, was highest in Cu, and was the same in Cd and Zn. In mixture of Cd and GSH, the content was highest in 1 mM GSH, and the content in 0.1 mM GSH was the same as that in Cd. When GSH was treated in mixture of Cu and GSH, the content increased as the concentration of GSH became higher, but the content was highest in 0.1 mM GSH, and lowest in 1 mM GSH in mixture of Zn and GSH. However, the content was high in all trials compared to the control, showing no significant differences (Fig. 7). The activity of rubisco activase, when GSH was treated, decreased compared to the control, but as the concentration of GSH became higher, the activity increased. When the heavy metals were treated, the activity in Cd decreased compared to the control, increased in Cu, and the activity in Zn was the same as that in the control. In mixture of Cd and GSH and Cu and GSH, the activity in 0.1 mM GSH was the highest, and was the lowest in 1 mM GSH. When GSH was treated in mixture of Zn and GSH, the activity increased, but activity was higher in 0.1 mM GSH. However, there were no significant differences in all trials compared to the control (Fig. 8).
Figure 7.
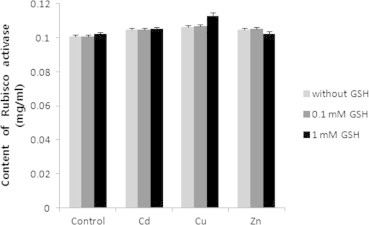
Effects of heavy metals and GSH on content of rubisco activase in leaves of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
Figure 8.
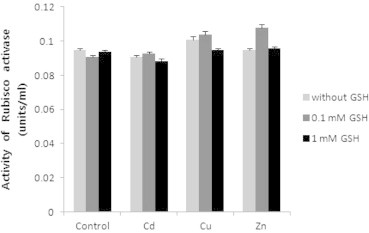
Effects of heavy metals and GSH on activity of rubisco activase in leaves of tobacco plants grown on MS medium containing Cd, Cu, Zn, and GSH for 5 weeks.
3.5. Effects of denaturing agents on the activity of rubisco
In the control, Cd + 1 mM GSH treated with six denaturing agents, the activity increased in all cases, and the activity increased most by EDTA in the control, and increased most by l-cysteine in Cd + 1 mM GSH. In 0.1 mM GSH, the activity decreased by five denaturing agents except l-cysteine, decreased most by thiourea while in 1 mM GSH, the activity increased by five denaturing agents except thiourea, and increased most by EDTA. In Cd, mixture of Cu and GSH, Zn and GSH, the activity decreased by all denaturing agents, and the activity decreased most by β-mercaptoethanol in Cd, Cu + 1 mM GSH, and decreased most by l-cysteine in Cu + 0.1 mM GSH, decreased most by urea and guanidine-HCl in Zn, and decreased most by thiourea in Zn + 0.1 mM GSH, and decreased most by guanidine-HCl in Zn + 1 mM GSH. In Cd + 0.1 mM GSH, the activity decreased greatly by urea and thiourea, and increased by l-cysteine and β-mercaptoethanol. In Cu, the activity increased by l-cysteine, urea and guanidine-HCl, and decreased by β-mercaptoethanol, EDTA and thiourea. Though six denaturing agents affected the activity of rubisco, in most cases, the activity was higher than that in the control (Table 1).
Table 1.
Effects of denaturing agents on rubisco activity.
| Rubisco activity (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Zn | GSH | No treatment | l-cysteine | β-Mercapto ethanol | EDTA | Urea | Thiourea | Guanidine-HCl |
| (mM) | ||||||||||
| 0 | 0 | 0 | 0 | 100 | 106 | 106 | 110 | 103 | 107 | 105 |
| 0.1 | 117 | 118 | 114 | 108 | 107 | 101 | 110 | |||
| 1 | 107 | 112 | 109 | 118 | 107 | 106 | 110 | |||
| 0 | 178 | 107 | 92 | 109 | 109 | 102 | 111 | |||
| 0.2 | 0.1 | 106 | 111 | 117 | 101 | 99 | 99 | 103 | ||
| 1 | 98 | 115 | 109 | 106 | 105 | 103 | 104 | |||
| 0 | 111 | 141 | 109 | 109 | 121 | 108 | 133 | |||
| 0.2 | 0.1 | 160 | 107 | 112 | 110 | 115 | 111 | 115 | ||
| 1 | 162 | 113 | 108 | 110 | 110 | 118 | 110 | |||
| 0 | 223 | 109 | 109 | 126 | 87 | 112 | 98 | |||
| 0.2 | 0.1 | 187 | 116 | 106 | 110 | 107 | 105 | 109 | ||
| 1 | 163 | 112 | 105 | 109 | 110 | 104 | 102 | |||
3.6. Effects of denaturing agents on the activity of rubisco activase
Rubisco activase activity in all trials decreased by six denaturing agents lower than that in the control, and decreased most in Cd + 1 mM GSH, mixture of Zn and GSH. In the control, the activity decreased least by β-mercaptoethanol, decreased most by thiourea while in 0.1 mM GSH, the activity decreased least by thiourea and decreased most by EDTA. In a mixture of Cd and GSH, Cu, the activity decreased least by l-cysteine while in mixture of Cd and GSH, the activity decreased most by EDTA and in Cu, the activity decreased most by urea, respectively. In 1 mM GSH, mixture of Cu and GSH, Zn + 1 mM GSH, the activity decreased most by guanidine-HCl while in 1 mM GSH, mixture of Cu and GSH, the activity decreased least by urea, and in Zn + 1 mM GSH, the activity decreased least by l-cysteine. In Zn, Zn + 0.1 mM GSH, the activity decreased most by l-cysteine while in Zn, the activity decreased least by urea, and in Zn + 0.1 mM GSH, the activity decreased least by EDTA. In most cases, the activity decreased most by EDTA and guanidine-HCl, and decreased least by l-cysteine and urea (Table 2).
Table 2.
Effects of denaturing agents on rubisco activase activity.
| Rubisco activase activity (%) |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cd | Cu | Zn | GSH | No treatment | l-Cysteine | β-Mercapto ethanol | EDTA | Urea | Thiourea | Guanidine-HCl |
| (mM) | ||||||||||
| 0 | 0 | 0 | 0 | 100 | 45 | 61 | 48 | 42 | 41 | 45 |
| 0.1 | 96 | 40 | 41 | 38 | 44 | 48 | 44 | |||
| 1 | 99 | 44 | 44 | 38 | 45 | 38 | 32 | |||
| 0 | 96 | 52 | 37 | 36 | 40 | 42 | 37 | |||
| 0.2 | 0.1 | 98 | 46 | 42 | 34 | 41 | 41 | 37 | ||
| 1 | 93 | 45 | 33 | 29 | 37 | 37 | 34 | |||
| 0 | 106 | 55 | 53 | 52 | 46 | 51 | 52 | |||
| 0.2 | 0.1 | 109 | 38 | 40 | 39 | 45 | 43 | 37 | ||
| 1 | 100 | 38 | 41 | 38 | 43 | 40 | 33 | |||
| 0 | 100 | 39 | 41 | 39 | 46 | 42 | 41 | |||
| 0.2 | 0.1 | 114 | 29 | 34 | 41 | 40 | 40 | 38 | ||
| 1 | 101 | 43 | 38 | 32 | 36 | 39 | 27 | |||
4. Discussion
Glutathione is involved in detoxification through conjugation with herbicide (Lamoureux and Rusness, 1989), and plays a role of antioxidant (Gill and Tuteja, 2010). The heavy metal ions showing the toxicity in plants (Nieboer and Richardson, 1980) are absorbed through roots, and thereafter metastasize to stems or leaves, not only involving in the hindrance of ion absorption, reduction of dry weight in lateral roots, main roots and chlorophyll content (Kelly et al., 1979) but also in the inhibition of the growth of roots (Kahle, 1993).
Considering the effects of Cd, Cu, and Zn on the growth of tobacco plants, in 0.2 mM Cd, Cu, and Zn, the growths were retarded more compared to the control, and the effect on growth of plants was different depending on heavy metals. The growth of Populus termula clone (Kieffer et al., 2008) was retarded remarkably at 20 μM Cd, showing the tendency of hindrance to growth at a relatively low concentration compared to this study. In addition, the growth of maize seedling (Rüegsegger and Brunold, 1992) was retarded remarkably at 200 μM Cd, showing the same as the result that the growth was retarded at 0.2 mM Cd in this study. Cu is highly toxic to plants even at a micromolar range of exposure (Cabral, 2003). There are reports that the growth of roots was retarded even at 1 μM Cu of relatively low concentration (Schat and Ten Bookum, 1992), and the report that fresh weight of Populus × euramericana clone Adda was reduced at more than 100 μM Cu (Borghia et al., 2007), both of which were similar to the result of this study showing that the growth was retarded at 0.2 mM Cu even if there are some differences in the concentration.
The growth of Brassica juncea and Cajanus cajan (Alia et al., 1995) increased at a relative low concentration less than 0.05 mM Zn, but was retarded at more than 0.1 mM Zn, which was similar to the result of this study showing that the growth was retarded at 0.2 mM Zn. In addition, in case of poplar clone I-214 (Baccio et al., 2005), not only the growth but also the number of leaves was decreased, which is considered due to the fact that Zn at low concentrations stimulates the growth by regulating the important metabolic process related to growth and development of plants (Stiborová et al., 1987), and also interferes with the metabolism essential for the growth of plants (Van Assche and Clijsters, 1990). Comparing the heavy metals in Cd, Cu, and Zn, GSH mixture of heavy metals and GSH in order to investigate the effect of GSH on impact of heavy metals affecting the growth of tobacco plant, the growth controlled by Cd, Cu, and Zn was recovered by GSH, and the concentration of GSH to recover the growth of plants was different depending on heavy metals. This is considered due to the fact that GSH is involved in physiological adaptation to environmental stress like a high heavy metal excess (Grill et al., 1989), and that phytochelatin formed based on GSH substrate (Wu et al., 2004) is involved in metabolism and detoxification of metal ions like Cd, Cu, and Zn in cells (Grill et al., 1985). In view of the report that GSH content decreased by Cd, Cu, and Zn (Márquez-García et al., 2012), we can see the fact that glutathione is a major mechanism for the toxic effects of heavy metals, which is supported by the results that the heavy metals and glutathione are closely connected, and that GSH is related with the synthesis of phytochelatin (Schützendübel and Polle, 2002).
On investigating the effects of Cd, Cu, and Zn on chlorophyll content and GSH effect, the content decreased by Cd and Cu, and increased by Zn, showing a different result, with an effect on the chlorophyll content of plants depending on heavy metals. The content of chlorophyll decreased at 20 μM Cd in pea (Sandalio et al., 2001) and at 50 μM Cd in Camellia sinensis bud (Mohanpuria et al., 2007), in all of which cases the concentration was very low compared to this study. This result is considered due to the fact that Cd served as a strong inhibitor of chlorophyll accumulation (Padmaja et al., 1990). Unlike the result of this study showing the decrease due to 0.2 mM Cu, in case of Populus × euramericana clone Adda (Borghia et al., 2007), the content of chlorophyll increased by Cu less than 500 μM and decreased by 1000 μM Cu. In the case of Zn at poplar clone I-214 (Baccio et al., 2005), the content of chlorophyll decreased by Zn less than 10 mM and was not changed at 10 mM, but there appeared the chlorosis, which was different from this study showing the increased content by 0.2 mM Zn.
The study at the level of secondary metabolites like jasmonic acid and (Popova and Vaklinova, 1988), salicylic acid (Pancheva and Popova, 1998) with regard to rubisco has been conducted, and glutathione is reported to detoxify the effects of heavy metals on enzymes. So, this study was carried out for rubisco and rubisco activase. The effect of GSH on effects of heavy metals investigated with comparing the content and activity of rubisco induced by heavy metals and GSH. The content of rubisco in all trials was remarkably low compared to the control, but increased by GSH. In addition, in case of Cd, Cu, and Zn, the content was all lower than that in 0.1 mM GSH, but the content of rubisco increased by mixture of heavy metals and GSH, from which we could see that GSH has recovered the hindrance due to heavy metals. This is considered due to the detoxification effect of GSH on heavy metals. The activity of rubisco was increased by GSH and heavy metals compared to the control. In the mixture of Cu and GSH the activity increased, but in the mixture of Cd and GSH, the activity decreased up to the level of the control, and in the mixture of Zn and GSH, the activity decreased, but did not reach the level of the control. It is considered that GSH shows the effect of detoxification on heavy metals selectively. In a study on the activity of rubisco by Cd, and interaction between glutathione and Cd, Pietrini et al. (2003) reported that the facilitation of antioxidative activity by Cd has something to do with the increase of glutathione, which shows a relationship with the effect of GSH on Cd in this study. They made a research on the effect of salicylic acid on rubisco induced by Cd in tobacco plant, and reported that SA serves the positive effect to improve the damage effect by Cd (Wang and Roh, 2012), which showed differently from the result of Cd in this study. In addition, unlike the result of this study showing a remarkable increase of activity by Zn, there is a report that the activity of rubisco decreases by Zn (Van Assche et al., 1980).
In order to investigate whether the results of heavy metals and GSH on rubisco are connected with rubisco activase due to the fact that rubisco is activated by rubisco activase, we studied the effect of GSH on the effects of heavy metals by measuring the content of rubisco activase. From the fact that the effects due to heavy metals and GSH are determined as non-existent as the content and activity of rubisco activase show no significant differences in all trials of heavy metals and GSH, it is considered that there is no detoxification effect by GSH on the content and activity of rubisco activase. The result of this study showed some differences compared to the result obtained by Roh and Chin (2005) who reported that the content and activity of rubisco activase decreased by Cd. In a report of Wang and Roh (2012) who studied the effect of salicylic acid on rubisco induced by Cd, the fact that the result on rubisco was the same as that on rubisco activase, showed that the rubisco and rubisco activase acted together in connection, which is different from the present result. So it is considered that rubisco is not attributable by rubisco activase.
It is known that l-cysteine and β-mercaptoethanol having the thiol group break up the disulfide bond, and urea, thiourea and guanidine-HCl cause the denaturation by forming a strong hydrogen bond with protein (Wang and Roh, 2012), and urea and guanidine-HCl decrease even the protein folding (Camacho and Thirumalai, 1996). Study on the effect of denaturing agent on rubisco activity showed that six denaturing agents affected the activity of rubisco, but in most cases, the activity was higher than that in the control. The activity in Cd decreased by all denaturing agents, but the activity in tobacco (Wang and Roh, 2012) increased by l-cysteine and β-mercaptoethanol and decreased by guanidine-HCl, showing the result different from this study. In addition, the structural change of rubisco occurred in spinach leaves by guanidine-HCl at relatively high concentrations compared to this study, and the activity decreased more than 50% by 0.2 M guanidine-HCl, and decreased up to 100% by 0.4 M guanidine-HCl (Jiang et al., 1997).
The activity of rubisco activase decreased by six denaturing agents in all trials, lower than that in the control, and in most cases, the activity decreased most by EDTA and guanidine-HCl, and decreased least by l-cysteine and urea. Unlike the result of this study, there were no effects of denaturing agents on the activity of rubisco activase in tobacco plant (Wang and Roh, 2012).
5. Conclusion
In conclusion, from the present study, the recovery to inhibitory effects of heavy metals is the result of the detoxification effect by GSH, and the lack of effects of GSH and heavy metals on content and activity of rubisco activase is considered to represent that the effect of GSH and heavy metals on rubisco is not based on connection with rubisco activase.
Footnotes
Peer review under responsibility of King Saud University.
References
- Alia Prasad K.V.S.K., Pardha Saradhi P. Effect of zinc on free radicals and proline in Brassica and Cajanus. Phytochemistry. 1995;39:45–47. [Google Scholar]
- Borghia M., Tognettib R., Montefortia G., Sebastiani L. Responses of Populus × euramericana (Pdeltoides × P. nigra) clone Adda to increasing copper concentrations. Environ. Exp. Bot. 2007;61:66–73. [Google Scholar]
- Bradford M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Cabral J.P. Copper toxicity to five Parmelia lichens in vitro. Environ. Exp. Bot. 2003;49:237–250. [Google Scholar]
- Camacho C.J., Thirumalai D. Denaturants can accelerate folding rates in a class of globular proteins. Protein Sci. 1996;5:1826–1832. doi: 10.1002/pro.5560050908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christie N.T., Costa M. In vitro assessment of the toxicity of metal compounds IV Disposition of metals in cells: Interactions with membranes, glutathione, metallothionein, and DNA. Biol. Trace Elem. Res. 1984;6:139–158. doi: 10.1007/BF02916931. [DOI] [PubMed] [Google Scholar]
- Cobbett C., Goldsbrough P. Phytochelatins and metallothioneins: roles in heavy metal detoxification and homeostasis. Ann. Rev. Plant Biol. 2002;53:159–182. doi: 10.1146/annurev.arplant.53.100301.135154. [DOI] [PubMed] [Google Scholar]
- Coleman J.O.D., Blake-Kalff M.M.A., Davies T.G.E. Detoxification of xenobiotics by plants: chemical modification and vacuolar compartmentation. Trends Plant Sci. 1997;2:144–151. [Google Scholar]
- Di Baccio D., Kopriva S., Sebastiani L., Rennenberg H. Does glutathione metabolism have a role in the defence of poplar against zinc excess? New Phytol. 2005;167:73–80. doi: 10.1111/j.1469-8137.2005.01462.x. [DOI] [PubMed] [Google Scholar]
- Dixon D.P., Cummins I., Cole D.J., Edwards R. Glutathione-mediated detoxification systems in plants. Plant Biol. 1998;1:258–266. doi: 10.1016/s1369-5266(98)80114-3. [DOI] [PubMed] [Google Scholar]
- Ellis R.J. The most abundant protein in the world. Trends Biochem. Sci. 1979;4:241–244. [Google Scholar]
- Gill S.S., Tuteja N. Reactive oxygen species and antioxidant machinery in biotic stress tolerance in crop plants. Plant Physiol. Biochem. 2010;48:909–930. doi: 10.1016/j.plaphy.2010.08.016. [DOI] [PubMed] [Google Scholar]
- Grill E., Winnacker E.L., Zenk M.H. Phytochelatins: the principal heavy-metal complexing peptides of higher plants. Science. 1985;230:674–676. doi: 10.1126/science.230.4726.674. [DOI] [PubMed] [Google Scholar]
- Grill E., Löffler S., Winnacker E.L., Zenk M.H. Phytochelatins, the heavy-metal-binding peptides of plants, are synthesized from glutathione by a specific γ-glutamylcysteine dipeptidyl transpeptidase (phytochelatin synthase) Proc. Natl. Acad. Sci. USA. 1989;86:6838–6842. doi: 10.1073/pnas.86.18.6838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R. Molecular physiology of plant sulfur metabolism. Planta. 1997;202:138–148. doi: 10.1007/s004250050112. [DOI] [PubMed] [Google Scholar]
- Hell R., Bergmann L. γ-Glutamylcysteine synthetase in higher plants: catalytic properties and subcellular localization. Planta. 1990;180:603–612. doi: 10.1007/BF02411460. [DOI] [PubMed] [Google Scholar]
- Heyno E., Klose C., Krieger-Liszkay A. Origin of cadmium-induced reactive oxygen species production: mitochondrial electron transfer versus plasma membrane NADPH oxidase. New Phytol. 2008;179:687–699. doi: 10.1111/j.1469-8137.2008.02512.x. [DOI] [PubMed] [Google Scholar]
- Inskeep W.P., Bloom P.R. Extinction coefficients of chlorophyll a and b in N,N-dimethylformamide and 80% acetone. Plant Physiol. 1985;77:483–485. doi: 10.1104/pp.77.2.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis S.C., Joser L.H.P., Hopper M.D. Cd uptake from solution by plants and its transport from roots to shoots. Plant Soil. 1976;44:179–191. [Google Scholar]
- Jiang R.F., Wang Z.X., Xu G.J. Substrate induced reactivation of spinach ribulose-1,5-bisphosphate carboxylase/oxygenase denatured by low concentrations of guanidine hydrochloride. Biochim. Biophys. Acta. 1997;1343:95–101. doi: 10.1016/s0167-4838(97)00125-8. [DOI] [PubMed] [Google Scholar]
- Kahle H. Response of roots of trees to heavy metals. Environ. Exp. Bot. 1993;33:99–119. [Google Scholar]
- Kelly J.M., Parker G.R., Mc Fee W.W. Heavy metal accumulation and growth of seedlings of five forest species as influenced by soil cadmium level. J. Environ. Qual. 1979;8:361–364. [Google Scholar]
- Kieffer P., Dommes J., Hoffmann L., Hausman J.F., Renaut J. Quantitative changes in protein expression of cadmium- exposed poplar plants. Proteomics. 2008;8:2514–2530. doi: 10.1002/pmic.200701110. [DOI] [PubMed] [Google Scholar]
- Lamoureux G.L., Rusness D.G. The role of glutathione and glutathione-S-transferases in pesticide metabolism, selectivity, and mode of action in plants and insects. In: Dolphin D., Poulson R., Avramovic O., editors. Wiley; New York: 1989. pp. 153–196. (Glutathione: Chemical Biochemical and Medical Aspects). [Google Scholar]
- Márquez-García B., Horemans N., Torronteras R., Córdoba F. Glutathione depletion in healthy cadmium-exposed Erica andevalensis. Environ. Exp. Bot. 2012;75:159–166. [Google Scholar]
- Mendoza-Cozatl D., Loza-Tavera H., Hernandez-Navarro A., Moreno-Sanchez R. Sulfur assimilation and glutathione metabolism under cadmium stress in yeast, protists and plants. FEMS Microbiol. Rev. 2005;29:653–671. doi: 10.1016/j.femsre.2004.09.004. [DOI] [PubMed] [Google Scholar]
- Mittler R. Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci. 2002;7:405–410. doi: 10.1016/s1360-1385(02)02312-9. [DOI] [PubMed] [Google Scholar]
- Mohanpuria P., Rana N.K., Yadav S.K. Cadmium induced oxidative stress influence on glutathione metabolic genes of Camellia sinensis (L) O. Kuntze. Environ. Toxicol. 2007;22:368–374. doi: 10.1002/tox.20273. [DOI] [PubMed] [Google Scholar]
- Murashige T., Skoog F. A revised medium for rapid growth and bio assays with tobacco tissue cultures. Physiol. Plant. 1962;15:473–497. [Google Scholar]
- Nieboer E., Richardson D.H.S. The replacement of the nondescript term heavy metal by a biologically and chemically significant classification of metal ions. Environ. Pollut. B Chem. Phys. 1980;1:2–26. [Google Scholar]
- Okorokov L.A., Lichko L.P., Kulaev I.S. Vacuoles: main compartments of potassium, magnesium, and phosphate ions in Saccharomyces carlsbergenis cells. J. Bacteriol. 1980;144:661–665. doi: 10.1128/jb.144.2.661-665.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padmaja K., Prasad D.D.K., Prasad A.R.K. Inhibition of chlorophyll synthesis in Phaseolus vulgaris L. seedlings by cadmium acetate. Photosynthetica. 1990;24:399–405. [Google Scholar]
- Påhlsson A.M.B. Toxicity of heavy metals (Zn, Cu, Cd, Pb) to vascular plants. Water Air Soil Pollut. 1989;47:287–319. [Google Scholar]
- Pancheva T.V., Popova L.P. Effect of salicylic acid on the synthesis of ribulose-1,5-bisphosphate carboxylase/oxygenase in barley leaves. J. Plant Physiol. 1998;152:381–386. [Google Scholar]
- Panda S.K., Chaudhury I., Khan M.H. Heavy metals induce lipid peroxidation and affect antioxidants in wheat leaves. Biol. Plant. 2003;46:289–294. [Google Scholar]
- Parry M.A.J., Keys A.J., Madgwick P.J., Carmo-Silva A.E., Andralojc P.J. Rubisco regulation: a role for inhibitors. J. Exp. Bot. 2008;59:1569–1580. doi: 10.1093/jxb/ern084. [DOI] [PubMed] [Google Scholar]
- Parry M.A.J., Andralojc P.J., Mitchell R.A.C., Madgwick P.J., Keys A.J. Manipulation of rubisco: the amount, activity, function and regulation. J. Exp. Bot. 2003;54:1321–1333. doi: 10.1093/jxb/erg141. [DOI] [PubMed] [Google Scholar]
- Pätsikkä E., Kairavuo M., Šeršen F., Aro E.M., Tyystjärvi E. Excess copper predisposes photosystem II to photoinhibition in vivo by outcompeting iron and causing decrease in leaf chlorophyll. Plant Physiol. 2002;129:1359–1367. doi: 10.1104/pp.004788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perchorowicz J.T., Raynes D.A., Jensen R.G. Light limitation of photosynthesis and activation of ribulose bisphosphate carboxylase in wheat seedlings. Proc. Nati. Acad. Sci. USA. 1981;78:2985–2989. doi: 10.1073/pnas.78.5.2985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietrini F., Iannelli M.A., Pasqualini S., Massacci A. Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav) Trin. ex Steudel. Plant Physiol. 2003;133:829–837. doi: 10.1104/pp.103.026518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popova L.P., Vaklinova S.G. Effect of jasmonic acid on the synthesis of ribulose-1,5-bisphosphate carboxylase/oxygenase in barley leaves. J. Plant Physiol. 1988;133:210–215. [Google Scholar]
- Portis A.R., Jr. Rubisco activase. Biochim. Biophys. Acta. 1990;1015:15–28. doi: 10.1016/0005-2728(90)90211-l. [DOI] [PubMed] [Google Scholar]
- Portis A.R., Jr. Rubisco activase: rubisco’s catalytic chaperone. Photosynth. Res. 2003;75:11–27. doi: 10.1023/A:1022458108678. [DOI] [PubMed] [Google Scholar]
- Qian H., Li J., Sun L., Chen W., Sheng G.D., Liu W., Fu Z. Combined effect of copper and cadmium on Chlorella vulgaris growth and photosynthesis-related gene transcription. Aquat. Toxicol. 2009;94:56–61. doi: 10.1016/j.aquatox.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Quartacci M.F., Pinzino C., Sgherri C.L.M., Dalla Vecchia F., Navari-Izzo F. Growth in excess copper induces changes in the lipid composition and fluidity of PSII-enriched membranes in wheat. Physiol. Plant. 2000;108:87–93. [Google Scholar]
- Racker E. Ribulose diphosphate carboxylase from spinach leaves: ribulose diphosphate + CO2 + H2O → 2 3-P-glycerate. Methods Enzy. 1962;5:266–270. [Google Scholar]
- Robinson S.P., Portis A.R., Jr. Adenosine triphosphate hydrolysis by purified rubisco activase. Arch. Biochem. Biophys. 1989;268:93–99. doi: 10.1016/0003-9861(89)90568-7. [DOI] [PubMed] [Google Scholar]
- Roh K.S., Chin H.S. Cadmium toxicity and calcium effect on growth and photosynthesis of tobacco. J. Life Sci. 2005;15:453–460. [Google Scholar]
- Roh K.S., Kim J.K., Song S.D., Chung H.S., Song J.S. Decrease of the activation and carbamylation of rubisco by high CO2 in kidney bean. Kor. J. Biotechnol. Bioeng. 1996;11:295–302. [Google Scholar]
- Romero-Puertas M.C., Rodriguez-Serrano M., Corpas F.J., Gomez M., Del Rio L.A., Sandalio L.M. Cadmium-induced subcellular accumulation of O•2- and H2O2 in pea leaves. Plant Cell Environ. 2004;27:1122–1134. [Google Scholar]
- Rüegsegger A., Brunold C. Effect of cadmium on γ-glutamylcysteine synthesis in maize seedlings. Plant Physiol. 1992;99:428–433. doi: 10.1104/pp.99.2.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sage R.F., Sharkey T.D., Seemann J.R. The in-vivo response of the ribulose-1,5-bisphosphate carboxylase activation state and the pool sizes of photosynthetic metabolites to elevated CO2 in Phaseolus vulgaris L. Planta. 1988;174:407–416. doi: 10.1007/BF00959528. [DOI] [PubMed] [Google Scholar]
- Sandalio L.M., Dalurzo H.C., Gómez M., Romero-Puertas M.C., del Río L.A. Cadmium-induced changes in the growth and oxidative metabolism of pea plants. J. Exp. Bot. 2001;52:2115–2126. doi: 10.1093/jexbot/52.364.2115. [DOI] [PubMed] [Google Scholar]
- Schat H., Ten Bookum W.M. Genetic control of copper tolerance in Silene vulgaris. Heredity. 1992;68:219–229. [Google Scholar]
- Schnyder H., Mächler F., Nösberger J. Influence of temperature and O2 concentration on photosynthesis and light activation of ribulosebisphosphate carboxylase oxygenase in intact leaves of white clover (Trifolium repens L) J. Exp. Bot. 1984;35:147–156. [Google Scholar]
- Schnyder H., Mächler F., Nösberger J. Regeneration of ribulose 1,5-bisphosphate and ribulose 1,5-bisphosphate carboxylase/oxygenase activity associated with lack of oxygen inhibition of photosynthesis at low temperature. J. Exp. Bot. 1986;37:1170–1179. [Google Scholar]
- Schützendübel A., Polle A. Plant responses to abiotic stresses: heavy metal-induced oxidative stress and protection by mycorrhization. J. Exp. Bot. 2002;53:1351–1365. [PubMed] [Google Scholar]
- Shen J.B., Orozco E.M., Ogren W.L. Expression of the two isoforms of spinach ribulose 1,5-bisphosphate carboxylase activase and essentiality of the conserved lysine in the consensus nucleotide-binding domain. J. Biol. Chem. 1991;266:8963–8968. [PubMed] [Google Scholar]
- Stiborová M., Ditrichová M., BŘEzinová A. Effect of heavy metal ions on growth and biochemical characteristics of photosynthesis of barley and maize seedlings. Biol. Plant. 1987;29:453–467. [Google Scholar]
- Thormalley P.J., Vasak M. Possible role for metallothionein in protection against radiation-induced oxidative stress. Kinetics and mechanism of its reaction with superoxide and hydroxy radicals. Biochem. Biophys. Acta. 1985;827:36–44. doi: 10.1016/0167-4838(85)90098-6. [DOI] [PubMed] [Google Scholar]
- Van Assche F., Clijsters H. Effects of metals on enzyme activity in plants. Plant Cell Environ. 1990;13:195–206. [Google Scholar]
- Van Assche F., Ceulemans R., Clijsters H. Zinc mediated effects on leaf CO2 diffusion conductances and net photosynthesis in Phaseolus vulgaris L. Photosynth. Res. 1980;1:171–180. doi: 10.1007/BF00020596. [DOI] [PubMed] [Google Scholar]
- Waalkes M.P. Cadmium carcinogenesis in review. J. Inorg. Biochem. 2000;79:241–244. doi: 10.1016/s0162-0134(00)00009-x. [DOI] [PubMed] [Google Scholar]
- Wang Y.S., Roh K.S. The reverse effect of salicylic acid on Cd-induced growth, chlorophyll, and rubisco/rubisco activase in tobacco. J. Life Sci. 2012;22:778–787. [Google Scholar]
- Wang Z.Y., Snyder G.W., Esau B.D., Portis A.R., Jr., Ogren W.L. Species-dependent variation in the interaction of substrate-bound ribulose-1,5-bisphosphate carboxylase/oxygenase (rubisco) and rubisco activase. Plant Physiol. 1992;100:1858–1862. doi: 10.1104/pp.100.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wishnick M., Lane M.D. Ribulose diphosphate carboxylase from spinach leaves. Methods Enzy. 1971;23:570–577. [Google Scholar]
- Wu F.B., Chen F., Wei K., Zhang G.P. Effect of cadmium on free amino acid, glutathione and ascorbic acid concentrations in two barley genotypes (Hordeum vulgare L) differing in cadmium tolerance. Chemosphere. 2004;57:447–454. doi: 10.1016/j.chemosphere.2004.06.042. [DOI] [PubMed] [Google Scholar]



