Abstract
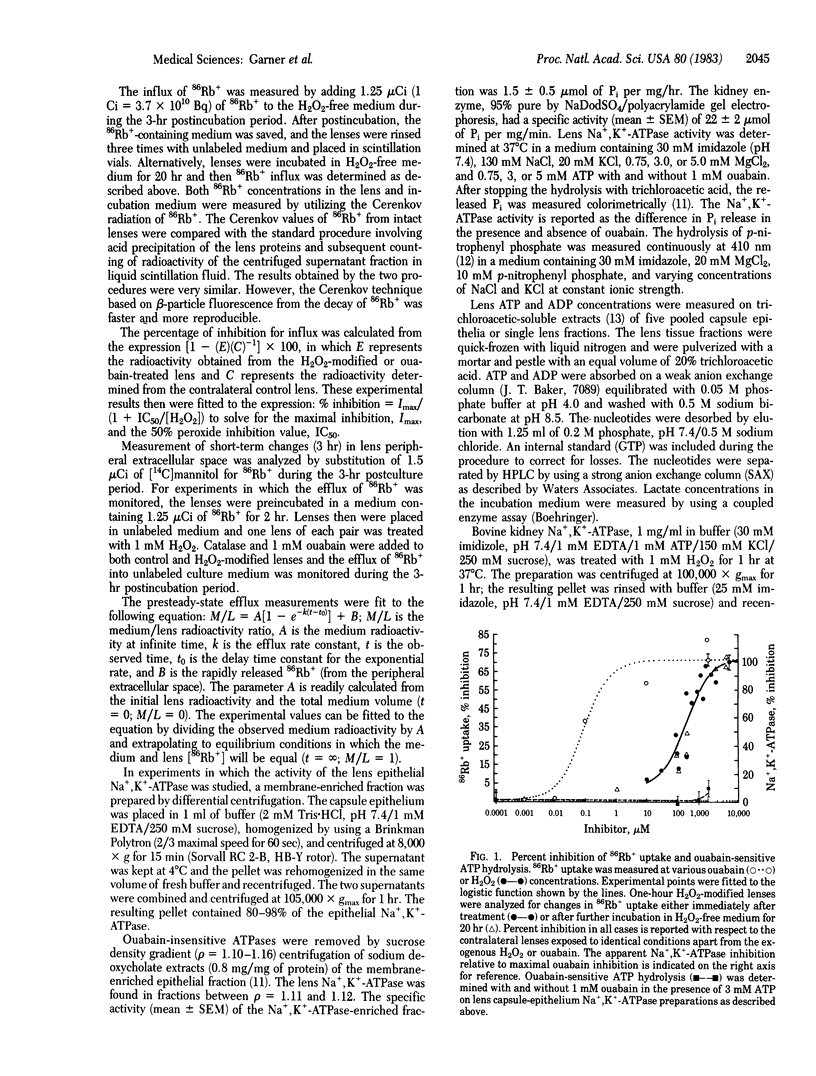
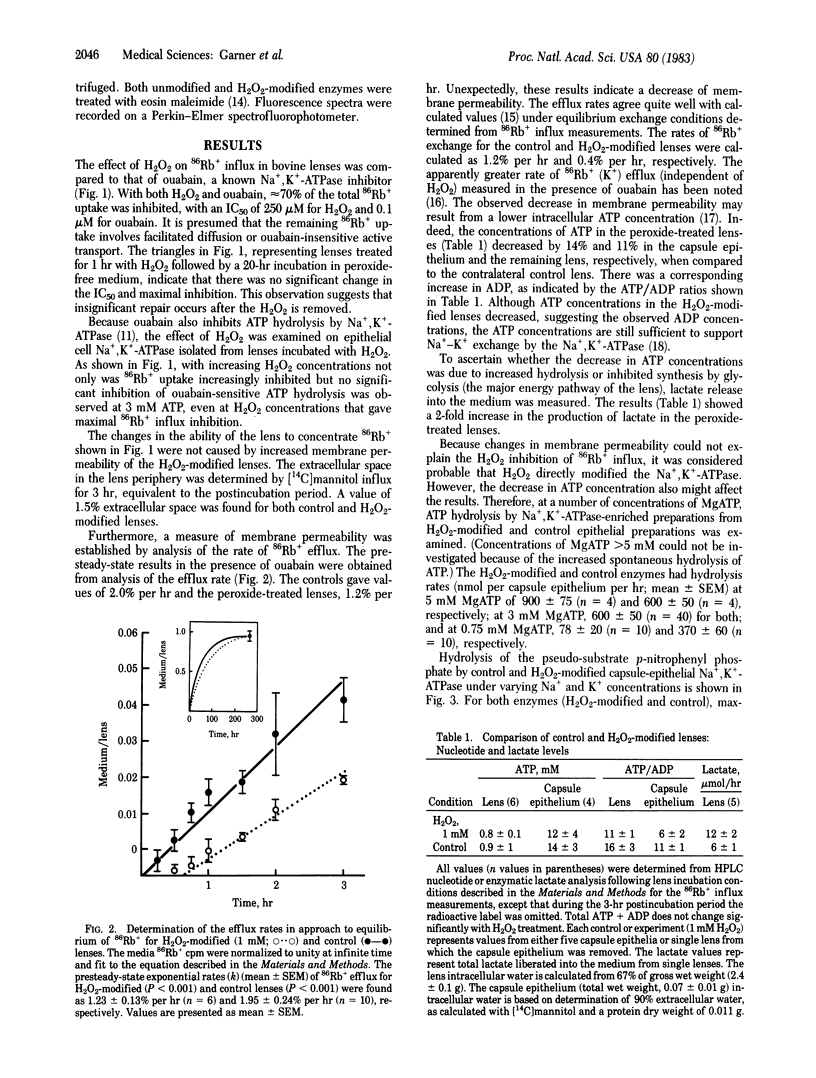
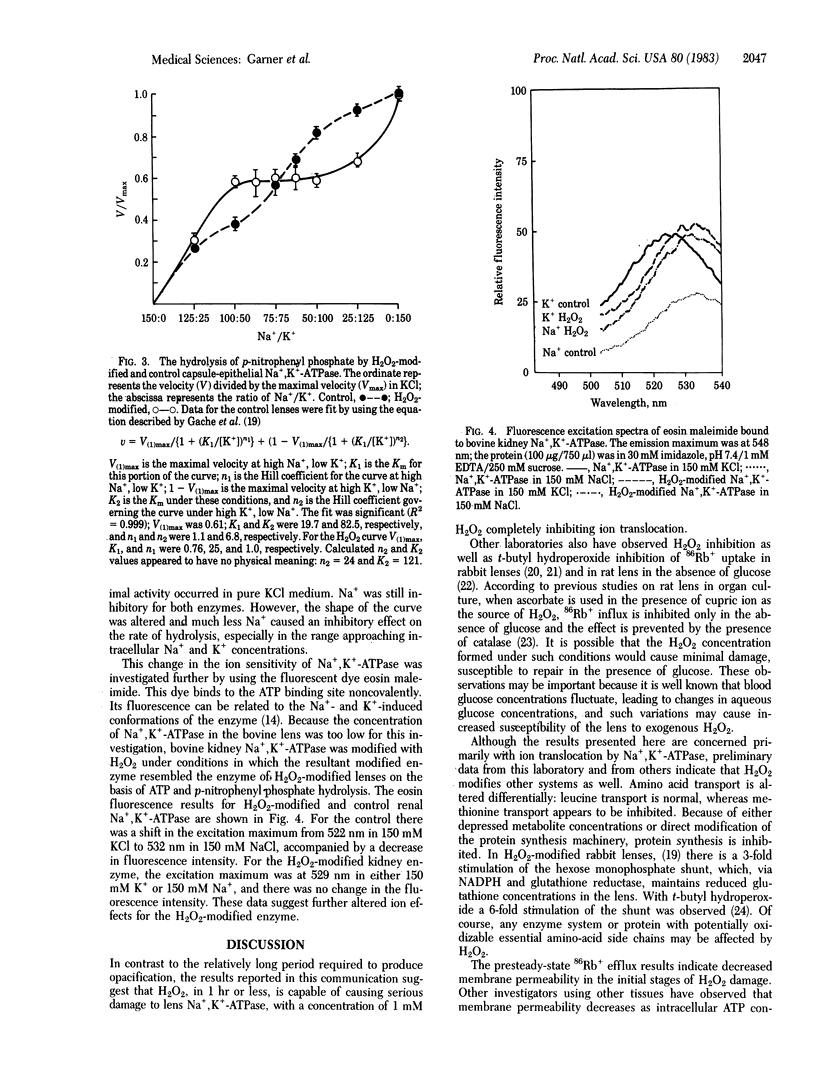
A 1-hr exposure of bovine lenses in organ culture to H2O2 concentrations in the range found in the aqueous fluid of patients with cataracts inhibits 86Rb+ influx. At 1 mM H2O2, complete inhibition was observed and further investigated. Membrane permeability is slightly decreased. Although lactate concentrations increase 2-fold, lens ATP concentrations decrease approximately equal to 10%, suggesting that glycolysis may be stimulated but ATP production is not able to keep up with the demand for energy. Examination of epithelial cell Mg2+-stimulated Na+,K+-ATPase isolated from the cultured lenses indicates H2O2-induced modification. At 5 mM MgATP, ATP hydrolysis is accelerated 30%; at 3 mM MgATP, hydrolysis is normal; and at 0.75 mM MgATP, it is inhibited 75%. p-Nitrophenyl phosphate hydrolysis and eosin maleimide binding indicate that K+ control of the enzyme is modified. Thus, a very early effect of H2O2 upon the lens, well before the formation of opacity, appears to be the uncoupling of Na+ and K+ transport from ATP hydrolysis.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cheng H. M., Chylack L. T., Jr, von Saltza I. Supplementing glucose metabolism in human senile cataracts. Invest Ophthalmol Vis Sci. 1981 Dec;21(6):812–818. [PubMed] [Google Scholar]
- Fukui H. N., Epstein D. L., Kinoshita J. H. Ascorbic acid effects on lens 86 rubidium transport. Exp Eye Res. 1973 Feb;15(2):249–253. doi: 10.1016/0014-4835(73)90126-7. [DOI] [PubMed] [Google Scholar]
- Fukui H. N. The effect of hydrogen peroxide on the rubidium transport of the rat lens. Exp Eye Res. 1976 Dec;23(6):595–599. doi: 10.1016/0014-4835(76)90217-7. [DOI] [PubMed] [Google Scholar]
- Garner M. H., Roy D., Rosenfeld L., Garner W. H., Spector A. Biochemical evidence for membrane disintegration in human cataracts. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1892–1895. doi: 10.1073/pnas.78.3.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giblin F. J., Chakrapani B., Reddy V. N. Glutathione and lens epithelial function. Invest Ophthalmol. 1976 May;15(5):381–393. [PubMed] [Google Scholar]
- Giblin F. J., McCready J. P., Reddy V. N. The role of glutathione metabolism in the detoxification of H2O2 in rabbit lens. Invest Ophthalmol Vis Sci. 1982 Mar;22(3):330–335. [PubMed] [Google Scholar]
- Giblin F. J., Nies D. E., Reddy V. N. Stimulation of the hexose monophosphate shunt in rabbit lens in response to the oxidation of glutathione. Exp Eye Res. 1981 Sep;33(3):289–298. doi: 10.1016/s0014-4835(81)80052-8. [DOI] [PubMed] [Google Scholar]
- Lane L. K., Potter J. D., Collins J. H. Large-scale purification of Na,K-ATPase and its protein subunits from lamb kidney medulla. Prep Biochem. 1979;9(2):157–170. doi: 10.1080/00327487908061681. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E. G., Fortes P. A. Inhibition of sodium and potassium adenosine triphosphatase by 2',3'-O-(2,4,6-trinitrocyclohexadienylidene) adenine nucleotides. Implications for the structure and mechanism of the Na:K pump. J Biol Chem. 1981 Mar 10;256(5):2357–2366. [PubMed] [Google Scholar]
- Patterson J. W. Effects of amino acid loading on lens amino acids, cations and water. Exp Eye Res. 1979 Jun;28(6):689–698. doi: 10.1016/0014-4835(79)90069-1. [DOI] [PubMed] [Google Scholar]
- Skou J. C., Esmann M. Eosin, a fluorescent probe of ATP binding to the (Na+ + K+)-ATPase. Biochim Biophys Acta. 1981 Oct 2;647(2):232–240. doi: 10.1016/0005-2736(81)90251-0. [DOI] [PubMed] [Google Scholar]
- Spector A., Garner W. H. Hydrogen peroxide and human cataract. Exp Eye Res. 1981 Dec;33(6):673–681. doi: 10.1016/s0014-4835(81)80107-8. [DOI] [PubMed] [Google Scholar]
- Spector A., Scotto R., Weissbach H., Brot N. Lens methionine sulfoxide reductase. Biochem Biophys Res Commun. 1982 Sep 16;108(1):429–434. doi: 10.1016/0006-291x(82)91884-8. [DOI] [PubMed] [Google Scholar]
- Thoft R. A., Kinoshita J. H. The rate of potassium exchange of the lens. Invest Ophthalmol. 1965 Oct;4(5):800–805. [PubMed] [Google Scholar]
- Wallick E. T., Lane L. K., Schwartz A. Biochemical mechanism of the sodium pump. Annu Rev Physiol. 1979;41:397–411. doi: 10.1146/annurev.ph.41.030179.002145. [DOI] [PubMed] [Google Scholar]


