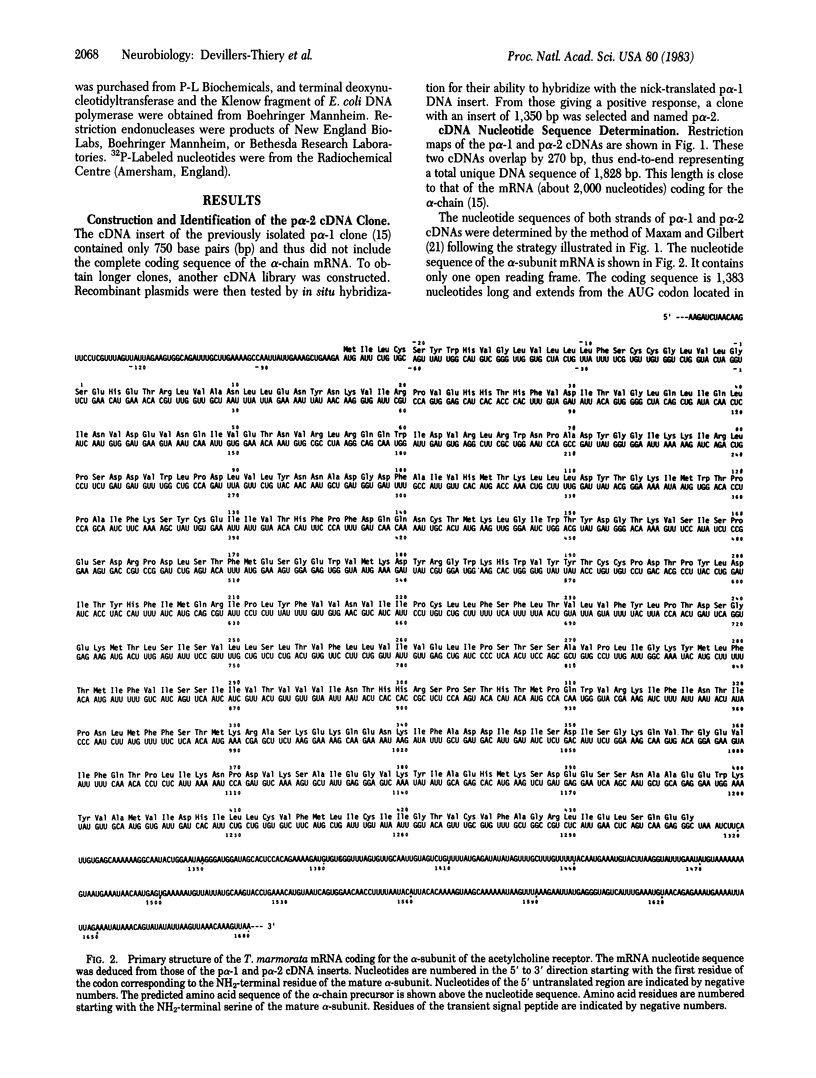
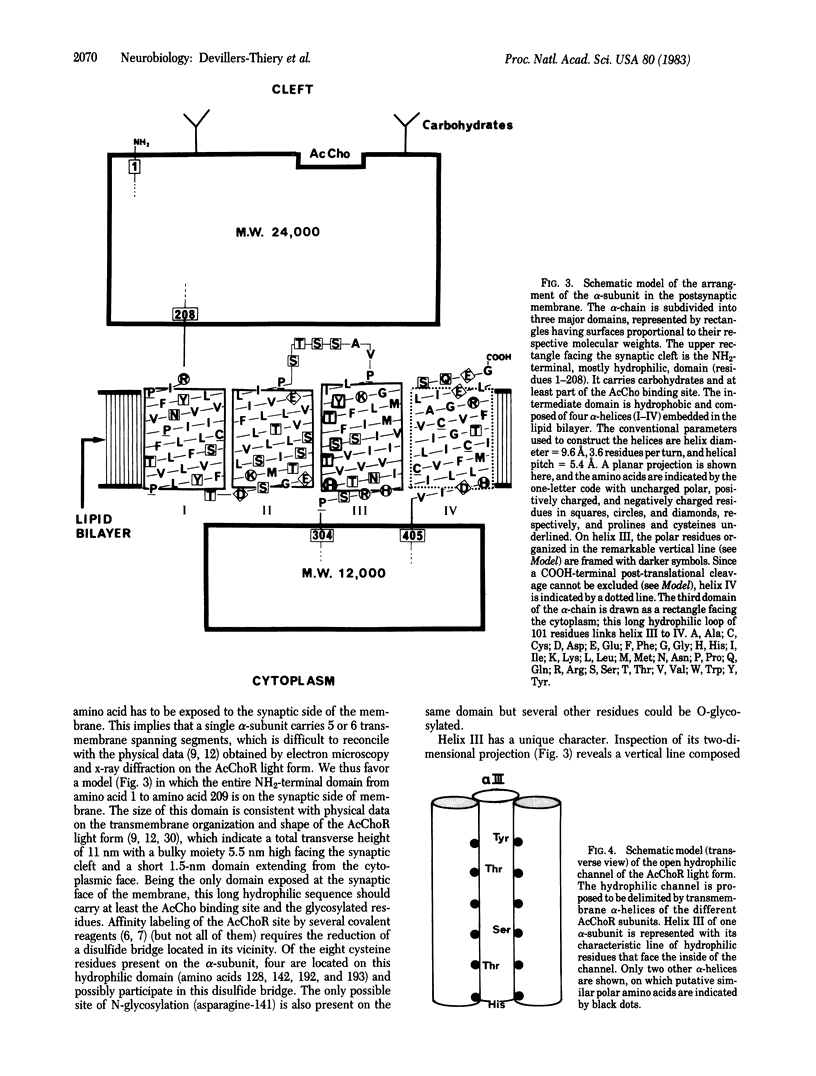
Abstract
A 1,350-base-pair-long cDNA clone, named p alpha-2, was isolated by hybridization to the previously characterized clone p alpha-1 and found to be specific for the alpha-subunit of the Torpedo marmorata acetylcholine receptor. The nucleotide sequences of both cDNA inserts were analyzed and the sequence of the complete coding region and part of the 5' and 3' untranslated regions of the alpha-chain mRNA was determined. The complete amino acid sequence of the alpha-chain precursor is presented and used to develop a model for the transmembrane organization of the polypeptide.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. In vitro synthesis, glycosylation, and membrane insertion of the four subunits of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5598–5602. doi: 10.1073/pnas.78.9.5598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anholt R., Lindstrom J., Montal M. Functional equivalence of monomeric and dimeric forms of purified acetylcholine receptors from Torpedo californica in reconstituted lipid vesicles. Eur J Biochem. 1980 Aug;109(2):481–487. doi: 10.1111/j.1432-1033.1980.tb04819.x. [DOI] [PubMed] [Google Scholar]
- Ballivet M., Patrick J., Lee J., Heinemann S. Molecular cloning of cDNA coding for the gamma subunit of Torpedo acetylcholine receptor. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4466–4470. doi: 10.1073/pnas.79.14.4466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blobel G., Dobberstein B. Transfer of proteins across membranes. I. Presence of proteolytically processed and unprocessed nascent immunoglobulin light chains on membrane-bound ribosomes of murine myeloma. J Cell Biol. 1975 Dec;67(3):835–851. doi: 10.1083/jcb.67.3.835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartaud J., Benedetti E. L. A morphological study of the cholinergic receptor protein from Torpedo marmorata in its membrane environment and in its detergent-extracted purified form. J Cell Sci. 1978 Feb;29:313–337. doi: 10.1242/jcs.29.1.313. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Englund P. T. Specific labeling of 3' termini with T4 DNA polymerase. Methods Enzymol. 1980;65(1):39–43. doi: 10.1016/s0076-6879(80)65008-3. [DOI] [PubMed] [Google Scholar]
- Changeux J. P. The acetylcholine receptor: an "allosteric" membrane protein. Harvey Lect. 1979 1980;75:85–254. [PubMed] [Google Scholar]
- Davis C. G., Gordon A. S., Diamond I. Specificity and localization of the acetylcholine receptor kinase. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3666–3670. doi: 10.1073/pnas.79.11.3666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Changeux J. P., Paroutaud P., Strosberg A. D. The amino-terminal sequence of the 40,000 molecular weight subunit of the acetylcholine receptor protein from Torpedo marmorata. FEBS Lett. 1979 Aug 1;104(1):99–105. doi: 10.1016/0014-5793(79)81092-3. [DOI] [PubMed] [Google Scholar]
- Dwyer T. M., Adams D. J., Hille B. The permeability of the endplate channel to organic cations in frog muscle. J Gen Physiol. 1980 May;75(5):469–492. doi: 10.1085/jgp.75.5.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelman D. M., Henderson R., McLachlan A. D., Wallace B. A. Path of the polypeptide in bacteriorhodopsin. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2023–2027. doi: 10.1073/pnas.77.4.2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giraudat J., Devillers-Thiery A., Auffray C., Rougeon F., Changeux J. P. Identification of a cDNA clone coding for the acetylcholine binding subunit of Torpedo marmorata acetylcholine receptor. EMBO J. 1982;1(6):713–717. doi: 10.1002/j.1460-2075.1982.tb01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunstein M., Hogness D. S. Colony hybridization: a method for the isolation of cloned DNAs that contain a specific gene. Proc Natl Acad Sci U S A. 1975 Oct;72(10):3961–3965. doi: 10.1073/pnas.72.10.3961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heidmann T., Oswald R., Changeux J. P. Le site de liaison de haute affinité de la chlorpromazine n'est présent qu'à un seul exemplaire par molécule de récepteur cholinergique et est commun aux quatre chaînes polypeptidiques. C R Seances Acad Sci III. 1982 Oct 11;295(5):345–349. [PubMed] [Google Scholar]
- Heidmann T., Sobel A., Popot J. L., Changeux J. P. Reconstitution of a functional acetylcholine receptor. Conservation of the conformational and allosteric transitions and recovery of the permeability response; role of lipids. Eur J Biochem. 1980 Sep;110(1):35–55. doi: 10.1111/j.1432-1033.1980.tb04839.x. [DOI] [PubMed] [Google Scholar]
- Kistler J., Stroud R. M., Klymkowsky M. W., Lalancette R. A., Fairclough R. H. Structure and function of an acetylcholine receptor. Biophys J. 1982 Jan;37(1):371–383. doi: 10.1016/S0006-3495(82)84685-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MONOD J., WYMAN J., CHANGEUX J. P. ON THE NATURE OF ALLOSTERIC TRANSITIONS: A PLAUSIBLE MODEL. J Mol Biol. 1965 May;12:88–118. doi: 10.1016/s0022-2836(65)80285-6. [DOI] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. Sequencing end-labeled DNA with base-specific chemical cleavages. Methods Enzymol. 1980;65(1):499–560. doi: 10.1016/s0076-6879(80)65059-9. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Furutani Y., Hirose T., Asai M., Inayama S., Miyata T., Numa S. Primary structure of alpha-subunit precursor of Torpedo californica acetylcholine receptor deduced from cDNA sequence. Nature. 1982 Oct 28;299(5886):793–797. doi: 10.1038/299793a0. [DOI] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Hirose T., Asai M., Takashima H., Inayama S., Miyata T. Primary structures of beta- and delta-subunit precursors of Torpedo californica acetylcholine receptor deduced from cDNA sequences. Nature. 1983 Jan 20;301(5897):251–255. doi: 10.1038/301251a0. [DOI] [PubMed] [Google Scholar]
- Oswald R., Changeux J. P. Ultraviolet light-induced labeling by noncompetitive blockers of the acetylcholine receptor from Torpedo marmorata. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3925–3929. doi: 10.1073/pnas.78.6.3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popot J. L., Cartaud J., Changeux J. P. Reconstitution of a functional acetylcholine receptor. Incorporation into artificial lipid vesicles and pharmacology of the agonist-controlled permeability changes. Eur J Biochem. 1981 Aug;118(2):203–214. [PubMed] [Google Scholar]
- Raftery M. A., Hunkapiller M. W., Strader C. D., Hood L. E. Acetylcholine receptor: complex of homologous subunits. Science. 1980 Jun 27;208(4451):1454–1456. doi: 10.1126/science.7384786. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Ross M. J., Klymkowsky M. W., Agard D. A., Stroud R. M. Structural studies of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1977 Nov;116(4):635–659. doi: 10.1016/0022-2836(77)90264-9. [DOI] [PubMed] [Google Scholar]
- Sumikawa K., Houghton M., Smith J. C., Bell L., Richards B. M., Barnard E. A. The molecular cloning and characterisation of cDNA coding for the alpha subunit of the acetylcholine receptor. Nucleic Acids Res. 1982 Oct 11;10(19):5809–5822. doi: 10.1093/nar/10.19.5809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandlen R. L., Wu W. C., Eisenach J. C., Raftery M. A. Studies of the composition of purified Torpedo californica acetylcholine receptor and of its subunits. Biochemistry. 1979 May 15;18(10):1845–1854. doi: 10.1021/bi00577a001. [DOI] [PubMed] [Google Scholar]
- Wennogle L. P., Changeux J. P. Transmembrane orientation of proteins present in acetylcholine receptor-rich membranes from Torpedo marmorata studied by selective proteolysis. Eur J Biochem. 1980 May;106(2):381–393. doi: 10.1111/j.1432-1033.1980.tb04584.x. [DOI] [PubMed] [Google Scholar]
- Wu W. C., Raftery M. A. Reconstitution of acetylcholine receptor function using purified receptor protein. Biochemistry. 1981 Feb 17;20(4):694–701. doi: 10.1021/bi00507a004. [DOI] [PubMed] [Google Scholar]
- von Heijne G. Membrane proteins: the amino acid composition of membrane-penetrating segments. Eur J Biochem. 1981 Nov;120(2):275–278. doi: 10.1111/j.1432-1033.1981.tb05700.x. [DOI] [PubMed] [Google Scholar]
- von Heijne G. On the hydrophobic nature of signal sequences. Eur J Biochem. 1981 May 15;116(2):419–422. doi: 10.1111/j.1432-1033.1981.tb05351.x. [DOI] [PubMed] [Google Scholar]



