Abstract
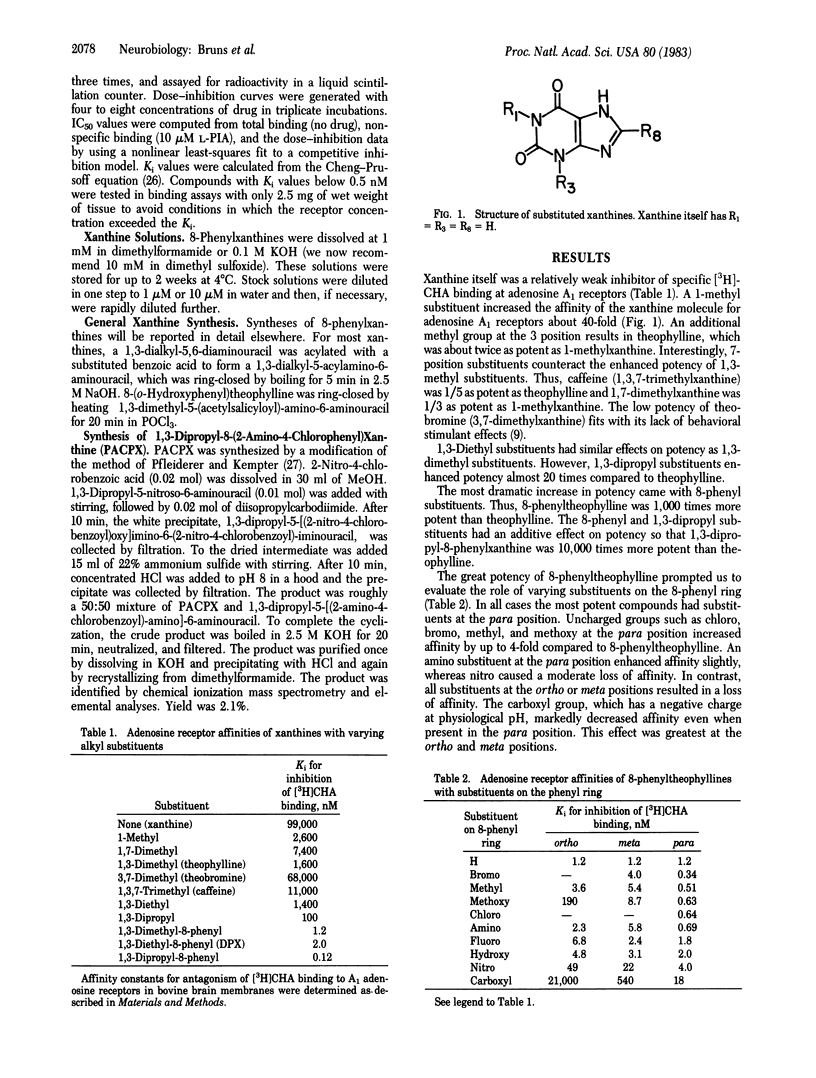
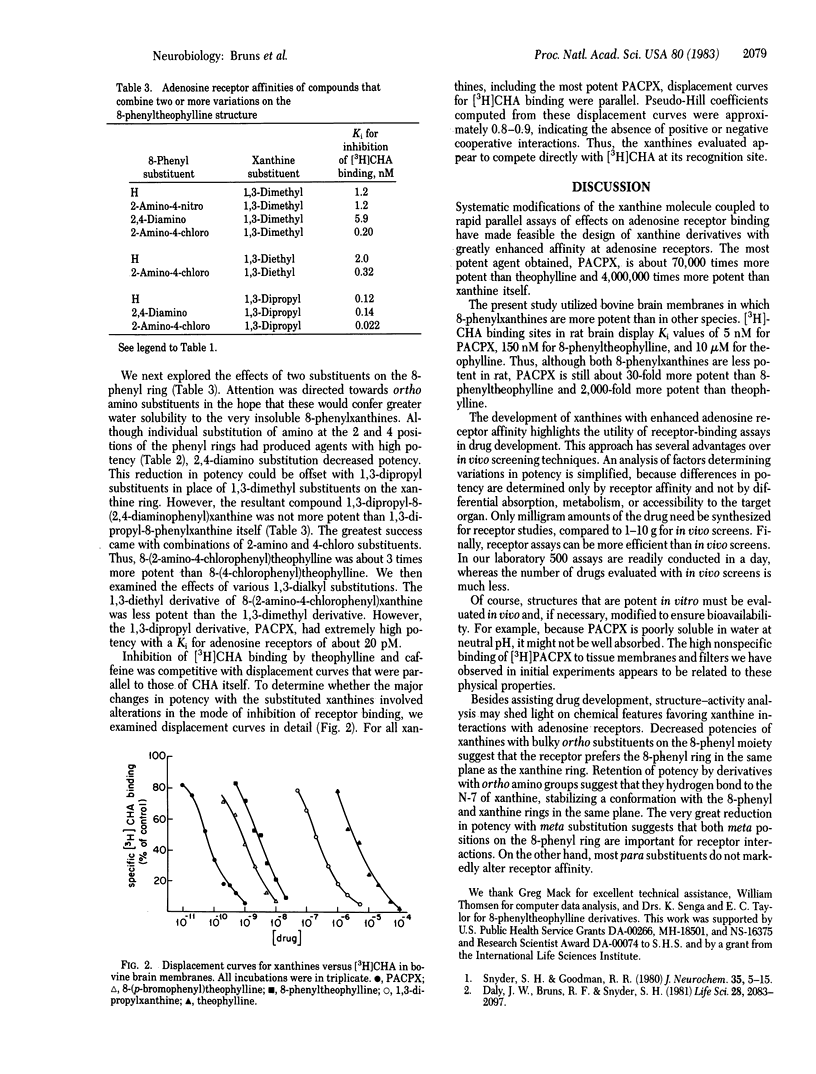
Structure-activity analysis of alkylxanthine derivatives at adenosine receptor binding sites has been employed to design more potent adenosine receptor antagonists. Receptor affinities of xanthines were determined by measuring inhibition of the binding of N6-[3H]cyclohexyladenosine to bovine brain membranes. 1,3-Dipropyl substitutions enhance potency compared to the 1,3-dimethyl substitution in theophylline. An 8-phenyl substituent produces a considerable increase in potency, which is augmented by certain para substitutions on the 8-phenyl ring. Combining an ortho amino with a para-chloro substituent on the 8-phenyl ring affords further increases in potency. Combining all of these substituents results in 1,3-dipropyl-8-(2-amino-4-chlorophenyl) xanthine, a compound of extraordinary receptor affinity, with a Ki for adenosine A1 receptors of 22 pM. It is 4,000,000 times more potent than xanthine itself and 70,000 times more potent than theophylline.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bruns R. F. Adenosine antagonism by purines, pteridines and benzopteridines in human fibroblasts. Biochem Pharmacol. 1981 Feb 15;30(4):325–333. doi: 10.1016/0006-2952(81)90062-9. [DOI] [PubMed] [Google Scholar]
- Bruns R. F., Daly J. W., Snyder S. H. Adenosine receptors in brain membranes: binding of N6-cyclohexyl[3H]adenosine and 1,3-diethyl-8-[3H]phenylxanthine. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5547–5551. doi: 10.1073/pnas.77.9.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman R. A., Miller D. J. Structure-activity relations for caffeine: a comparative study of the inotropic effects of the methylxanthines, imidazoles and related compounds on the frog's heart. J Physiol. 1974 Nov;242(3):615–634. doi: 10.1113/jphysiol.1974.sp010726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Daly J. W., Bruns R. F., Snyder S. H. Adenosine receptors in the central nervous system: relationship to the central actions of methylxanthines. Life Sci. 1981 May 11;28(19):2083–2097. doi: 10.1016/0024-3205(81)90614-7. [DOI] [PubMed] [Google Scholar]
- Gavish M., Goodman R. R., Snyder S. H. Solubilized adenosine receptors in the brain: regulation of guanine nucleotides. Science. 1982 Mar 26;215(4540):1633–1635. doi: 10.1126/science.6280275. [DOI] [PubMed] [Google Scholar]
- Goodman R. R., Cooper M. J., Gavish M., Snyder S. H. Guanine nucleotide and cation regulation of the binding of [3H]cyclohexyladenosine and [3H]diethylphenylxanthine to adenosine A1 receptors in brain membranes. Mol Pharmacol. 1982 Mar;21(2):329–335. [PubMed] [Google Scholar]
- Goodman R. R., Synder S. H. Autoradiographic localization of adenosine receptors in rat brain using [3H]cyclohexyladenosine. J Neurosci. 1982 Sep;2(9):1230–1241. doi: 10.1523/JNEUROSCI.02-09-01230.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Londos C., Cooper D. M., Wolff J. Subclasses of external adenosine receptors. Proc Natl Acad Sci U S A. 1980 May;77(5):2551–2554. doi: 10.1073/pnas.77.5.2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy K. M., Snyder S. H. Heterogeneity of adenosine A1 receptor binding in brain tissue. Mol Pharmacol. 1982 Sep;22(2):250–257. [PubMed] [Google Scholar]
- Phillis J. W., Wu P. H. The role of adenosine and its nucleotides in central synaptic transmission. Prog Neurobiol. 1981;16(3-4):187–239. doi: 10.1016/0301-0082(81)90014-9. [DOI] [PubMed] [Google Scholar]
- Schwabe U., Trost T. Characterization of adenosine receptors in rat brain by (-)[3H]N6-phenylisopropyladenosine. Naunyn Schmiedebergs Arch Pharmacol. 1980 Sep;313(3):179–187. doi: 10.1007/BF00505731. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Goodman R. R. Multiple neurotransmitter receptors. J Neurochem. 1980 Jul;35(1):5–15. doi: 10.1111/j.1471-4159.1980.tb12483.x. [DOI] [PubMed] [Google Scholar]
- Snyder S. H., Katims J. J., Annau Z., Bruns R. F., Daly J. W. Adenosine receptors and behavioral actions of methylxanthines. Proc Natl Acad Sci U S A. 1981 May;78(5):3260–3264. doi: 10.1073/pnas.78.5.3260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielman W. S., Thompson C. I. A proposed role for adenosine in the regulation of renal hemodynamics and renin release. Am J Physiol. 1982 May;242(5):F423–F435. doi: 10.1152/ajprenal.1982.242.5.F423. [DOI] [PubMed] [Google Scholar]
- Stone T. W. Physiological roles for adenosine and adenosine 5'-triphosphate in the nervous system. Neuroscience. 1981;6(4):523–555. doi: 10.1016/0306-4522(81)90145-7. [DOI] [PubMed] [Google Scholar]
- Vapaatalo H., Onken D., Neuvonen P. J., Westermann E. Stereospecificity in some central and circulatory effects of phenylisopropyl-adenosine (PIA). Arzneimittelforschung. 1975 Mar;25(3):407–410. [PubMed] [Google Scholar]
- Williams M., Risley E. A. Biochemical characterization of putative central purinergic receptors by using 2-chloro[3H]adenosine, a stable analog of adenosine. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6892–6896. doi: 10.1073/pnas.77.11.6892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu P. H., Phillis J. W., Balls K., Rinaldi B. Specific binding of 2-[3H]chloroadenosine to rat brain cortical membranes. Can J Physiol Pharmacol. 1980 May;58(5):576–579. doi: 10.1139/y80-096. [DOI] [PubMed] [Google Scholar]
- van Calker D., Müller M., Hamprecht B. Adenosine regulates via two different types of receptors, the accumulation of cyclic AMP in cultured brain cells. J Neurochem. 1979 Nov;33(5):999–1005. doi: 10.1111/j.1471-4159.1979.tb05236.x. [DOI] [PubMed] [Google Scholar]



