Sir,
A plethora of newer anti-cancer drugs with novel mechanisms of action and less well-known side-effect profile has arrived.[1] Adjuvant chemotherapy in patients of solid organ and hematological malignancy with newer anti-cancer agents to maintain the remission has become the standard management protocol.[2] Rapidly growing cells are the collateral targets of chemotherapy, consequently the skin, hair follicles, and nail matrix are frequently affected by chemotherapy. We hereby report the cutaneous toxicity of multikinase inhibitors (MKI) and their management strategy with dose titration of anti-cancer agents, i.e., sorafenib and sunitinib.
Five patients with metastatic renal cell carcinoma with resection of involved kidney (mean age: 58.2 years; range: 43-66 years) were referred to our outpatient department from a tertiary cancer hospital or painful bullous lesions and erosions on palms, soles, and other weight-bearing areas. Out of the five patients, three patients were taking oral sorafenib 400 mg (2 × 200 mg tablets) twice daily and other two were receiving sunitinib 25 mg twice daily. The average period for development of skin toxicity was 3-6 weeks after starting the treatment. The demographic information of the patients regarding age, sex, type of malignancy, and chief dermatological complaints are summarized in Table 1. Mucosal involvement was not reported in any patient. Biopsy was obtained from a bulla in all the patients and from a callosity in two patients under local anesthesia after informed consent, fixed in 10% buffered formalin, embedded in paraffin and stained with hematoxylin and eosin (unnecessary) None of the patient gave history of excessive hair loss.
Table 1.
Clinical features of patients with cutaneous drug reaction secondary to anticancer agent (column for duration between start of drug and onset of skin symptoms; column for underlying malignancy not required as all were suffering from renal cell carcinoma - mentioned in starting of article)

Tender tense vesicles, bullae and erosions on palms, soles, and fingers were seen in all patients [Figure 1a and b], and two patients had a few bullae on ankles and knees. In patients with long-standing lesions, some of the pressure points showed painful yellowish callosities resembling circumscribed palmoplantar keratoderma. At one or two places, such callosity was seen to form the roof of the blister. Mucosal examination did not reveal any abnormality. Routine blood investigations including complete hemogram, serum biochemistry, and urinalysis were within normal reference limits.
Figure 1.

(a and b) Blister on erythematous base seen on volar aspect of palms and fingers
Histopathological examination of the lesion showed upper epidermal multi-loculated blister due to ballooning of keratinocytes [Figure 2]. In one of the biopsies, there was extensive ballooning change throughout the epidermis with necrosis and formation of sub-epidermal blister [Figure 3]. Biopsy form the long-standing callosity-like plaque showed irregular psoriasiform hyperplasia with thickened parakeratotic stratum corneum reflecting a sub-acute eczematous process [Figure 4].
Figure 2.
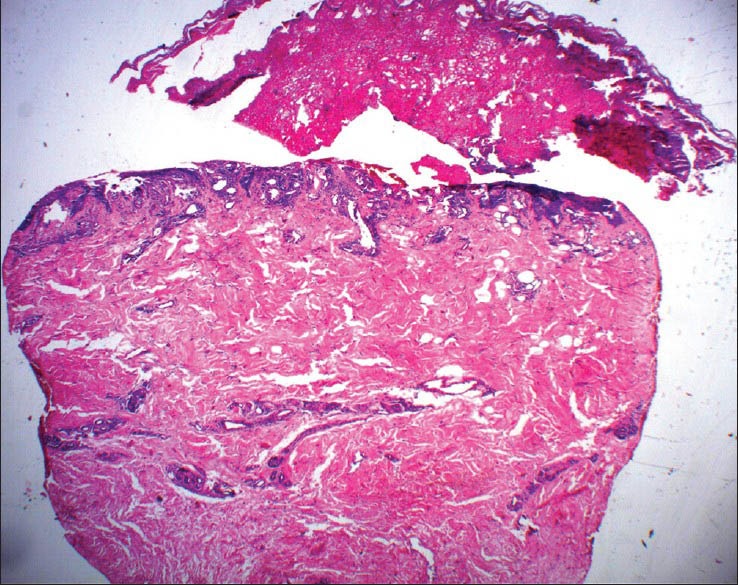
Scanner view of skin biopsy showing ballooning degeneration of the epidermis with lymphocytic infiltrate in the upper part of the dermis (H and E, ×10)
Figure 3.

Epidermis showing hyperplastic changes and broad parakerotosis with interspersed plasma globules and sparse superficial infiltrate of lymphocytes and eosinophils. Parakeratotic layer showing large plasma globules (arrow) with severe pyknotic eosinophils (H and E, ×20)
Figure 4.

Biopsy from callosity-like plaque showing non-specific changes regular acanthosis, broad parakerotosis suggesting a long-standing lesion (H and E, ×20)
All the patients were advised to avoid pressure on weight-bearing areas and were prescribed potent topical steroid clobetasol propionate (0.05%) ointment to be applied twice a day to affected areas until resolution. The patients were prescribed moisturizing creams and bland emollients for symptomatic relief.
Sunitinib and sorafenib are examples of a new class of anti-cancer agent namely, multi-targeted oral small molecule tyrosine kinase inhibitors, resulting in inhibition of tumor angiogenesis. Pharmacological studies have shown them to be selective inhibitor of vascular endothelial growth factor receptor (VEGFR)-2 and VEGFR-3, platelet-derived growth factor receptor (PDGFR)-β, Raf, FLT-3, and C- kit.[3]
Currently, sorafenib and sunitinib are approved for treatment of metastatic renal cell carcinoma and unresectable hepatocellular carcinoma.[4,5] Seborrheic dermatitis-like rash, pruritus, erythema, xerosis, stomatitis, subungual splinter hemorrhages, alopecia, modification of hair growth or pigmentation, skin discoloration, and hand-foot skin reaction have been reported in clinical trials of sorafenib and sunitinib.[6]
Out of all these, hand-foot skin reaction (HFSR; palmar-plantar erythrodysesthesia; acral erythema) is the most symptomatic, as it affects activities of daily living ADL in patients taking oral sorafenib and sunitinib. Other reported side-effects for sorafenib are keratoacanthoma[7,8] leukocytoclastic vasculitis,[9] squamous cell carcinoma,[10] and alopecia. None of our cases had any other cutaneous adverse effects. HFSR associated with sorafenib and sunitinib therapy affects friction and weight-bearing acral surfaces more focally than the classic hand-foot syndrome,[11] which has been reported with capecitabine[12] cytarabine, 5-fluorouracil, and methotrexate.[13] HFSR secondary to chemotherapy has been graded by National Cancer Institute - Cancer Therapy Evaluation Program under the initiative of ? (NCI- CTCAE v3.0) [Table 2].[14] (CTCAE expansion to be mentioned: Common Terminology Criteria for Adverse Events version 3.0).
Table 2.
Grading of hand-foot skin reaction and suggested management protocol (Quote reference for findings column as it is taken from)

From the above discussion, it is evident that grade 1 and grade 2 cutaneous reactions do not require discontinuation of treatment as majority of patient will fall in these two grades of reaction. Exact pathogenesis for hand-foot skin reaction due to MKI therapy is yet to be elucidated. Some authors have proposed direct cytotoxic effects of the drug through eccrine sweat glands correlating with acral location of this dermatoses.[15] Treatment strategy includes avoiding friction and weight, using thick cotton gloves and socks, potent topical steroid ointment mixed with moisturizing creams. For disabling pain, oral pregabalin or topical lignocaine (2%) has been advocated.[16]
Sunitinib is yet another multi-targeted tyrosine kinase inhibitor with anti-tumor and anti-angiogenic activities and inhibits VEGFR-2, stem cell factor receptor (c-KIT), fetal liver tyrosine kinase receptor-3, and PDGFR α and β.[17] Cutaneous side-effects include acral erythema and hair depigmentation, most likely due to a direct toxic effect and blockade of stem cell factor or c-kit signaling pathway. Seborrheic dermatitis is another side-effect associated with sunitinib.[18] Hair depigmentation is a known side-effect of sunitinib due to inhibition of melanocyte function through blockage of c-Kit signaling pathway.[19]
Knowledge of dermatological side-effects of newer anti-cancer drugs having molecular specificity will enable the dermatologist and primary care physician to tackle them more efficiently and will allow the medical oncologist to titrate the offending drug in tumor burdened patients. This will aid us in rational management of cutaneous side-effects with obviating the need of withdrawing these potentially life-prolonging medications.
REFERENCES
- 1.Kamil N, Kamil S, Ahmed SP, Ashraf R, Khurram M, Ali MO. Toxic effects of multiple anticancer drugs on skin. Pak J Pharm Sci. 2010;23:7–14. [PubMed] [Google Scholar]
- 2.Alley E, Green R, Schuchter L. Cutaneous toxicities of cancer therapy. Curr Opin Oncol. 2002;14:212–6. doi: 10.1097/00001622-200203000-00012. [DOI] [PubMed] [Google Scholar]
- 3.Mendel DB, Laird AD, Xin X, Louie SG, Christensen JG, Li G, et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: Determination of a pharmacokinetic/pharmacodynamic relationship. Clin Cancer Res. 2003;9:327–37. [PubMed] [Google Scholar]
- 4.van der Veldt AA, Haanen JB, van den Eertwegh AJ, Boven E. Targeted therapy for renal cell cancer: Current perspectives. Discov Med. 2010;10:394–405. [PubMed] [Google Scholar]
- 5.Biró K, Küronya Z. Recent advancements in the treatment of renal cell carcinoma – Focus on international guidelines. Magy Onkol. 2010;54:369–76. doi: 10.1556/MOnkol.54.2010.4.11. [DOI] [PubMed] [Google Scholar]
- 6.Bhojani N, Jeldres C, Patard JJ, Perrotte P, Suardi N, Hutterer G, et al. Toxicities associated with the administration of sorafenib, sunitinib, and temsirolimus and their management in patients with metastatic renal cell carcinoma. Eur Urol. 2008;53:917–30. doi: 10.1016/j.eururo.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 7.Kong HH, Cowen EW, Azad NS, Dahut W, Gutierrez M, Turner ML. Keratoacanthomas associated with sorafenib therapy. J Am Acad Dermatol. 2007;56:171–2. doi: 10.1016/j.jaad.2006.10.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jantzem H, Dupre-Goetghebeur D, Spindler P, Merrer J. Sorafenib-induced multiple eruptive keratoacanthomas. Ann Dermatol Venereol. 2009;136:894–7. doi: 10.1016/j.annder.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 9.Chung NM, Gutierrez M, Turner ML. Leukocytoclastic vasculitis masquerading as hand-foot syndrome in a patient treated with sorafenib. Arch Dermatol. 2006;142:1510–1. doi: 10.1001/archderm.142.11.1510. [DOI] [PubMed] [Google Scholar]
- 10.Dubauskas Z, Kunishige J, Prieto VG, Jonasch E, Hwu P, Tannir NM. Cutaneous squamous cell carcinoma and inflammation of actinic keratoses associated with sorafenib. Clin Genitourin Cancer. 2009;7:20–3. doi: 10.3816/CGC.2009.n.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee WJ, Lee JL, Chang SE, Lee MW, Kang YK, Choi JH, et al. Cutaneous adverse effects in patients treated with the multitargeted kinase inhibitors sorafenib and sunitinib. Br J Dermatol. 2009;161:1045–51. doi: 10.1111/j.1365-2133.2009.09290.x. [DOI] [PubMed] [Google Scholar]
- 12.Vasudevan B. An unusual case of capecitabine hyperpigmentation: Is hyperpigmentation a part of hand-foot syndrome or a separate entity? Indian J Pharmacol. 2010;42:326–8. doi: 10.4103/0253-7613.70401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nagore E, Insa A, Sanmartín O. Antineoplastic therapy-induced palmar plantar erythrodysesthesia (‘hand-foot’) syndrome. Incidence, recognition and management. Am J Clin Dermatol. 2000;1:225–34. doi: 10.2165/00128071-200001040-00004. [DOI] [PubMed] [Google Scholar]
- 14.National Cancer Institute. Common terminology criteria for adverse events v3.0. [Last accessed 2011 Feb 12]. Available from: http://www.ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf .
- 15.Lai SE, Kuzel T, Lacouture ME. Hand-foot and stump syndrome to sorafenib. J Clin Oncol. 2007;25:341–3. doi: 10.1200/JCO.2006.08.9565. [DOI] [PubMed] [Google Scholar]
- 16.Lacouture ME, Wu S, Robert C, Atkins MB, Kong HH, Guitart J, et al. Evolving strategies for the management of hand-foot skin reaction associated with the multitargeted kinase inhibitors sorafenib and sunitinib. Oncologist. 2008;13:1001–11. doi: 10.1634/theoncologist.2008-0131. [DOI] [PubMed] [Google Scholar]
- 17.Abrams TJ, Lee LB, Murray LJ, Pryer NK, Cherrington JM. SU11248 inhibits KIT and platelet-derived growth factor receptor beta in preclinical models of human small cell lung cancer. Mol Cancer Ther. 2003;2:471–8. [PubMed] [Google Scholar]
- 18.Yang CH, Lin WC, Chuang CK, Chang YC, Pang ST, Lin YC, et al. Hand-foot skin reaction in patients treated with sorafenib: A clinicopathological study of cutaneous manifestations due to multitargeted kinase inhibitor therapy. Br J Dermatol. 2008;158:592–6. doi: 10.1111/j.1365-2133.2007.08357.x. [DOI] [PubMed] [Google Scholar]
- 19.Moss KG, Toner GC, Cherrington JM, Mendel DB, Laird AD. Hair depigmentation is a biological readout for pharmacological inhibition of KIT in mice and humans. J Pharmacol Exp Ther. 2003;307:476–80. doi: 10.1124/jpet.103.052530. [DOI] [PubMed] [Google Scholar]


