Recent evidence points to a role for Rif1 (Rap1-interacting factor) in DNA replication and genomic stability; however, the mechanism by which Rif1 functions has remained elusive. Hiraga el al. now report that budding yeast Rif1 controls DNA replication genome-wide and describe how Rif1 opposes Dbf4-dependent kinase (DDK) function by directing Protein Phosphatase 1 (PP1)-mediated dephosphorylation of the MCM complex. PP1 interaction sites are evolutionarily conserved within the Rif1 sequence; thus, the authors propose that replication control by Rif1 through PP1 is a conserved mechanism.
Keywords: DNA replication, protein phosphorylation, protein kinase, protein phosphatase, PP1
Abstract
Initiation of eukaryotic DNA replication requires phosphorylation of the MCM complex by Dbf4-dependent kinase (DDK), composed of Cdc7 kinase and its activator, Dbf4. We report here that budding yeast Rif1 (Rap1-interacting factor 1) controls DNA replication genome-wide and describe how Rif1 opposes DDK function by directing Protein Phosphatase 1 (PP1)-mediated dephosphorylation of the MCM complex. Deleting RIF1 partially compensates for the limited DDK activity in a cdc7-1 mutant strain by allowing increased, premature phosphorylation of Mcm4. PP1 interaction motifs within the Rif1 N-terminal domain are critical for its repressive effect on replication. We confirm that Rif1 interacts with PP1 and that PP1 prevents premature Mcm4 phosphorylation. Remarkably, our results suggest that replication repression by Rif1 is itself also DDK-regulated through phosphorylation near the PP1-interacting motifs. Based on our findings, we propose that Rif1 is a novel PP1 substrate targeting subunit that counteracts DDK-mediated phosphorylation during replication. Fission yeast and mammalian Rif1 proteins have also been implicated in regulating DNA replication. Since PP1 interaction sites are evolutionarily conserved within the Rif1 sequence, it is likely that replication control by Rif1 through PP1 is a conserved mechanism.
DNA replication in eukaryotes initiates from multiple chromosomal loci called replication origins. Replication origins initiate at different times in S phase that are influenced by the transcriptional status of nearby chromatin, availability of proteins limiting for DNA replication, and chromosome context effects such as centromere or telomere proximity (Aparicio 2013; Mechali et al. 2013). Origin activation (or “firing”) requires two protein kinases: CDK (cyclin-dependent kinase) and DDK (Dbf4-dependent kinase). In the budding yeast Saccharomyces cerevisiae, DDK is composed of the catalytic subunit Cdc7 and its activator, Dbf4. Dbf4 is one of the factors that limits replication initiation and so plays a role in execution of the replication timing program (Mantiero et al. 2011; Tanaka et al. 2011). Dbf4 levels are controlled by cell cycle-regulated transcription and destruction and peak during S phase (Oshiro et al. 1999; Weinreich and Stillman 1999; Ferreira et al. 2000). Despite this tight cell cycle control, a low level of DDK activity exists during G1 phase, and Dbf4 protein is recruited to early origins (Katou et al. 2006; Tanaka et al. 2011). Preferential recruitment of Dbf4 may therefore be one of the key steps defining early origins.
Mcm4 is a subunit of the hexameric MCM complex, which is loaded onto replication origins during G1 phase. Phosphorylation of Mcm4 protein by DDK is a critical step in initiating replication, as it triggers recruitment of Cdc45 and GINS, a four-subunit protein complex, to create the CMG (Cdc45–MCM–GINS) complex that forms the active replicative helicase (Fu et al. 2011). The N-terminal domain of Mcm4 plays an inhibitory role in CMG assembly that is alleviated by DDK phosphorylation (Sheu and Stillman 2010). Either removal of this Mcm4 N-terminal domain or the introduction of phosphomimetic mutations is sufficient to bypass the requirement for DDK in CMG formation and DNA replication, identifying Mcm4 as the only DDK target whose phosphorylation is essential for DNA replication (Sheu and Stillman 2010).
Rif1 (Rap1-interacting factor 1) was originally identified as a telomeric chromatin component required for telomere length regulation in budding yeast through its physical interaction with Rap1 (Hardy et al. 1992; Shi et al. 2013). It has since been demonstrated that S. cerevisiae Rif1 suppresses activation of the DNA damage checkpoint near telomeres (Xue et al. 2011; Ribeyre and Shore 2012) and affects telomere replication time (Lian et al. 2011). The presence of Rif1 is evolutionarily conserved, but in other eukaryotes, Rif1 has been shown to play nontelomeric roles, such as directing the pathways used in DNA double-strand break repair and DNA recombination (Chapman et al. 2013; Di Virgilio et al. 2013; Escribano-Diaz et al. 2013). Recent studies have implicated the fission yeast (Schizosaccharomyces pombe) and mammalian Rif1 proteins in the regulation of DNA replication genome-wide. Deleting the S. pombe rif1+ gene suppresses the block to replication caused by mutations in DDK subunits (Hayano et al. 2012). Removal of S. pombe or mammalian Rif1 also derails the replication temporal program, affecting the order in which origins initiate replication (Cornacchia et al. 2012; Hayano et al. 2012; Yamazaki et al. 2012). Rif1 binds most strongly to telomeric regions within the fission yeast genome but can also be detected at hundreds of internal sites. The relationship between Rif1-binding sites and origins affected by the rif1Δ mutation is ill-defined, however, and the mechanism through which Rif1 affects origin replication initiation time and imposes the requirement for DDK has been unclear. Mammalian Rif1 moreover seems to be involved in large-scale chromatin organization, but again the mechanism is unclear (Cornacchia et al. 2012; Yamazaki et al. 2012). In budding yeast, a role for Rif1 in global control of DNA replication has not previously been reported.
Eukaryotic Rif1 proteins are evolutionarily divergent but do share some features, in particular HEAT (Huntingtin, elongation factor 3, Protein Phosphatase 2A, and yeast kinase TOR1) repeats as well as SILK and RVXF motifs (Xue et al. 2011; Sreesankar et al. 2012). HEAT is a tandem repeat of ∼50 amino acids found in a wide variety of eukaryotic proteins. HEAT repeats are proposed to form a “solenoid” domain functioning in protein–protein interactions (Andrade et al. 2001). SILK and RVXF motifs are conserved sequence signatures of Protein Phosphatase 1 (PP1)-interacting proteins that mediate binding to PP1 (Bollen et al. 2010). Consistent with the presence of PP1 interaction motifs, Rif1 was reported to interact physically with PP1 in budding yeast (Breitkreutz et al. 2010), but the physiological significance of PP1–Rif1 interaction has been unclear.
PP1 is a conserved Ser/Thr protein phosphatase with multiple functions in eukaryotes (Cohen 2002). Most eukaryotes contain several PP1 isoforms, but S. cerevisiae has a single PP1 encoded by the essential gene GLC7. PP1 proteins have indiscriminate phosphatase activity in vitro, and in vivo are directed by PP1 targeting proteins to physiologically relevant substrates or cellular locations. PP1 targeting proteins are diverse, generally showing no sequence similarity beyond the presence of at least one PP1 interaction (e.g., RVXF or SILK) motif (Wakula et al. 2003). These PP1 interaction motifs are also found in other PP1-interacting proteins, including PP1 substrates and inhibitory regulators (Bollen et al. 2010). For all such PP1-interacting proteins, physical interaction via PP1 interaction motifs is important for them to direct PP1 function in vivo.
As the sole S. cerevisiae PP1, Glc7 is essential for cell viability. Glc7 has been implicated in many cellular pathways, including glycogen metabolism (Feng et al. 1991), control of budding (Black et al. 1995), premeiotic DNA synthesis (Ramaswamy et al. 1998), mitotic control (Hisamoto et al. 1994; Black et al. 1995), and recovery from checkpoint arrest (Bazzi et al. 2010). Compared with protein kinases, little is known about the involvement of protein phosphatases in the regulation of DNA replication.
In this study, we show that the S. cerevisiae Rif1 protein controls DNA replication genome-wide and that Rif1 exerts this control through PP1-mediated dephosphorylation of the MCM complex early in the cell cycle. We confirm that Rif1 and Glc7 interact, implicating Rif1 as a previously unidentified PP1 substrate targeting subunit. Targeting of PP1 by Rif1 appears itself to be controlled by DDK through phosphorylation to inactivate the Rif1 PP1 interaction motifs. By both controlling and being controlled by DDK phosphorylation, Rif1 contributes to the sharp rise in DDK-mediated phosphorylation of Mcm4 that allows cells to begin replication.
Results
A rif1Δ mutant escapes from a cdc7 block and partially suppresses cdc7-1 temperature sensitivity
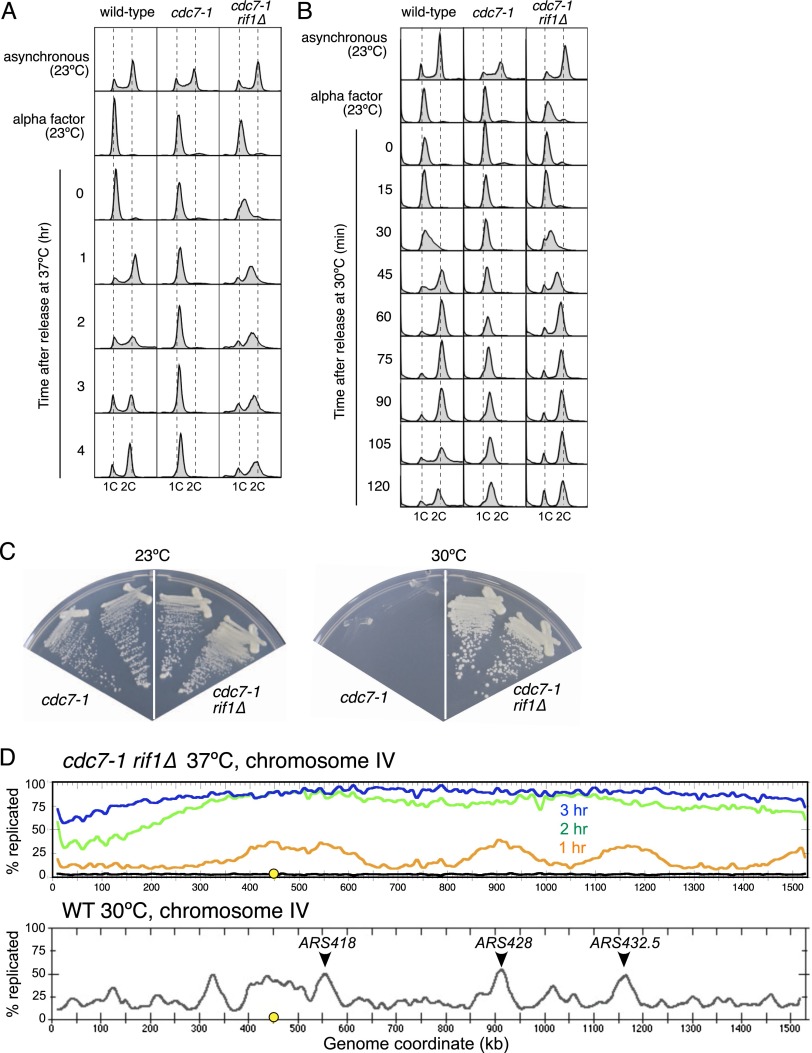
In the course of an attempt to synchronize rif1Δ cells using the cdc7-1 temperature-sensitive allele, we noticed that the cdc7-1 rif1Δ strain escapes a cdc7ts (37°C) block. To test the effect of RIF1 deletion on cdc7-1 arrest systematically, wild-type, cdc7-1, and cdc7-1 rif1Δ strains were arrested in G1 phase at 23°C using α-factor and then released at 37°C, and their DNA content was followed by flow cytometric analysis (Fig. 1A). Wild-type cells quickly transit S phase and enter a second cell cycle 2–3 h after removal of α-factor. DNA content did not change on release of the cdc7-1 mutant strain, confirming that these cells fail to begin DNA replication. In contrast, the majority of cdc7-1 rif1Δ cells began DNA replication (as demonstrated by their exit from the 1C peak) and, after 2–3 h, had accumulated as a near-2C population; however, the majority of cdc7-1 rif1Δ cells did not complete mitosis, suggesting that DNA replication was incomplete after 4 h at 37°C. When released at 30°C, the cdc7-1 rif1Δ strain (Fig. 1B, right) began and completed DNA replication with kinetics similar to a wild-type strain (Fig. 1B, left), suggesting that the cdc7-1 defect is largely suppressed at this temperature. Unlike the cdc7-1 strain, the cdc7-1 rif1Δ strain grows well at 30°C (Fig. 1C, right plate), indicating that the loss of Rif1 suppresses the cdc7-1 defect and allows cell cycle progression at 30°C.
Figure 1.
Deletion of RIF1 partially suppresses temperature-sensitive defects of cdc7-1. (A) Wild-type (AW31), cdc7-1 (RM14-3a), and cdc7-1 rif1Δ (HYLS1) strains were arrested with α-factor and released at 37°C. Cells were sampled, and DNA content was analyzed by flow cytometry. (B) As in A but released at 30°C. (C) Growth of cdc7-1 (RM14-3a) and cdc7-1 rif1Δ (HYLS1) strains at 23°C and 30°C. See also Supplemental Figure S1. (D, top panel) DNA replication profile of chromosome IV in the cdc7-1 rif1Δ strain (HYLS1) after release from α-factor for 1 h (orange), 2 h (green), and 3 h (blue) at 37°C. The black curve shows the extent of replication in G1 control. The Y-axis shows the percent replication. (Bottom panel) The gray curve shows the DNA replication profile of chromosome IV in wild-type cells in early S phase 40 min after release from α-factor (redrawn from McCune et al. 2008). Early initiating replication origins ARS418, ARS428, and ARS432.5 are indicated. Yellow circles indicate CEN4. See also Supplemental Figure S2.
RIF1 deletion cannot, however, completely bypass the need for DDK function at 37°C (data not shown), indicating that, at elevated temperatures, the replication that occurs in the rif1Δ strain depends on residual DDK activity. Moreover, deleting RIF1 does not suppress the lethality caused by deleting CDC7 or DBF4 (Supplemental Fig. S1). In summary, deletion of S. cerevisiae RIF1 can partially compensate for compromised DDK function but does not remove the requirement for DDK altogether.
Replication initiation within telomeric and nontelomeric chromosome regions enables the rif1Δ mutant to escape a cdc7ts block
S. cerevisiae Rif1 regulates telomere function (Hardy et al. 1992), and we previously showed that replication origins near telomeres are prematurely activated in a rif1Δ mutant (Lian et al. 2011). We therefore tested whether, in the cdc7-1 rif1Δ strain, replication depends on deregulated telomere-proximal origins or genome-wide initiation events. To distinguish between these possibilities, we used an isotopic labeling method (Alvino et al. 2007) to monitor the appearance of replicated DNA in the cdc7-1 rif1Δ strain released from α-factor at 37°C (Fig. 1D, top panel; Supplemental Fig. S2). Consistent with our flow cytometry analysis (Fig. 1A), chromosomal DNA replicated slowly and neared completion after 3 h. Importantly, 1 h after release, we observed zones of replicated DNA not only close to telomeres but also within internal chromosomal regions (e.g., chromosome IV 350–630 kb), implying that at 37°C in the cdc7-1 rif1Δ strain, replication begins from internal as well as telomere-proximal origins.
The chromosome regions that are first to be replicated in the cdc7-1 rif1Δ strain at 37°C generally correspond to chromosome domains that are early replicating in the normal S phase of a wild-type strain (Fig. 1D, bottom panel; McCune et al. 2008). This replication pattern indicates that the sites most prone to replication initiation at 37°C in cdc7-1 rif1Δ are likely to correspond to normally early initiating origins; for example, origins ARS418, ARS428, and ARS432.5 (arrowheads in Fig. 1D; Nieduszynski et al. 2007; Siow et al. 2012).
Rif1 suppresses Mcm4 phosphorylation
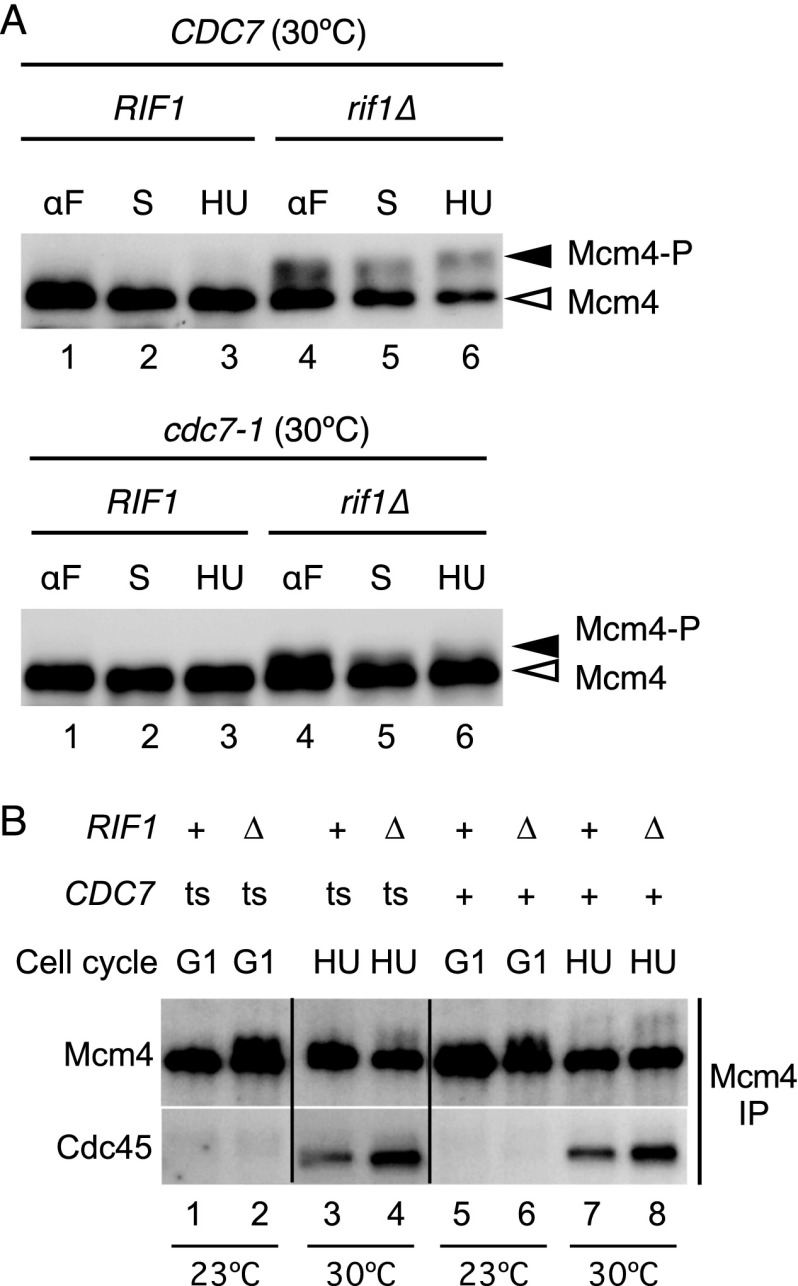
The data above suggest that Rif1 regulates DNA replication by counteracting DDK activity. Mcm4 is the major target of DDK in DNA replication control, so we investigated whether Rif1 affects the phosphorylation status of Mcm4. In whole-cell extracts from wild-type yeast, phosphorylated forms of a Flag-tagged Mcm4 protein are barely detectable because only a small fraction of Mcm4 protein is phosphorylated (Fig. 2A, top panel, lanes 1–3; Sheu and Stillman 2006). Deleting RIF1 increased the fraction of Mcm4 protein that displayed a slower mobility in SDS-PAGE analysis (Fig. 2A, top panel, lanes 4–6). This slower mobility form corresponds to hyperphosphorylated Mcm4, as it is lost upon phosphatase treatment (Supplemental Fig. S3A). Therefore, loss of Rif1 function leads to hyperphosphorylation of Mcm4 protein. Importantly, this hyperphosphorylation depends on DDK function, since the amount of Mcm4 showing lowest mobility is reduced if Cdc7 activity is compromised (in a cdc7-1 rif1Δ strain at 30°C) (Fig. 2A, bottom panel). Lack of Rif1 therefore does not substitute for the DDK requirement but rather compensates for reduced DDK activity—a result consistent with the observation that RIF1 deletion does not suppress lethality of cdc7Δ or dbf4Δ (Supplemental Fig. S1). In the rif1Δ strain, the majority of chromatin-associated Mcm4 appears to be hyperphosphorylated (Supplemental Fig. S3B), suggesting that Rif1 preferentially affects chromatin-bound Mcm4.
Figure 2.
Phosphorylation of Mcm4 protein is controlled by Rif1. (A) Phosphorylation of Mcm4-3Flag protein analyzed by Western blotting. (Top panel) CDC7 cells grown at 30°C were arrested with α-factor (αF) and released with or without hydroxyurea at 30°C. S-phase samples were taken at a mid-S-phase time point (S); hydroxyurea-arrested samples were taken 90 min after release (HU). (Bottom panel) cdc7-1 cells were grown and arrested initially at 23°C, followed by 1 h of incubation at 30°C before collection of α-factor sample and release at 30°C. Strains used were SHY361 (CDC7 RIF1), SHY363 (CDC7 rif1Δ), SHY386 (cdc7-1 RIF1), and SHY360 (cdc7-1 rif1Δ). (B) Western blot analysis of Cdc45 coimmunoprecipitated with Mcm4. Strains with Mcm4-Flag and Cdc45-HA were synchronized with α-factor at 23°C. G1 samples were collected, and then cultures were shifted to 30°C before being released into the medium containing hydroxyurea for 90 min at 30°C. Protein extracts were prepared, and Mcm4-Flag protein was immunoprecipitated as described in the Supplemental Material. Strains used were HYLS111 (CDC7 RIF1), HYLS113 (CDC7 rif1Δ), HYLS108 (cdc7-1 RIF1), and HYLS109 (cdc7-1 rif1Δ). See also Supplemental Figure S3.
Rif1 therefore restricts DDK-mediated phosphorylation of Mcm4 even during G1 phase in cells blocked with α-factor (Fig. 2A). DDK activity is generally low during G1 due to low Dbf4 levels (Oshiro et al. 1999; Weinreich and Stillman 1999; Ferreira et al. 2000), although the presence of some functional DDK during G1 phase has been demonstrated (Katou et al. 2006; Tanaka et al. 2011). The increase in Mcm4 phosphorylation caused by deleting Rif1 was not due to increased or precocious Dbf4 expression, as Western analysis revealed no change in Dbf4 levels in the rif1Δ mutant in either S phase (Supplemental Fig. S3C) or G1 phase (data not shown).
Next, we examined whether increased Mcm4 phosphorylation is productive for the DNA replication process. One consequence of DDK-mediated Mcm4 phosphorylation is Cdc45 recruitment by the MCM complex to form CMG, the active replicative DNA helicase (Gambus et al. 2006; Moyer et al. 2006). We tested whether elevated Mcm4 phosphorylation in the absence of Rif1 leads to increased CMG complex formation. In the rif1Δ strain, we found a significant increase in the amount of Cdc45 protein interacting with Mcm4 protein during S phase in both CDC7+ and cdc7-1 contexts (as assessed by analyzing Cdc45 coimmunoprecipitating with Mcm4) (Fig. 2B, cf. lanes 4,8 and 3,7, respectively). Deregulated Mcm4 phosphorylation in the absence of Rif1 therefore appears to allow increased CMG formation, potentially stimulating additional origin initiation events that could be related to S-phase deregulation in the absence of Rif1 (Lian et al. 2011; Xue et al. 2011; Cornacchia et al. 2012; Yamazaki et al. 2012). Mcm4–Cdc45 interaction was not detected in G1 phase in rif1Δ cells despite the fact that Mcm4 phosphorylation is increased. Presumably other cell cycle controls prevent premature Mcm4 phosphorylation from triggering aberrant DNA replication during G1 phase in the rif1Δ strain. In summary, Rif1 seems to regulate DNA replication by repressing Mcm4 phosphorylation.
Rif1 C terminus physically interacts with Dbf4
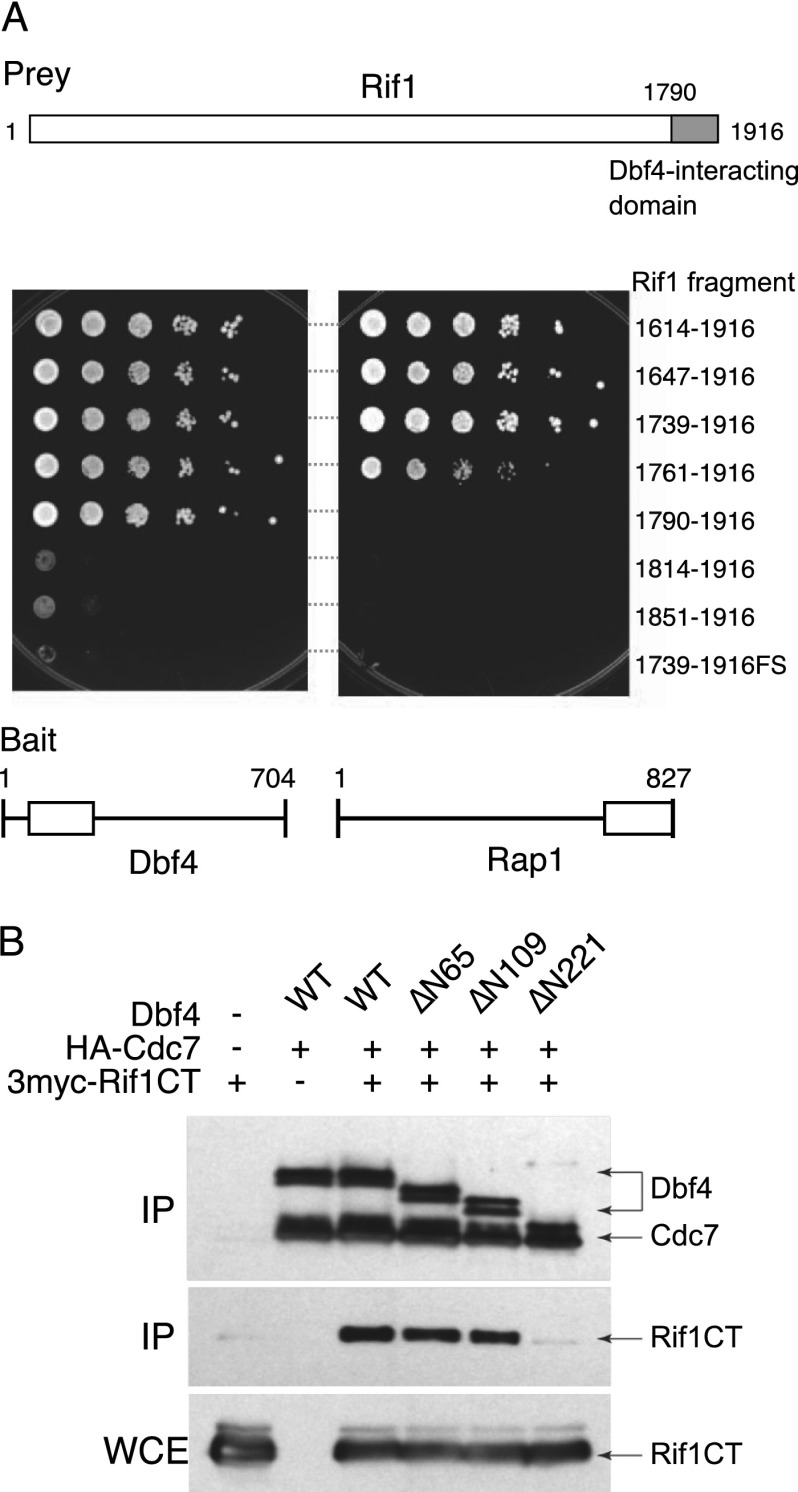
In investigating how Rif1 counteracts DDK function, we first considered the possibility that Rif1 binds to DDK directly and negatively regulates its kinase activity. A two-hybrid-based investigation of Dbf4 had revealed that it interacts with the C-terminal domain of Rif1. Further mapping identified Rif1 residues 1790–1916 as the minimal region required for interaction with a BRCT domain in the N-terminal region of the Dbf4 protein (Fig. 3A, left plate; Supplemental Fig. S4B). This 1790–1916 region overlaps the C-terminal Rif1 domain that interacts with Rap1 protein at telomeres. However, the minimal Rif1 domain required for Dbf4 interaction (residues 1790–1916) is shorter than that required for Rap1 interaction (residues 1761–1916) (Fig. 3A, right plate), suggesting that Rif1 interacts with Dbf4 and Rap1 through distinct molecular mechanisms.
Figure 3.
The C-terminal domain of Rif1 interacts with Dbf4. (A) Two-hybrid analysis of Rif1–Dbf4 and Rif1–Rap1 interactions using a HIS3 reporter. Bait plasmids contain the Dbf4 BRCT domain (amino acids 67–227; left panel) or the Rap1 C-terminal domain (amino acids 653–827; right panel). Prey plasmids contain a series of Rif1 C-terminal fragments as indicated at the right. 1739–1916FS represents a 1-nucleotide insertion causing a frameshift at position 1745. The gray-filled region in the Rif1 cartoon at the top illustrates the minimal region required for Dbf4 interaction. (B) Coimmunopurification of Rif1 protein with the Cdc7–Dbf4 complex. HA-tagged Cdc7, a series of full-length (wild-type [WT]) or N-terminally truncated Dbf4 proteins, and a myc-tagged Rif1 C-terminal fragment were coexpressed in insect Sf9 cells, followed by immunopurification of HA-Cdc7. Copurifying proteins were detected by Western blotting. As previously demonstrated, all three N-terminally truncated Dbf4 fragments interact with Cdc7 protein and stimulate its kinase activity (Gabrielse et al. 2006). See also Supplemental Figure S4.
To confirm this interaction biochemically, full-length HA-tagged Cdc7 protein, a series of truncated Dbf4 proteins, and the C-terminal fragment of Rif1 (residues 1735–1916; referred to as Rif1-CT) were coexpressed in insect cells. Cdc7 was immunoprecipitated from the cell extracts and tested for recovery of Dbf4 and Rif1-CT. We found that the Rif1-CT was recovered with Cdc7 and that this coprecipitation depended on the presence of Dbf4 with an intact BRCT domain (BRCT domain is residues 118–221 in Dbf4) (Fig. 3B; Supplemental Fig. S4A). This result was therefore consistent with the two-hybrid analysis, indicating that the C-terminal region of Rif1 can bind to DDK through an interaction with Dbf4.
A physical interaction between Rif1 and Dbf4 appeared to favor the suggestion that Rif1 restrains replication by binding DDK and repressing its activity. We found, however, that addition of purified Rif1-CT (fused to GST) does not affect in vitro kinase activity of DDK toward Mcm4 (Supplemental Fig. S4C). Moreover, overexpressing the Rif1 C-terminal domain in yeast did not affect growth of a cdc7-1 strain, the binding of Dbf4 to an origin, or Dbf4 binding to chromatin (data not shown), as tested using established assays (Dowell et al. 1994; Sheu and Stillman 2006). These results indicate that the Rif1 C terminus is not detrimental to DDK function, although, as we were unable to express or test GST-fused full-length Rif1, we cannot exclude the possibility that full-length Rif1 might be capable of repressing DDK activity.
Mapping of Rif1 domains required to counteract DDK
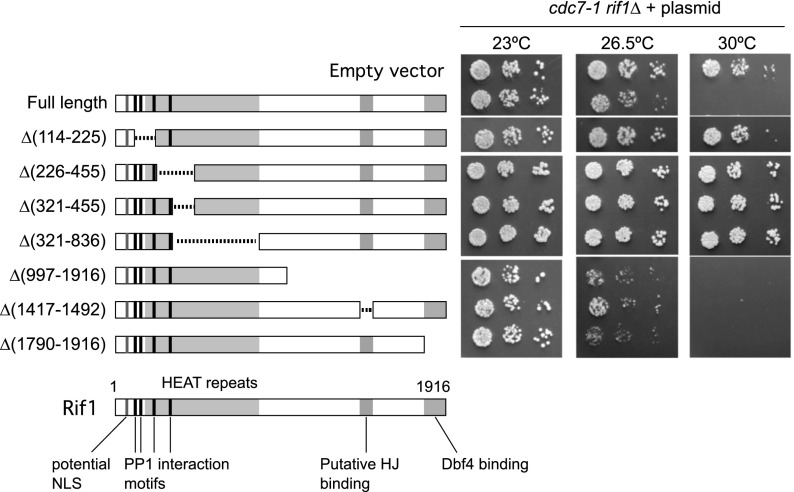
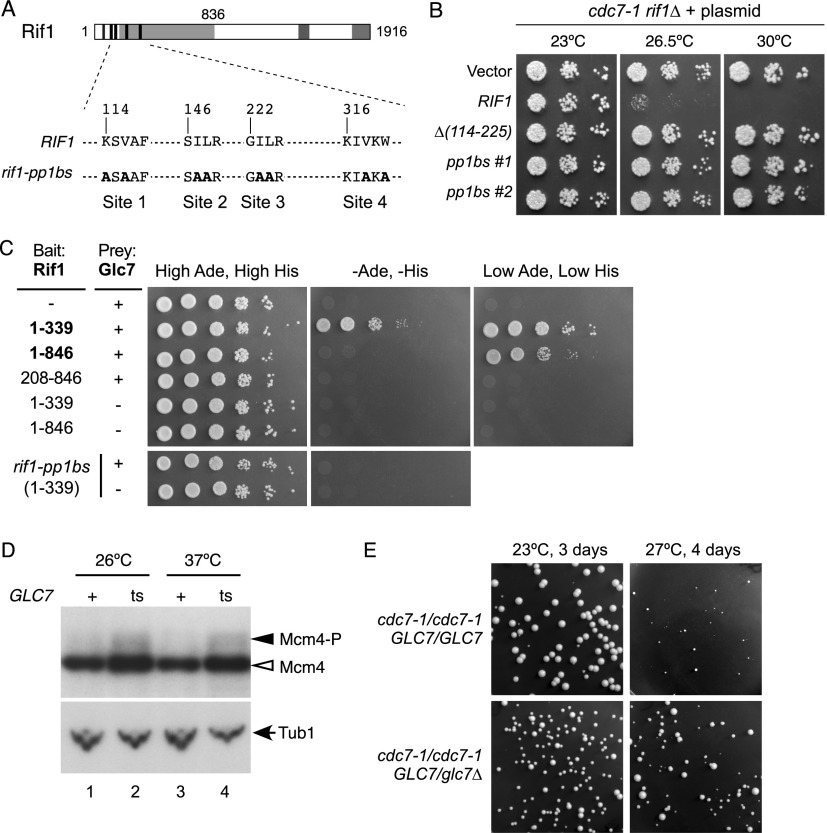
To identify domains of the Rif1 protein important for regulating DNA replication and DDK function, we constructed a series of RIF1 alleles with internal deletions or terminal truncations (Fig. 4). These constructs were designed to remove putative functional elements, including the C-terminal Dbf4-interacting region identified above, the HEAT repeats (Xu et al. 2010; Sreesankar et al. 2012), potential PP1 interaction motifs in the N-terminal domain (Sreesankar et al. 2012), and a region with weak homology with a putative Holliday junction (HJ)-binding domain in vertebrate Rif1 (Xu et al. 2010).
Figure 4.
Identifying Rif1 domains required for repression of DNA replication. Plasmids containing RIF1 alleles with an internal deletion or truncation were transformed into a cdc7-1 rif1Δ strain (SHY553). Serial dilutions of cultures were spotted onto SC-Ura plates and incubated at the indicated temperatures. See also Supplemental Figure S5.
We tested the effect of these constructs on growth of a cdc7-1 strain by introducing centromeric plasmids with the corresponding alleles into the cdc7-1 rif1Δ strain. If a mutated RIF1 allele retains the replication-repressive function of wild-type RIF1, it will prevent the cdc7-1 strain from growing at the restrictive temperature of 30°C (Fig. 4, full-length). If, on the other hand, the RIF1 allele cannot repress replication, it will permit cdc7-1 cells to grow at 30°C (Fig. 4, empty vector). We found that any deletion within its N-terminal half made Rif1 nonfunctional for replication repression (Fig. 4), including relatively small deletions covering the PP1 interaction motifs Δ(114–225) or the HEAT repeats Δ(321–455), suggesting that these features are important for the function of Rif1 in regulating DNA replication. The abundance of Rif1-Δ(114–225) was not reduced compared with wild-type Rif1 (Supplemental Fig. S5), so loss of repressive activity is not due to instability of this mutant protein.
To our surprise, the entire C-terminal half of Rif1, including the region mediating Dbf4 interaction, was found to be dispensable for replication repression (Fig. 4, 30°C panel, bottom three strains). In fact, rather than alleviating its repressive effect, removal of the C-terminal Dbf4-interacting domain appeared to make Rif1 even more repressive toward replication. Specifically, these deletions exacerbated the temperature sensitivity of the cdc7-1 mutant so that it grew very poorly even at 26.5°C, a temperature at which a cdc7-1 RIF1 strain can grow moderately well (Fig. 4, 26.5°C panel, cf. Δ[1790–1916] and Δ[997–1916] with full-length). This enhanced repressive effect suggests that the C-terminal region of Rif1 may down-regulate its repressive function in replication.
PP1 mediates DNA replication control by Rif1
Our deletion analysis highlighted a potential PP1-interacting region of Rif1 as important for replication repression. Sequences capable of PP1 interaction are called SILK and RVXF motifs, their consensus sequences more precisely expressed as (S/G)IL(K/R) and (K/R)x1(V/I)x2(F/W), where x1 may or may not be present. Two potential PP1 interaction motifs have been described in the N-terminal region of Rif1: KSVAF (at position 114) and SILR (at 146) (Sreesankar et al. 2012). We noticed two further potential PP1 interaction motifs in the N-terminal region: GILR (at 222) and KIVKW (at 316) (Fig. 5A). To investigate the significance of these PP1 interaction motifs, we constructed a RIF1 allele in which all of the four putative PP1 interaction motifs were mutated (rif1-pp1bs) (Fig. 5A). These mutations completely reproduced the effect of RIF1 deletion with respect to replication repression (Fig. 5B); that is, a cdc7-1 strain carrying rif1-pp1bs was able to grow at 30°C, like cdc7-1 rif1Δ or cdc7-1 rif1-Δ(114–225) (Fig. 5B). The rif1-pp1bs gene product was expressed at levels similar to wild-type Rif1 (Supplemental Fig. S5). Its phenotype demonstrates that the N-terminal PP1 interaction motifs are essential for Rif1 function in regulating DNA replication and strongly suggests that repression of replication by Rif1 is mediated through PP1.
Figure 5.
PP1 mediates DNA replication control by Rif1. (A) Protein sequence illustrating mutations in the four Rif1 PP1 interaction motifs (rif1-pp1bs). (B) Growth of the cdc7-1 rif1Δ strain carrying the rif1-pp1bs allele, tested as in Figure 4. #1 and #2 show different isolates. See also Supplemental Figure S5. (C) Two-hybrid analysis of Rif1–Glc7 interaction using ADE2 and HIS3 reporters. Bait plasmids contain the indicated Rif1 fragments (top panels) or a Rif1 fragment (amino acids 1–339) mutated at the PP1-binding sites as in A (bottom panels). Prey plasmid contains a Glc7 fragment (amino acids 1–287). Full-length Glc7 is 312 amino acids. Neither full-length Glc7 nor full-length Rif1 two-hybrid constructs were obtained during plasmid construction. See also Supplemental Figure S6. (D) Mcm4 phosphorylation status in the GLC7 (SHY557) and glc7-10 (SHY559) strains, analyzed by Western blotting. Strains were grown at 26°C and arrested with α-factor, and then, after further incubation for 1 h at either 26°C or 37°C, whole-cell extracts were prepared. (E) Comparison of growth of cdc7-1 diploid strains either homozygous (above) or heterozygous (below) for GLC7 at 23°C and 27°C. See also Supplemental Figure S6.
Rif1 has previously been reported to interact physically with Glc7, the sole PP1 in budding yeast (Breitkreutz et al. 2010). We sought to confirm this interaction using a Rif1–Glc7 two-hybrid assay. Growth on selective −adenine −histidine medium (Fig. 5C, top middle panel) demonstrated interaction between a slightly truncated Glc7 prey fragment (amino acids 1–287) and a Rif1 bait fragment containing the PP1 interaction motifs (amino acids 1–339). A weaker interaction was observed between Glc7 and a longer Rif1 fragment (amino acids 1–846), as indicated by growth under less stringent selection conditions (low levels of adenine and histidine) (Fig. 5C, right panel). Mutating the Rif1 PP1 interaction motifs prevented the two-hybrid interaction, as expected if Rif1 directly recruits Glc7 protein (Fig. 5C, bottom middle panel; Supplemental Fig. S6A). Our two-hybrid analysis therefore confirmed interaction between Rif1 and Glc7 protein domains, dependent on the Rif1 PP1 interaction motifs.
On the basis of our results, we hypothesized that Rif1 represses DNA replication through PP1, counteracting DDK by directing Glc7-mediated dephosphorylation of the MCM complex. If this model is correct, Glc7 will be required to restrict phosphorylation of Mcm4 protein, in particular during G1 phase, when the effect of the rif1Δ mutation is especially marked. Whole-cell extracts were prepared from α-factor-arrested glc7-10 cells (Andrews and Stark 2000), and phosphorylation of Mcm4 protein was compared with wild-type cells. Since glc7-10 is temperature-sensitive for growth, we prepared extracts from cells grown at the permissive temperature (26°C) and after shift for 1 h to the restrictive temperature (37°C). Mcm4 phosphorylation is increased in glc7-10 cells (Fig. 5D, lanes 2,4), demonstrating that PP1 plays a role in suppressing Mcm4 phosphorylation during G1 phase. Increased Mcm4 phosphorylation was seen at both 26°C and 37°C, consistent with the observation that, even at permissive temperature, the glc7-10 allele compromises PP1 function and results in slow growth (data not shown).
If Glc7 acts to reverse DDK-mediated phosphorylation of the MCM complex, then reducing levels of active Glc7 might alleviate the growth defect of a cdc7-1 strain, similar to the rif1Δ mutation. To test this possibility, we examined the effect of removing one copy of the GLC7 gene in a cdc7-1/cdc7-1 diploid background. The cdc7-1/cdc7-1 GLC7+/glc7Δ heterozygote grew better than cdc7-1/cdc7-1 GLC7+/GLC7+ at 27°C (Fig. 5E), indicating that reduced Glc7 levels can partially compensate for compromised DDK activity. Conversely, we would predict that increased Glc7 phosphatase activity might interfere with replication when DDK activity is limited. We found that even a mild increase in Glc7 levels, caused by the introduction of a centromeric plasmid bearing the GLC7 gene, compromised growth of a cdc7-1 strain at 26°C (Supplemental Fig. S6B), although growth of a CDC7+ strain was unaffected by the same plasmid. The detrimental effect of additional Glc7 depended on the presence of Rif1, since the GLC7 plasmid did not interfere with growth of the cdc7-1 rif1Δ mutant at temperatures where its growth is marginal (30°C–32°C) (Supplemental Fig. S6C). Overall, these tests confirmed that the cdc7-1 mutant, whose growth is limited by available DDK, is extremely sensitive to Glc7 phosphatase levels but only if Rif1 is present. These results therefore support the suggestion that Rif1 binds Glc7 and directs it to dephosphorylate Mcm4.
Rif1–PP1 interaction may be regulated by DDK phosphorylation
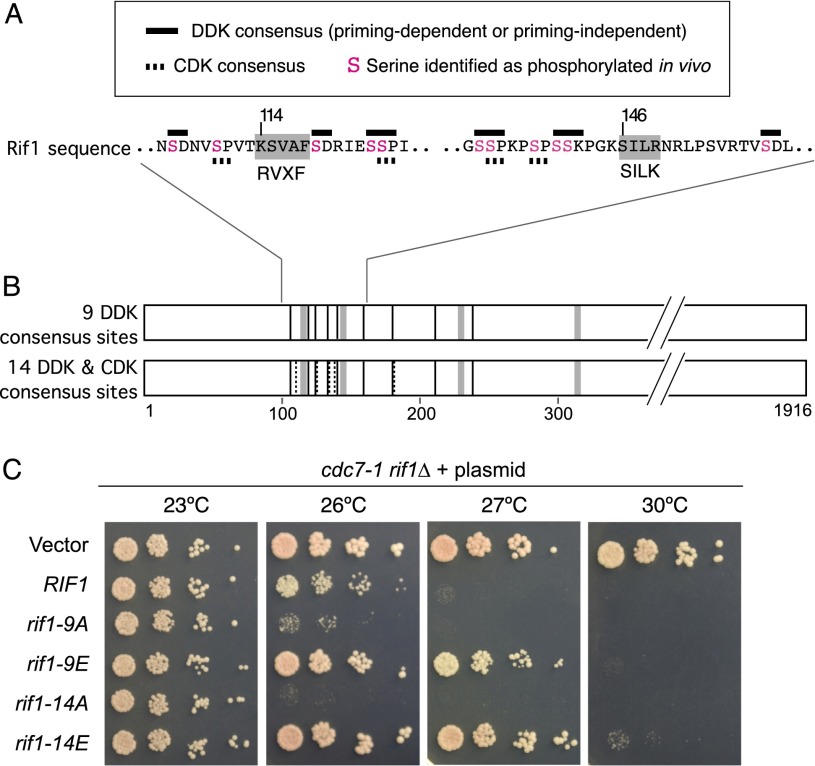
The Rif1 C-terminal domain can interact with Dbf4 (Fig. 3), but the Rif1 C terminus is dispensable for replication repression and apparently plays a negative role that restricts Rif1 function (Fig. 4). We considered whether the physical interaction between Rif1 and Dbf4 might reflect the fact that Rif1 is a substrate of DDK rather than a regulator of DDK activity. There are nine potential DDK phosphorylation sites between amino acids 100 and 250 in the N-terminal domain of Rif1, six of which are clustered around the PP1-binding motifs at 114 and 146 (Fig. 6A,B; Sasanuma et al. 2008; Wan et al. 2008). The DDK consensus target sequence is S or T followed by a negatively charged residue, D or E. DDK can also carry out “priming-dependent” phosphorylation when the target S or T is followed by a phosphorylated S or T (Randell et al. 2010). DDK phosphorylates a number of such priming-dependent sites on Mcm4, with priming phosphorylations mediated by CDK or Mec1. Five potential CDK phosphorylation sites exist in this region of Rif1 (Fig. 6A,B).
Figure 6.
Phosphorylation at DDK consensus sequences regulates replication repression by Rif1. (A) The sequence surrounding the first two Rif1 RVXF and SILK motifs (shaded gray), with DDK target consensus sequences indicated by black bars, and CDK target consensus sequences indicated by broken bars. Serine residues identified as phosphorylated in vivo are shown in pink. (B) The top Rif1 cartoon illustrates the locations of the nine potential DDK target sites mutated. Vertical black lines indicate the nine potential DDK phosphorylation sites between 100 and 250 amino acids in the Rif1 sequence. Vertical gray bars indicate PP1 interaction motifs 1–4. The bottom Rif1 cartoon illustrates the locations of the 14 potential DDK and CDK target sites mutated. Vertical dashed lines indicate consensus CDK phosphorylation sites, with other annotations as above. See also Supplemental Figure S7. (C) Plasmids containing rif1-9A, rif1-9E, rif1-14A, or rif1-14E alleles mutated at the sites shown in B were transformed into cdc7-1 rif1Δ strain (SHY553). Serial dilutions of cultures were spotted onto SC-Ura plates and incubated at the indicated temperatures.
Proteomic analysis of Rif1 protein purified from yeast cells (A Sridhar and AD Donaldson, in prep.) showed that all six of the potential DDK sites marked in Figure 6A are indeed phosphorylated in vivo (phospho-serine residues marked in pink). Of particular interest is phosphorylated residue Ser119, immediately adjacent to the KSVAF motif at position 114 [peptide SVAFS(ph)DRIESSPIYR was identified with a MASCOT score of 47.62, Ser119 being the phosphorylated residue with a probability of 0.78]. Phosphorylation close to an RVXF motif has been shown to disrupt PP1-interacting function (Kuntziger et al. 2006). These observations suggested that interaction between Rif1 and PP1 may be negatively regulated by DDK phosphorylation. To test this possibility, we generated alleles of Rif1 in which either the nine DDK sites between amino acids 100 and 250 or all 14 CDK and DDK sites (Fig. 6B) were substituted with nonphosphorylatable alanine or phosphomimetic glutamate residues, creating rif1-9A, rif1-9E, rif1-14A, and rif1-14E alleles (sequences shown in Supplemental Fig. S7A). If phosphorylation is required to down-regulate the Rif1–Glc7 interaction, then we would predict that the rif1-9A and rif1-14A gene products will constitutively target PP1 activity to counteract DDK and will therefore be repressive toward replication. As predicted, the rif1-9A and rif1-14A alleles were hyperrepressive toward growth of the cdc7-1 strain (Fig. 6C), showing a phenotype resembling the rif1-Δ(1790–1916) truncation mutant (Fig. 4). This hyperrepressive effect is not caused by increased protein levels (Supplemental Fig. S7B). Conversely, rif1-9E and rif1-14E alleles alleviated the cdc7-1 growth defect, partially mimicking the effect of rif1Δ by allowing robust growth at 27°C, consistent with compromised interaction between PP1 and the phosphorylated form of Rif1. Neither rif1-9E nor rif1-14E permitted strong growth of the cdc7-1 strain at 30°C (unlike rif1Δ), perhaps because glutamate substitution does not fully mimic phosphorylation or because some other aspect of control is missing. For both nonphosphorylatable and phosphomimetic alleles, mutating the nine DDK consensus sites resulted in a clear phenotype, which was mildly enhanced by additionally mutating the CDK sites. Phosphorylation of Rif1 by DDK therefore appears to be largely sufficient for regulating interaction with PP1, with phosphorylation by CDK additionally making a contribution. In summary, the results of our investigation suggest a model in which Rif1 represses DNA replication by directing PP1 to dephosphorylate the MCM complex (illustrated in Fig. 7A), with the Rif1–PP1 interaction itself likely to be regulated by DDK phosphorylation.
Figure 7.
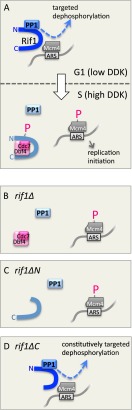
Model for the role of Rif1 as a PP1 substrate targeting subunit. (A) Proposed effect of Rif1 and PP1 on Mcm4 phosphorylation state during G1 phase (top) and S phase (bottom). (B–D) Model for the effects of Rif1 mutations on Mcm4 phosphorylation in late G1 phase. See the text for discussion.
Discussion
Here we demonstrated that budding yeast Rif1 is involved in regulating DNA replication genome-wide, consistent with findings in fission yeast and mammalian cells and suggesting that DNA replication control by Rif1 is conserved through eukaryotes (Cornacchia et al. 2012; Hayano et al. 2012; Yamazaki et al. 2012). Rif1 restrains replication by counteracting the action of DDK, and loss of Rif1 partially compensates for impaired DDK function in a cdc7-1 mutant strain. In particular, we found that deleting Rif1 increases Mcm4 phosphorylation levels and concomitantly alleviates the temperature sensitivity in growth of cdc7-1 cells. Depletion of Rif1 leads to increased Cdc7-mediated phosphorylation of Mcm4 protein even in G1 phase, when DDK activity is limited. PP1-binding motifs in the Rif1 N-terminal region are essential for replication repression, implicating PP1 in the mechanism. We confirmed an earlier report that Rif1 can interact physically with Glc7, the S. cerevisiae PP1 (Breitkreutz et al. 2010). Reduced PP1 levels alleviate the cdc7-1 growth defect, and PP1 appears to act in the same pathway as Rif1, since cells with reduced PP1 activity also show elevated Mcm4 phosphorylation.
What is the relationship between Rif1 and PP1 in replication control? PP1 has little innate substrate specificity and requires a substrate targeting subunit to impose specificity and direct it to cellular targets (Bollen et al. 2010). We propose that Rif1 represents a previously unidentified PP1 substrate targeting subunit that binds Glc7 and directs it to dephosphorylate Mcm4, thereby countering the action of DDK (Fig. 7A). We suggest that during G1 phase of the cell cycle, when DDK activity is low, Glc7 is recruited by the PP1-interacting motifs in the Rif1 N terminus and directed to dephosphorylate Mcm4, reversing any precocious DDK-mediated phosphorylation (Fig. 7A, top panel). In this study, we examined Mcm4 as the best-characterized DDK substrate and the only one essential for replication initiation. It seems likely, however, that Rif1 may also target Glc7 to prevent premature phosphorylation of other DDK substrates, in particular other MCM subunits (Bruck and Kaplan 2009). The simplest interpretation of our data is that PP1 directly dephosphorylates Mcm4, but we cannot exclude the possibility that the effect is indirect; for example, through PP1-mediated activation of a different phosphatase.
Our investigation also suggested how replication repression by Rif1 may be switched off as cells enter S phase. When Dbf4 levels rise and DDK becomes activated, the rate of Mcm4 phosphorylation will be increased, promoting origin activation (P-Mcm4 in Fig. 7A, bottom panel). We propose that, as cells enter S phase, Dbf4 also binds the C terminus of Rif1 (Fig. 3), recruiting Cdc7 and causing phosphorylation of the cluster of DDK target sites in the Rif1 N terminus, leading to inactivation of the PP1-binding motifs (Rif1-P in Fig. 7A, bottom panel). As a result, Glc7 is released from Rif1 and no longer directed to dephosphorylate Mcm4, favoring the buildup of DDK-mediated Mcm4 phosphorylation and consequent origin activation. We identified multiple phosphorylated serine residues within DDK target consensus sequences in the PP1-interacting region of Rif1 (Sasanuma et al. 2008; Wan et al. 2008). One such phosphorylation site (Ser119) is immediately adjacent to an RVXF PP1 interaction motif (KSVAF; 114–118) and corresponds to a priming-independent DDK consensus. Phosphorylation adjacent to an RVXF motif has been reported to disable PP1 binding (Kuntziger et al. 2006), and the effects of nonphosphorylatable and phosphomimetic Rif1 mutants support the notion that Rif1–Glc7 interaction is down-regulated by DDK phosphorylation. Such a dual mechanism—in which DDK both phosphorylates Mcm4 and down-regulates an Mcm4 phosphatase—would reinforce the switch from low to high DDK activity as cells pass from G1 into S phase, ensuring that DDK targets undergo a quick and robust shift from “hypophosphorylated” to “hyperphosphorylated” status.
Effects of mutating Rif1 domains
The effects of the various mutant constructs support our model. We propose that, when RIF1 is deleted, Glc7 can no longer dephosphorylate Mcm4. As a consequence, any premature phosphorylation that occurs cannot be reversed and therefore accumulates, predisposing cells and origins to replication initiation (Fig. 7B). Our model is supported by the observation that deleting RIF1 alleviates the defects of cdc7-1 in particular, since Rif1 exacerbates effects of low DDK activity by promoting the reversal of DDK phosphorylation. It is therefore as expected that removing Rif1 is beneficial in the cdc7-1 context. In fact, a cdc7-1 strain is probably especially sensitive to the presence of Rif1, since limited DDK activity may also cause Rif1 itself to remain unphosphorylated so that Glc7 is constitutively associated with Rif1 and targeted to dephosphorylate Mcm4. Our model therefore explains why deleting Rif1 is particularly effective in ameliorating cdc7-1 temperature sensitivity.
Removal of the N-terminal domain of Rif1 or mutation of the PP1 interaction motifs causes effects similar to the deletion mutant rif1Δ. Such a mutated Rif1 is unable to direct Glc7 to dephosphorylate DDK substrates, relieving cdc7-1 temperature sensitivity (Figs. 4, 5A,B, 7C) in a similar way to rif1Δ.
Removal of the Rif1 C-terminal domain, in contrast, had a mild but reproducible negative impact on cdc7-1 growth at semipermissive temperature (Fig. 4). This phenotype also supports our model, which predicts that the rif1ΔC mutant is unable to recruit DDK, as it lacks the Dbf4-interacting domain (Fig. 7D). As a result, the Rif1 N-terminal domain may remain unphosphorylated so that Glc7 is targeted to dephosphorylate DDK substrates throughout the cell cycle. Nonphosphorylatable rif1-9A and rif1-14A mutations caused an effect similar to rif1ΔC, consistent with their constitutive targeting of PP1 to dephosphorylate Mcm4. Cells with wild-type Cdc7 can presumably accumulate sufficient DDK to overwhelm such constitutive dephosphorylation activity, but defects caused by limiting DDK will be accentuated (Fig. 7D), consistent with our observation that rif1ΔC exacerbates cdc7-1 temperature sensitivity.
Our deletion analysis also indicated that the conserved HEAT repeats in the N-terminal half of Rif1 are required for replication repression (Fig. 4). HEAT repeats form a “solenoid” domain that mediates protein–protein interactions (Andrade et al. 2001). We suspect that the HEAT repeats may be involved in directing Glc7 to the appropriate targets, including the MCM complex. Mcm4 itself contains two RVXF motifs (positions 148 and 343), which may also contribute to Glc7 recruitment.
Backup S-phase controls and regulation of replication timing
A rif1Δ mutant does not begin replication during α-factor arrest despite the fact that Mcm4 is already hyperphosphorylated at this cell cycle stage (Fig. 2A). This control presumably reflects the fact that multiple events are required for replication initiation so that the precocious buildup of DDK-mediated MCM phosphorylation alone cannot trigger replication. In particular, CDK-mediated phosphorylation of Sld2 and Sld3 is required for origin initiation (Tanaka et al. 2007; Zegerman and Diffley 2007). It will be of interest to test whether removing Rif1 causes replication to initiate in α-factor in the context of mutated Sld2 and Sld3 variants that bypass the requirement for CDK to initiate replication (Tanaka et al. 2007; Zegerman and Diffley 2007).
Previous investigations showed that deleting S. pombe rif1+ suppresses mutations in DDK and that S. pombe and mammalian Rif1 affect genome-wide replication timing (Cornacchia et al. 2012; Hayano et al. 2012; Yamazaki et al. 2012). A previous study of the S. cerevisiae rif1Δ replication program showed a shortened interval between replication of early and late sequences, with telomeric origins in particular replicating aberrantly early (Lian et al. 2011). Loss of Rif1-mediated targeting of PP1 to dephosphorylate Mcm4 could conceivably cause a derailed replication program, since the level of DDK-mediated MCM phosphorylation is one of the factors likely to affect the initiation time of specific origins (Mantiero et al. 2011; Tanaka et al. 2011).
Spatial localization of Rif1
The effect of S. pombe Rif1 on origins is related to the proximity of chromosomal binding sites (Hayano et al. 2012), but intranuclear spatial localization may also be important (Yamazaki et al. 2012). S. cerevisiae Rif1 is reported to be localized at the nuclear periphery through association with peripherally localized telomere clusters (Gotta et al. 1996; Hiraga et al. 2008) and by palmitoylation-mediated nuclear envelope anchoring (Park et al. 2011). It will be interesting to study whether localization of Glc7 is affected by Rif1 and whether the spatial localization of Rif1 is related to its impact on replication origin initiation. During mitosis, Glc7 is localized by regulatory subunits to counteract the activity of Ipl1 kinase (Pinsky et al. 2006).
Evolutionary conservation of PP1 interaction motifs in Rif1
PP1 interaction motifs exist in Rif1 proteins from yeasts through to higher eukaryotes (Supplemental Fig. S7C) despite the generally limited conservation of Rif1 protein sequence. S. pombe Rif1 contains RVXF and SILK motifs in its N-terminal domain, like S. cerevisiae Rif1 (Supplemental Fig. S7C), even though the overall similarity of this region is low. The conservation raises the possibility that S. pombe Rif1 also represses DNA replication through PP1. Notably, the RVXF and SILK motifs of fission yeast Rif1 are both flanked by potential DDK phosphorylation sequences (Supplemental Fig. S7C,D). Both mammalian and Drosophila Rif1 proteins also have an N-terminal RVXF motif (Supplemental Fig. S7C) in addition to previously reported SILK and RVXF motifs close to their C termini (Sreesankar et al. 2012). We suggest that the role of mammalian Rif1 in repressing DNA replication also involves targeting of PP1 to counteract DDK phosphorylation. Consistent with this possibility, MCM phosphorylation is increased in human cells depleted for Rif1 (Yamazaki et al. 2012). Interestingly, PP1 was recently shown to be responsible for rapid reversal of DDK-dependent phosphorylation of Mcm4 in Xenopus egg extract (Poh et al. 2014).
Originally discovered for its role at yeast telomeres and later identified as important for controlling DNA replication, Rif1 is now known to mediate various additional chromosome stability functions, including DNA break repair pathway choice (Chapman et al. 2013; Di Virgilio et al. 2013; Escribano-Diaz et al. 2013) and down-regulation of checkpoint activity close to telomeres (Xue et al. 2011; Ribeyre and Shore 2012). All of these control mechanisms could potentially involve regulated dephosphorylation of specific proteins. It will be intriguing to discover whether phosphatase targeting is a common theme among the manifold cellular roles of Rif1.
Materials and methods
Yeast strains and plasmids
Yeast strains and plasmids are listed in Supplemental Material along with construction and two-hybrid assay procedures.
Microarray analysis of genomic DNA replication profiles
Isotopic density transfer experiments and microarray hybridizations were performed as described (Pohl et al. 2012). Genome-wide percent replication values were normalized, and the data were smoothed as described (Alvino et al. 2007; Feng et al. 2009).
Analysis of Mcm4 phosphorylation
In vivo Mcm4 phosphorylation was analyzed in either whole-cell extracts or chromatin fractions. Whole-cell extracts were prepared essentially as described (Kushnirov 2000); details are in the Supplemental Material. Chromatin fractions were prepared as described (Sheu and Stillman 2006).
Coimmunoprecipitation experiments and DDK assays
A series of Dbf4 proteins, HA-Cdc7, and Rif1-CT were expressed in insect Sf9 cells, and the whole-cell extracts were immunoprecipitated with anti-HA monoclonal 12CA5. Kinase assays were performed as described (Weinreich and Stillman 1999). See the Supplemental Material for additional details.
Acknowledgments
We thank Professor Mike Stark for advice and for strains and plasmids. Professor David Shore gifted yeast strains. This work was supported by Cancer Research UK grant A13356 and Biotechnology and Biological Sciences Research Council grant BB/K006304/1 to A.D.D., and National Institute of General Medical Sciences grant (18926) to B.J.B. and M.K.R.
Footnotes
Supplemental material is available for this article.
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.231258.113.
References
- Alvino GM, Collingwood D, Murphy JM, Delrow J, Brewer BJ, Raghuraman MK 2007. Replication in hydroxyurea: It's a matter of time. Mol Cell Biol 27: 6396–6406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrade MA, Petosa C, O'Donoghue SI, Muller CW, Bork P 2001. Comparison of ARM and HEAT protein repeats. J Mol Biol 309: 1–18 [DOI] [PubMed] [Google Scholar]
- Andrews PD, Stark MJ 2000. Type 1 protein phosphatase is required for maintenance of cell wall integrity, morphogenesis and cell cycle progression in Saccharomyces cerevisiae. J Cell Sci 113: 507–520 [DOI] [PubMed] [Google Scholar]
- Aparicio OM 2013. Location, location, location: It's all in the timing for replication origins. Genes Dev 27: 117–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzi M, Mantiero D, Trovesi C, Lucchini G, Longhese MP 2010. Dephosphorylation of γ H2A by Glc7/Protein Phosphatase 1 promotes recovery from inhibition of DNA replication. Mol Cell Biol 30: 131–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black S, Andrews PD, Sneddon AA, Stark MJ 1995. A regulated MET3-GLC7 gene fusion provides evidence of a mitotic role for Saccharomyces cerevisiae Protein Phosphatase 1. Yeast 11: 747–759 [DOI] [PubMed] [Google Scholar]
- Bollen M, Peti W, Ragusa MJ, Beullens M 2010. The extended PP1 toolkit: Designed to create specificity. Trends Biochem Sci 35: 450–458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breitkreutz A, Choi H, Sharom JR, Boucher L, Neduva V, Larsen B, Lin ZY, Breitkreutz BJ, Stark C, Liu G, et al. 2010. A global protein kinase and phosphatase interaction network in yeast. Science 328: 1043–1046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruck I, Kaplan D 2009. Dbf4–Cdc7 phosphorylation of Mcm2 is required for cell growth. J Biol Chem 284: 28823–28831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman JR, Barral P, Vannier JB, Borel V, Steger M, Tomas-Loba A, Sartori AA, Adams IR, Batista FD, Boulton SJ 2013. RIF1 is essential for 53BP1-dependent nonhomologous end joining and suppression of DNA double-strand break resection. Mol Cell 49: 858–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen PT 2002. Protein phosphatase 1—targeted in many directions. J Cell Sci 115: 241–256 [DOI] [PubMed] [Google Scholar]
- Cornacchia D, Dileep V, Quivy JP, Foti R, Tili F, Santarella-Mellwig R, Antony C, Almouzni G, Gilbert DM, Buonomo SB 2012. Mouse Rif1 is a key regulator of the replication-timing programme in mammalian cells. EMBO J 31: 3678–3690 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Virgilio M, Callen E, Yamane A, Zhang W, Jankovic M, Gitlin AD, Feldhahn N, Resch W, Oliveira TY, Chait BT, et al. 2013. Rif1 prevents resection of DNA breaks and promotes immunoglobulin class switching. Science 339: 711–715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowell SJ, Romanowski P, Diffley JF 1994. Interaction of Dbf4, the Cdc7 protein kinase regulatory subunit, with yeast replication origins in vivo. Science 265: 1243–1246 [DOI] [PubMed] [Google Scholar]
- Escribano-Diaz C, Orthwein A, Fradet-Turcotte A, Xing M, Young JT, Tkac J, Cook MA, Rosebrock AP, Munro M, Canny MD, et al. 2013. A cell cycle-dependent regulatory circuit composed of 53BP1-RIF1 and BRCA1-CtIP controls DNA repair pathway choice. Mol Cell 49: 872–883 [DOI] [PubMed] [Google Scholar]
- Feng ZH, Wilson SE, Peng ZY, Schlender KK, Reimann EM, Trumbly RJ 1991. The yeast GLC7 gene required for glycogen accumulation encodes a type 1 protein phosphatase. J Biol Chem 266: 23796–23801 [PubMed] [Google Scholar]
- Feng W, Bachant J, Collingwood D, Raghuraman MK, Brewer BJ 2009. Centromere replication timing determines different forms of genomic instability in Saccharomyces cerevisiae checkpoint mutants during replication stress. Genetics 183: 1249–1260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira MF, Santocanale C, Drury LS, Diffley JF 2000. Dbf4p, an essential S phase-promoting factor, is targeted for degradation by the anaphase-promoting complex. Mol Cell Biol 20: 242–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu YV, Yardimci H, Long DT, Ho TV, Guainazzi A, Bermudez VP, Hurwitz J, van Oijen A, Scharer OD, Walter JC 2011. Selective bypass of a lagging strand roadblock by the eukaryotic replicative DNA helicase. Cell 146: 931–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabrielse C, Miller CT, McConnell KH, DeWard A, Fox CA, Weinreich M 2006. A Dbf4p BRCA1 C-terminal-like domain required for the response to replication fork arrest in budding yeast. Genetics 173: 541–555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambus A, Jones RC, Sanchez-Diaz A, Kanemaki M, van Deursen F, Edmondson RD, Labib K 2006. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat Cell Biol 8: 358–366 [DOI] [PubMed] [Google Scholar]
- Gotta M, Laroche T, Formenton A, Maillet L, Scherthan H, Gasser SM 1996. The clustering of telomeres and colocalization with Rap1, Sir3, and Sir4 proteins in wild-type Saccharomyces cerevisiae. J Cell Biol 134: 1349–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardy CF, Sussel L, Shore D 1992. A RAP1-interacting protein involved in transcriptional silencing and telomere length regulation. Genes Dev 6: 801–814 [DOI] [PubMed] [Google Scholar]
- Hayano M, Kanoh Y, Matsumoto S, Renard-Guillet C, Shirahige K, Masai H 2012. Rif1 is a global regulator of timing of replication origin firing in fission yeast. Genes Dev 26: 137–150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraga S, Botsios S, Donaldson AD 2008. Histone H3 lysine 56 acetylation by Rtt109 is crucial for chromosome positioning. J Cell Biol 183: 641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hisamoto N, Sugimoto K, Matsumoto K 1994. The Glc7 type 1 protein phosphatase of Saccharomyces cerevisiae is required for cell cycle progression in G2/M. Mol Cell Biol 14: 3158–3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katou Y, Kaneshiro K, Aburatani H, Shirahige K 2006. Genomic approach for the understanding of dynamic aspect of chromosome behavior. Methods Enzymol 409: 389–410 [DOI] [PubMed] [Google Scholar]
- Kuntziger T, Rogne M, Folstad RL, Collas P 2006. Association of PP1 with its regulatory subunit AKAP149 is regulated by serine phosphorylation flanking the RVXF motif of AKAP149. Biochemistry 45: 5868–5877 [DOI] [PubMed] [Google Scholar]
- Kushnirov VV 2000. Rapid and reliable protein extraction from yeast. Yeast 16: 857–860 [DOI] [PubMed] [Google Scholar]
- Lian HY, Robertson ED, Hiraga S, Alvino GM, Collingwood D, McCune HJ, Sridhar A, Brewer BJ, Raghuraman MK, Donaldson AD 2011. The effect of Ku on telomere replication time is mediated by telomere length but is independent of histone tail acetylation. Mol Biol Cell 22: 1753–1765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantiero D, Mackenzie A, Donaldson A, Zegerman P 2011. Limiting replication initiation factors execute the temporal programme of origin firing in budding yeast. EMBO J 30: 4805–4814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCune HJ, Danielson LS, Alvino GM, Collingwood D, Delrow JJ, Fangman WL, Brewer BJ, Raghuraman MK 2008. The temporal program of chromosome replication: Genomewide replication in clb5Δ Saccharomyces cerevisiae. Genetics 180: 1833–1847 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mechali M, Yoshida K, Coulombe P, Pasero P 2013. Genetic and epigenetic determinants of DNA replication origins, position and activation. Curr Opin Genet Dev 23: 124–131 [DOI] [PubMed] [Google Scholar]
- Moyer SE, Lewis PW, Botchan MR 2006. Isolation of the Cdc45/Mcm2-7/GINS (CMG) complex, a candidate for the eukaryotic DNA replication fork helicase. Proc Natl Acad Sci 103: 10236–10241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieduszynski CA, Hiraga S, Ak P, Benham CJ, Donaldson AD 2007. OriDB: A DNA replication origin database. Nucleic Acids Res 35: D40–D46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshiro G, Owens JC, Shellman Y, Sclafani RA, Li JJ 1999. Cell cycle control of Cdc7p kinase activity through regulation of Dbf4p stability. Mol Cell Biol 19: 4888–4896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S, Patterson EE, Cobb J, Audhya A, Gartenberg MR, Fox CA 2011. Palmitoylation controls the dynamics of budding-yeast heterochromatin via the telomere-binding protein Rif1. Proc Natl Acad Sci 108: 14572–14577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinsky BA, Kotwaliwale CV, Tatsutani SY, Breed CA, Biggins S 2006. Glc7/Protein Phosphatase 1 regulatory subunits can oppose the Ipl1/aurora protein kinase by redistributing Glc7. Mol Cell Biol 26: 2648–2660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poh WT, Chadha GS, Gillespie PJ, Kaldis P, Blow JJ 2014. Xenopus Cdc7 executes its essential function early in S phase and is counteracted by checkpoint-regulated Protein Phosphatase 1. Open Biol 4: 130138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohl TJ, Brewer BJ, Raghuraman MK 2012. Functional centromeres determine the activation time of pericentric origins of DNA replication in Saccharomyces cerevisiae. PLoS Genet 8: e1002677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramaswamy NT, Li L, Khalil M, Cannon JF 1998. Regulation of yeast glycogen metabolism and sporulation by Glc7p protein phosphatase. Genetics 149: 57–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Randell JC, Fan A, Chan C, Francis LI, Heller RC, Galani K, Bell SP 2010. Mec1 is one of multiple kinases that prime the Mcm2–7 helicase for phosphorylation by Cdc7. Mol Cell 40: 353–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeyre C, Shore D 2012. Anticheckpoint pathways at telomeres in yeast. Nat Struct Mol Biol 19: 307–313 [DOI] [PubMed] [Google Scholar]
- Sasanuma H, Hirota K, Fukuda T, Kakusho N, Kugou K, Kawasaki Y, Shibata T, Masai H, Ohta K 2008. Cdc7-dependent phosphorylation of Mer2 facilitates initiation of yeast meiotic recombination. Genes Dev 22: 398–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu YJ, Stillman B 2006. Cdc7–Dbf4 phosphorylates MCM proteins via a docking site-mediated mechanism to promote S phase progression. Mol Cell 24: 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu YJ, Stillman B 2010. The Dbf4–Cdc7 kinase promotes S phase by alleviating an inhibitory activity in Mcm4. Nature 463: 113–117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi T, Bunker RD, Mattarocci S, Ribeyre C, Faty M, Gut H, Scrima A, Rass U, Rubin SM, Shore D, et al. 2013. Rif1 and Rif2 shape telomere function and architecture through multivalent Rap1 interactions. Cell 153: 1340–1353 [DOI] [PubMed] [Google Scholar]
- Siow CC, Nieduszynska SR, Muller CA, Nieduszynski CA 2012. OriDB, the DNA replication origin database updated and extended. Nucleic Acids Res 40: D682–D686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sreesankar E, Senthilkumar R, Bharathi V, Mishra RK, Mishra K 2012. Functional diversification of yeast telomere associated protein, Rif1, in higher eukaryotes. BMC Genomics 13: 255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S, Umemori T, Hirai K, Muramatsu S, Kamimura Y, Araki H 2007. CDK-dependent phosphorylation of Sld2 and Sld3 initiates DNA replication in budding yeast. Nature 445: 328–332 [DOI] [PubMed] [Google Scholar]
- Tanaka S, Nakato R, Katou Y, Shirahige K, Araki H 2011. Origin association of Sld3, Sld7, and Cdc45 proteins is a key step for determination of origin-firing timing. Curr Biol 21: 2055–2063 [DOI] [PubMed] [Google Scholar]
- Wakula P, Beullens M, Ceulemans H, Stalmans W, Bollen M 2003. Degeneracy and function of the ubiquitous RVXF motif that mediates binding to protein phosphatase-1. J Biol Chem 278: 18817–18823 [DOI] [PubMed] [Google Scholar]
- Wan L, Niu H, Futcher B, Zhang C, Shokat KM, Boulton SJ, Hollingsworth NM 2008. Cdc28–Clb5 (CDK-S) and Cdc7–Dbf4 (DDK) collaborate to initiate meiotic recombination in yeast. Genes Dev 22: 386–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinreich M, Stillman B 1999. Cdc7p–Dbf4p kinase binds to chromatin during S phase and is regulated by both the APC and the RAD53 checkpoint pathway. EMBO J 18: 5334–5346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Muniandy P, Leo E, Yin J, Thangavel S, Shen X, Ii M, Agama K, Guo R, Fox D 3rd, et al. 2010. Rif1 provides a new DNA-binding interface for the Bloom syndrome complex to maintain normal replication. EMBO J 29: 3140–3155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Rushton MD, Maringele L 2011. A novel checkpoint and RPA inhibitory pathway regulated by Rif1. PLoS Genet 7: e1002417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki S, Ishii A, Kanoh Y, Oda M, Nishito Y, Masai H 2012. Rif1 regulates the replication timing domains on the human genome. EMBO J 31: 3667–3677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegerman P, Diffley JF 2007. Phosphorylation of Sld2 and Sld3 by cyclin-dependent kinases promotes DNA replication in budding yeast. Nature 445: 281–285 [DOI] [PubMed] [Google Scholar]