Abstract
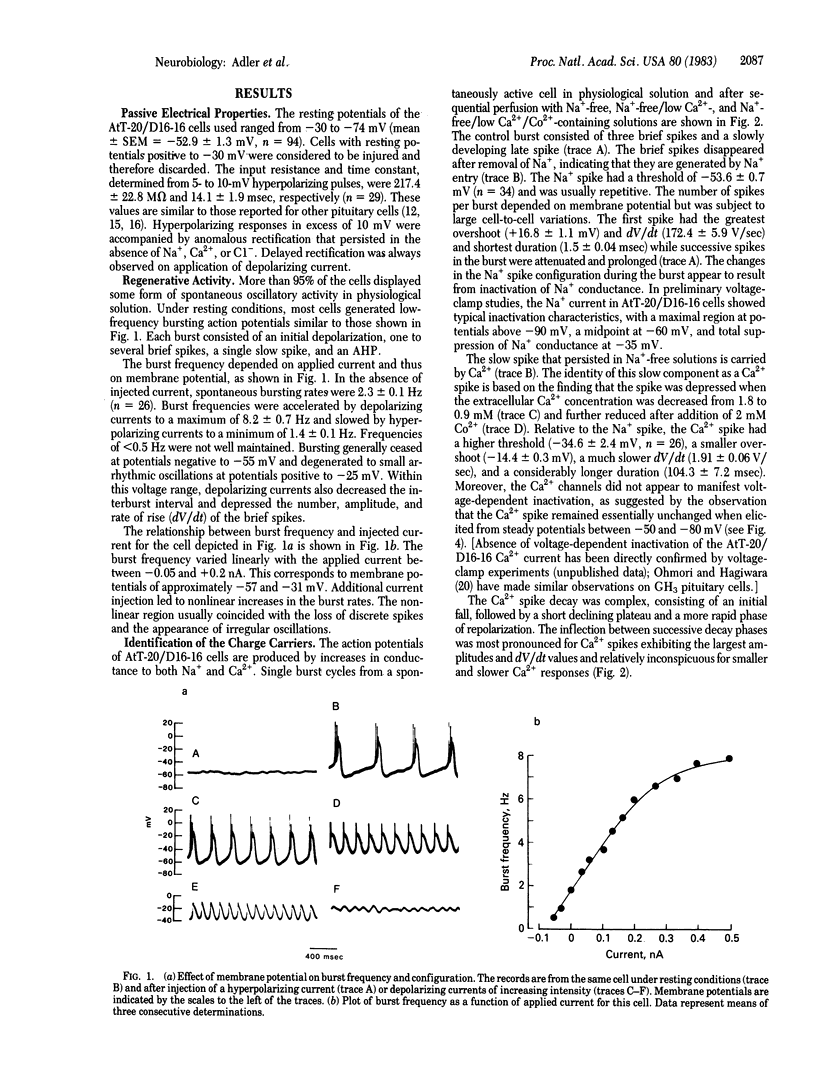
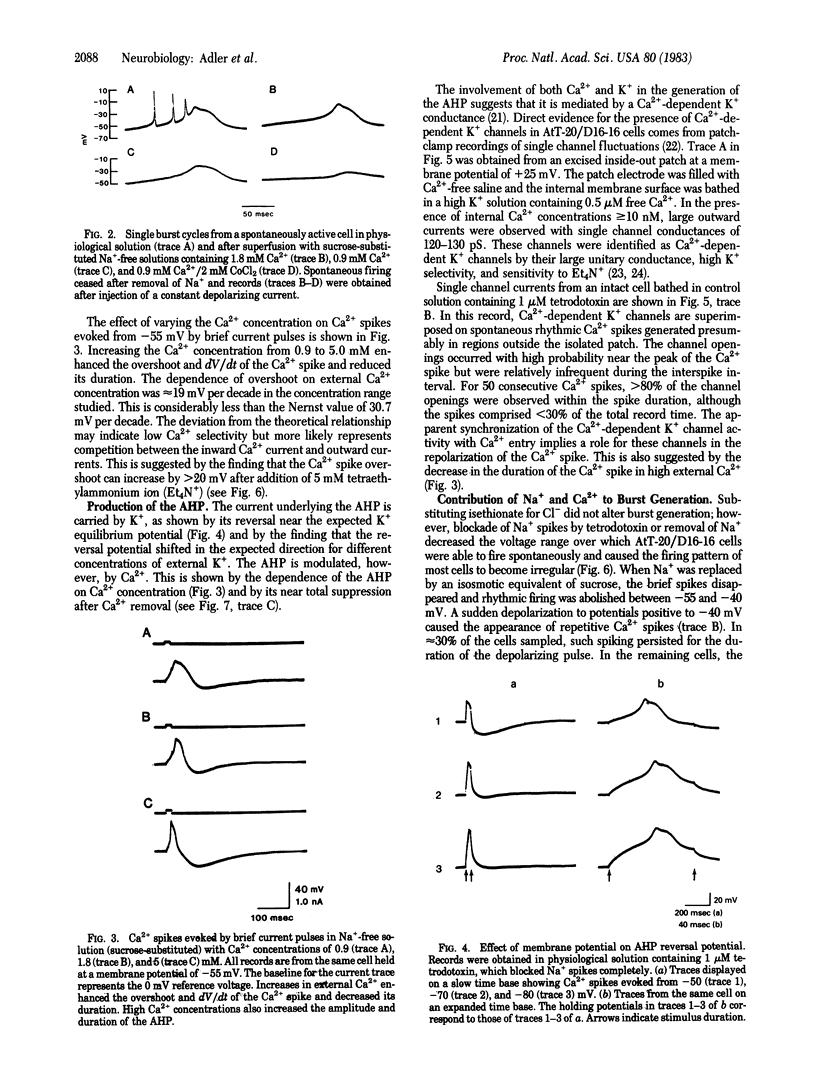
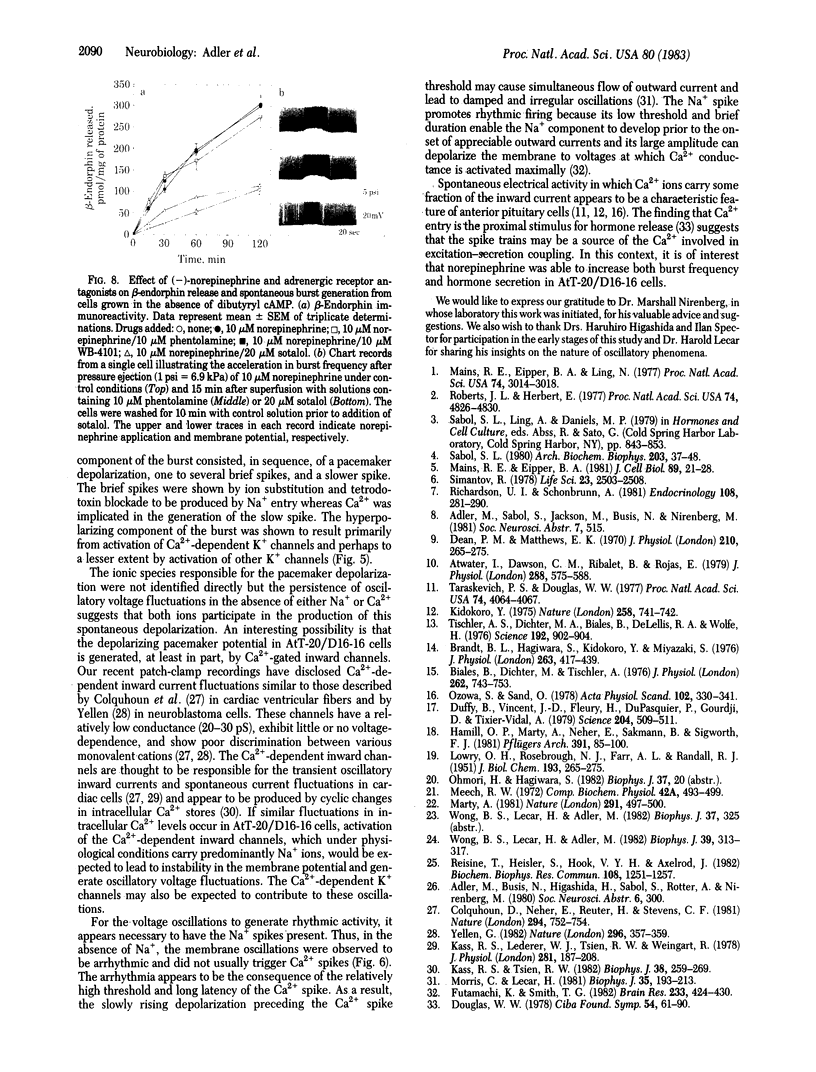
The electrophysiological properties of the mouse anterior pituitary cell line AtT-20/D16-16 were investigated with intracellular and patch-clamp techniques. Clonal AtT-20/D16-16 cells were found to be electrically excitable, with most cells exhibiting spontaneous bursting action potentials. The mean burst rates varied from 1.4 Hz at -55mV to 8.2 Hz at -25mV, showing an approximately linear frequency-current relationship in the low current range. The bursts consisted of one to several fast Na+ spikes superimposed on a slow pacemaker potential, followed by a Ca2+ spike and a Ca2+-sensitive afterhyperpolarization. Removal of either Na+ or Ca2+ from the bathing medium led to cessation of spontaneous activity and the appearance of arrhythmic firing patterns. Single channel recordings revealed the presence of Ca2+-dependent K+ channels with unitary conductances of approximately equal to 130 pS in physiological medium. These channels were activated by both intracellular Ca2+ and membrane depolarization. Addition of norepinephrine (10 microM) led to increases in burst frequency and beta-endorphin secretion mediated by activation of beta-adrenergic receptors. Our results, in conjunction with previous work, suggest that the Ca2+ that enters the cell during the burst may be involved in hormone secretion.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwater I., Dawson C. M., Ribalet B., Rojas E. Potassium permeability activated by intracellular calcium ion concentration in the pancreatic beta-cell. J Physiol. 1979 Mar;288:575–588. [PMC free article] [PubMed] [Google Scholar]
- Biales B., Dichter M., Tischler A. Electrical excitability of cultured adrenal chromaffin cells. J Physiol. 1976 Nov;262(3):743–753. doi: 10.1113/jphysiol.1976.sp011618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt B. L., Hagiwara S., Kidokoro Y., Miyazaki S. Action potentials in the rat chromaffin cell and effects of acetylcholine. J Physiol. 1976 Dec;263(3):417–439. doi: 10.1113/jphysiol.1976.sp011638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colquhoun D., Neher E., Reuter H., Stevens C. F. Inward current channels activated by intracellular Ca in cultured cardiac cells. Nature. 1981 Dec 24;294(5843):752–754. doi: 10.1038/294752a0. [DOI] [PubMed] [Google Scholar]
- Dean P. M., Matthews E. K. Electrical activity in pancreatic islet cells: effect of ions. J Physiol. 1970 Sep;210(2):265–275. doi: 10.1113/jphysiol.1970.sp009208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. W. Stimulus-secretion coupling: variations on the theme of calcium-activated exocytosis involving cellular and extracellular sources of calcium. Ciba Found Symp. 1978;(54):61–90. doi: 10.1002/9780470720356.ch4. [DOI] [PubMed] [Google Scholar]
- Dufy B., Vincent J. D., Fleury H., Du Pasquier P., Gourdji D., Tixier-Vidal A. Membrane effects of thyrotropin-releasing hormone and estrogen shown by intracellular recording from pituitary cells. Science. 1979 May 4;204(4392):509–511. doi: 10.1126/science.107590. [DOI] [PubMed] [Google Scholar]
- Futamachi K., Smith T. G., Jr Action of tetrodotoxin on pacemaker conductances in Aplysia neurons. Brain Res. 1982 Feb 11;233(2):424–430. doi: 10.1016/0006-8993(82)91218-5. [DOI] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Kass R. S., Lederer W. J., Tsien R. W., Weingart R. Role of calcium ions in transient inward currents and aftercontractions induced by strophanthidin in cardiac Purkinje fibres. J Physiol. 1978 Aug;281:187–208. doi: 10.1113/jphysiol.1978.sp012416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kass R. S., Tsien R. W. Fluctuations in membrane current driven by intracellular calcium in cardiac Purkinje fibers. Biophys J. 1982 Jun;38(3):259–269. doi: 10.1016/S0006-3495(82)84557-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kidokoro Y. Spontaneous calcium action potentials in a clonal pituitary cell line and their relationship to prolactin secretion. Nature. 1975 Dec 25;258(5537):741–742. doi: 10.1038/258741a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A. Coordinate, equimolar secretion of smaller peptide products derived from pro-ACTH/endorphin by mouse pituitary tumor cells. J Cell Biol. 1981 Apr;89(1):21–28. doi: 10.1083/jcb.89.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mains R. E., Eipper B. A., Ling N. Common precursor to corticotropins and endorphins. Proc Natl Acad Sci U S A. 1977 Jul;74(7):3014–3018. doi: 10.1073/pnas.74.7.3014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marty A. Ca-dependent K channels with large unitary conductance in chromaffin cell membranes. Nature. 1981 Jun 11;291(5815):497–500. doi: 10.1038/291497a0. [DOI] [PubMed] [Google Scholar]
- Meech R. W. Intracellular calcium injection causes increased potassium conductance in Aplysia nerve cells. Comp Biochem Physiol A Comp Physiol. 1972 Jun 1;42(2):493–499. doi: 10.1016/0300-9629(72)90128-4. [DOI] [PubMed] [Google Scholar]
- Morris C., Lecar H. Voltage oscillations in the barnacle giant muscle fiber. Biophys J. 1981 Jul;35(1):193–213. doi: 10.1016/S0006-3495(81)84782-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa S., Sand O. Electric activity of rat anterior pituitary cells in vitro. Acta Physiol Scand. 1978 Mar;102(3):330–341. doi: 10.1111/j.1748-1716.1978.tb06080.x. [DOI] [PubMed] [Google Scholar]
- Reisine T., Heisler S., Hook V. Y., Axelrod J. Multireceptor-induced release of adrenocorticotropin from anterior pituitary tumor cells. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1251–1257. doi: 10.1016/0006-291x(82)92134-9. [DOI] [PubMed] [Google Scholar]
- Richardson U. I., Schonbrunn A. Inhibition of adrenocorticotropin secretion by somatostatin in pituitary cells in culture. Endocrinology. 1981 Jan;108(1):281–290. doi: 10.1210/endo-108-1-281. [DOI] [PubMed] [Google Scholar]
- Roberts J. L., Herbert E. Characterization of a common precursor to corticotropin and beta-lipotropin: cell-free synthesis of the precursor and identification of corticotropin peptides in the molecule. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4826–4830. doi: 10.1073/pnas.74.11.4826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabol S. L. Storage and secretion of beta-endorphin and related peptides by mouse pituitary tumor cells: regulation by glucocorticoids. Arch Biochem Biophys. 1980 Aug;203(1):37–48. doi: 10.1016/0003-9861(80)90151-4. [DOI] [PubMed] [Google Scholar]
- Simantov R. Basal and potassium stimulated, calcium dependent, endorphins release from pituitary cells. Life Sci. 1978 Dec 18;23(25):2503–2508. doi: 10.1016/0024-3205(78)90175-3. [DOI] [PubMed] [Google Scholar]
- Taraskevich P. S., Douglas W. W. Action potentials occur in cells of the normal anterior pituitary gland and are stimulated by the hypophysiotropic peptide thyrotropin-releasing hormone. Proc Natl Acad Sci U S A. 1977 Sep;74(9):4064–4067. doi: 10.1073/pnas.74.9.4064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler A. S., Dichter M. A., Biales B., DeLellis R. A., Wolfe H. Neural properties of cultured human endocrine tumor cells of proposed neural crest origin. Science. 1976 May 28;192(4242):902–904. doi: 10.1126/science.179139. [DOI] [PubMed] [Google Scholar]
- Wong B. S., Lecar H., Adler M. Single calcium-dependent potassium channels in clonal anterior pituitary cells. Biophys J. 1982 Sep;39(3):313–317. doi: 10.1016/S0006-3495(82)84522-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen G. Single Ca2+-activated nonselective cation channels in neuroblastoma. Nature. 1982 Mar 25;296(5855):357–359. doi: 10.1038/296357a0. [DOI] [PubMed] [Google Scholar]




